2.1. Preparation and Characterization of Nanoemulsions
One of the first considerations when developing NEs as drug delivery systems is the solubility and stability of the drug in oils, as it acts as a driving force for the selection of the oil phase [
25]. Therefore, solubility of curcumin in soybean oil, alone and in combination with MCT was tested, considering their use in many formulations present on the market [
26]. Nanoemulsions prepared only with soybean oil (containing long chain triglycerides—LCT) are characterized with bigger droplet size when comparing to the NEs based on the combination of soybean oil and MCT. Such a result could probably be explained by differences in viscosities between the oil phases. Lower viscosity of MCT and MCT–soybean oil mixtures vs. soybean oil alone means that oil droplets made of lower viscosity oils can be disrupted more efficiently during high pressure homogenization [
27]. A fixed concentration of oil phase, being 20%
w/
w, was chosen due to higher triglyceride content in these emulsions compared to those containing 10% of the oil phase, lower phospholipid–triglyceride ratio and less interference with lipoprotein lipase activity [
28]. Moreover, our aim was to choose the oil phase that would allow the incorporation of the highest concentration of curcumin, bearing in mind prospective in vivo pharmacokinetic studies on an animal model, and also a fact that there is no consensus on curcumin recommended doses range.
Solubility of curcumin in soybean oil alone, as well as in its mixtures with MCT was not satisfactory (<1.5 mg/mL), while the addition of lecithin resulted in slightly higher curcumin solubility (˂4 mg/mL). With the fixed concentration of the oil phase set at 20%, drug loading would be low with the use of oil mixture alone. Therefore, benzyl alcohol, a co-solvent mixable with oils, was used to maximize the incorporation of curcumin, and achieve the concentration of 0.75 mg/mL of curcumin in the NEs, given that the solubility of curcumin in benzyl alcohol was much higher compared to the oils and their mixtures (25 mg/mL). Finally, mixture of soybean oil and MCT in 1:4 ratio (
w/
w) was chosen as the oil phase, relaying additionally on our previous experience, in terms of providing NEs with small droplet size, good stability, and lower viscosity [
16,
29].
The composition of the aqueous phase has been dictated by the composition of the oil phase. Sodium oleate had to be added as an additional stabilizer; otherwise, NEs would fall apart within a week of preparing. Due to its addition, the pH of the formulations was about 8, which was unfavorable for curcumin’s stability [
2,
30], and consequently the pH of the NEs’ aqueous phase was set at around 7 by 0.1 M HCl.
Concordant with applied experimental design (
Table 1), 25 unloaded nanoemulsion formulations were prepared using hot HPH and their physicochemical characterization was performed. Mean droplet size of all NEs was in the range of 106–171 nm, with PDI below 0.2 and ZP about −40 mV, suggesting that developed NEs could be suitable for parenteral use.
2.2. Experimental Design
Considering the impact of droplet size on the in vivo fate of nanoemulsions, their long term stability and success of aseptic filtration, as well as the significance of droplets size distribution for NEs’ safety profile [
25,
31,
32], Z-ave and PDI were chosen as response variables in experimental design. This strategy was employed to study the evolution of droplet size and size distribution in relation to process parameters and formulation factors aiming to find the optimal combination of input variables yielding the NEs with smallest Z-ave and PDI. Specifically, this approach allowed to elucidate the impact of formulation factors (PEG-PLs type and concentration) and process parameters (HPH pressure and number of cycles) on the NEs critical properties (prospective critical quality attributes), and to point out at the same time to any interactions between the independent variables, which could not be seen by employing one factor at the time approach.
Following the applied D-optimal experimental design (
Table 1), a total of 25 runs were proposed [15 (model points) + 5 (to estimate lack of fit) + 5 (replicates)] and then randomly performed. For the dependent variables, Z-ave and PDI, the effects corresponding to the investigated factors (PEG-PL type and concentration, HPH pressure and number of cycles) and their interactions were calculated (to see Z-ave and PDI for the NEs defined by experimental design, please check
Table 1). Factors and interactions with insignificant (
p > 0.05) influence on estimated responses were excluded from the generated models, except model terms required to support hierarchy, thus raising the reduced factorial models whose equations, in terms of coded factors, are given below in Equation (1) (Z-ave) and Equation (2) (PDI):
The ANOVA analysis showed the models generated for Z-Ave (model F value = 266.99) and PDI (model F value = 9.22) were significant (p < 0.05) with the non-significant lack of fit relative to the pure error. The proposed models had appropriate R2, adjusted R2, and adequate precision values (Z-Ave: 0.9956, 0.9919, 47.903; PDI: 0.7546, 0.6728, 10.195, respectively), indicating that two responses were well described by the proposed models and the models could be used to navigate the design space.
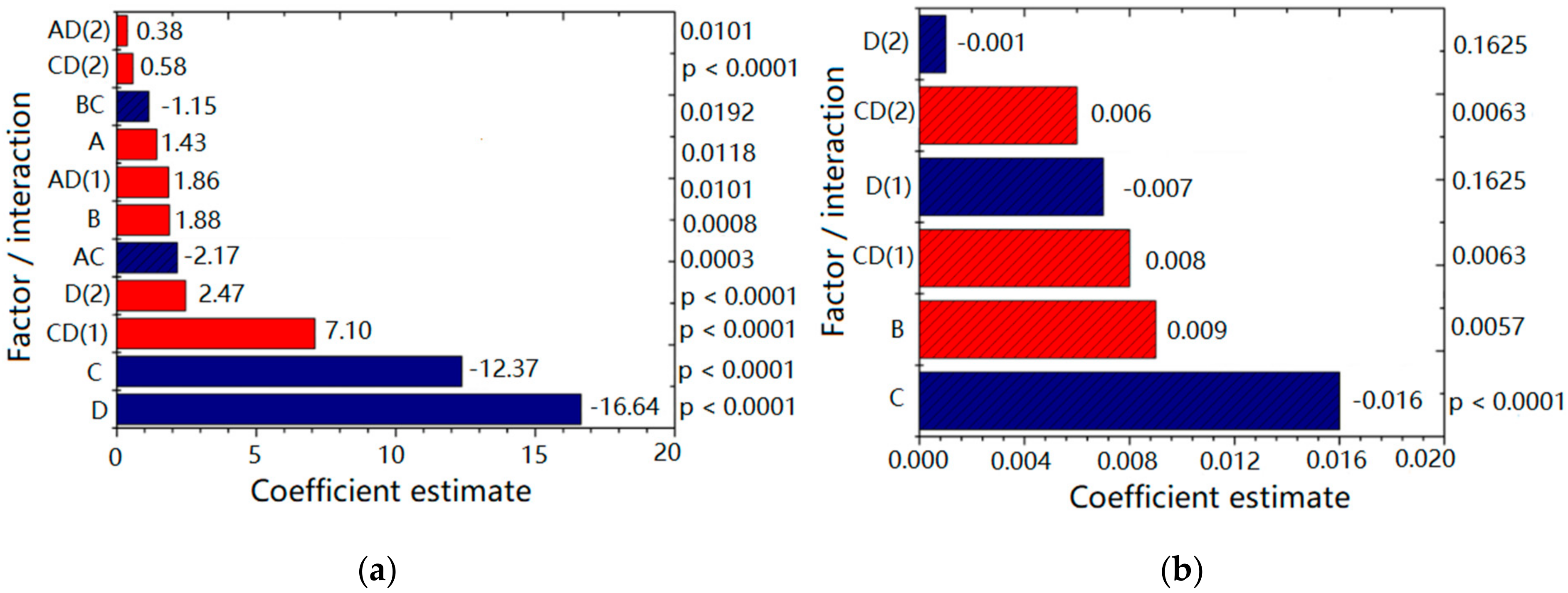
Figure 1 depicts plots for the factors and interactions (with corresponding coefficient values and
p values) influencing Z-ave (
Figure 1a) and PDI (
Figure 1b) of designed NEs. Higher coefficient value indicated a more pronounced effect on the investigated response, whereby a sign in front of coefficient indicated the direction of the model term influence; the negative sign pointed to the antagonistic effect of the adjoining factor. As can be seen from
Figure 1a, the response Z-ave was significantly (
p < 0.05) influenced by all the investigated factors in the following order: number of HPH cycles [D] > HPH pressure [C] > PEG-PL concentration [B] > PEG-PL type [A], with the process parameters (D and C) having a more pronounced effect compared to the formulation factors (A and B). By increasing the HPH pressure, Z-ave decreased (antagonistic effect). Higher homogenization pressure imposed bigger sheer force and turbulence on the processed formulation resulting in droplet size reduction [
31]. Similarly, more HPH cycles usually led to Z-ave reduction, whereas the increase of PEG-PL concentration and the use of PEG5000-DPPE (compared to PEG2000-DSPE) led to increase of droplet size (synergistic effect). Furthermore, not only investigated factors alone, but also their interactions (HPH pressure/number of HPH cycles—CD; PEG-PL type/HPH pressure—AC; PEG—PL concentration/HPH pressure—BC; PEG-PL type/number of HPH cycles—AD) were shown to influence Z-ave at a significant level (
p < 0.05), with CD being the most significant (
Figure 1a).
On the other hand, PDI was significantly (
p < 0.05) influenced by HPH pressure [C] and PEG-PL concentration [B], in the declining order (
Figure 1b). Namely, PEG-PL concentration had a positive effect, meaning that the droplet size distribution was wider when 0.3% of PEG-PL was used compared to the 0.1%. On the contrary, the increase of HPH pressure produced narrower (desired) PDI (antagonistic effect). The number of HPH cycles [D] individually was not a significant factor for PDI, but it was kept in the model because the interaction between HPH pressure and the number of HPH cycles—CD has been shown as significant (
Figure 1b).
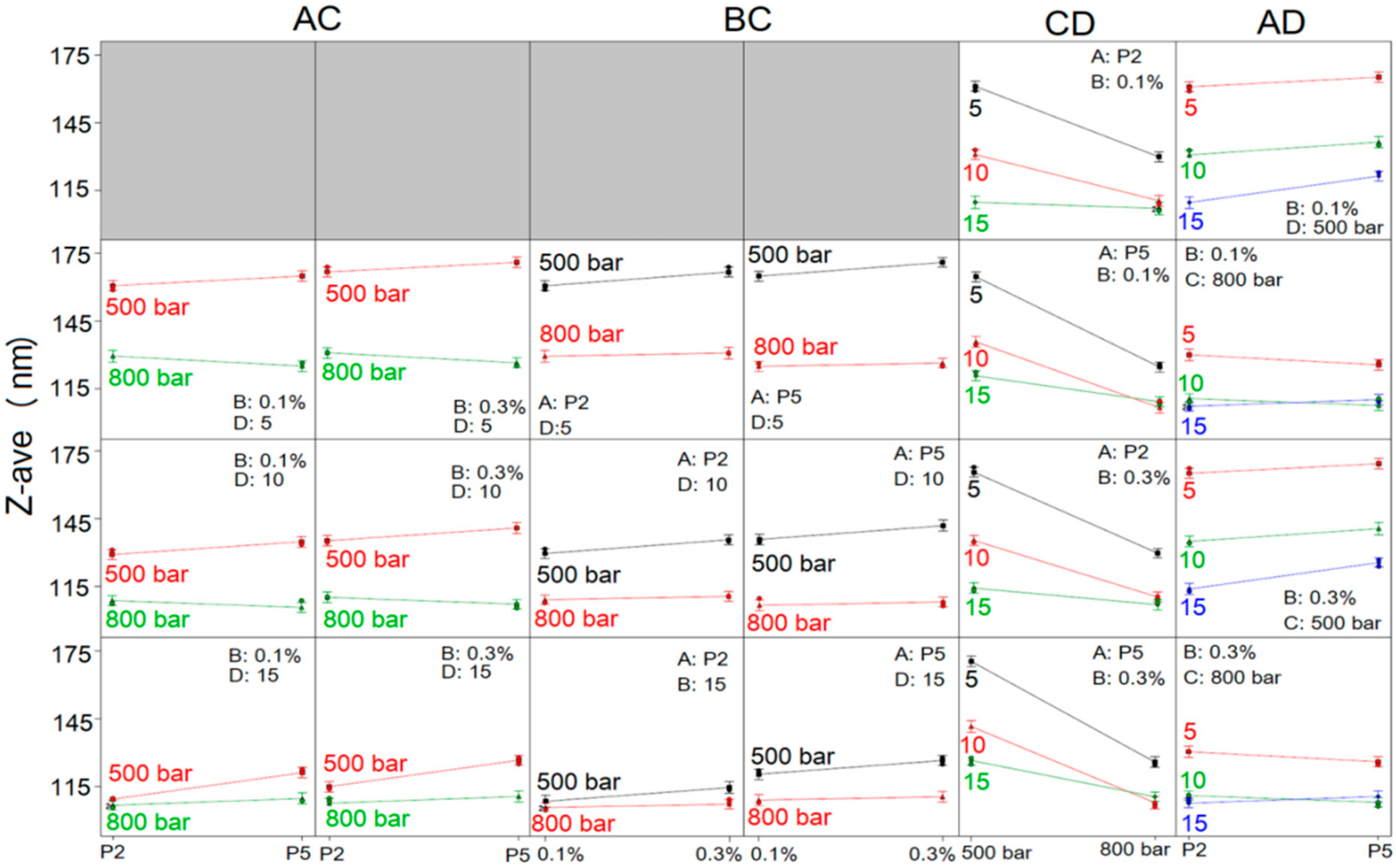
To comprehend the full impact of investigated factors on NE droplet size and size distribution, the interactions between the factors were thoroughly studied, and the constructed interaction plots are shown in
Figure 2 and
Figure 3. It can be observed, through AC interaction plots (
Figure 2), that the mean droplet size of NEs was lower when HPH pressure used for NE preparation was 800 bar compared to 500 bar, regardless of the type or concentration of PEG-PL or the number of homogenization cycles, whereby the difference in the Z-ave of NEs obtained under two different pressures was higher when fewer HPH cycles were employed (5 or 10 instead of 15 cycles). Furthermore, Z-ave of NEs prepared with PEG2000-DSPE at 500 bar was lower, as opposed to NEs formulated with PEG5000-DPPE, whether 0.1% or 0.3% of PEG-PL was used. On the contrary, when HPH was performed at 800 bar, Z-ave of NEs containing PEG5000-DPPE was slightly lower after 5 and 10 cycles of homogenization and slightly higher after 15 homogenization cycles, compared to NEs with PEG2000-DSPE, regardless of the PEG-PL concentration. From the plots describing the influence of BC interaction (
Figure 2), it could be seen that the Z-ave of NEs produced with both PEG-PL types at HPH pressure of 500 bar, increased when the PEG-PL concentration increased from 0.1% to 0.3%, regardless of the employed number of homogenization cycles. When the HPH pressure of 800 bar was used, Z-ave of all obtained NEs was almost unchanged with increasing PEG-PL concentration.
Looking further at the CD interaction (
Figure 2), it could be confirmed that with both PEG-PL types, at both concentrations, pressure of 800 bar delivered NEs with smaller droplets compared to 500 bar pressure, irrespective of the number of homogenization cycles; the decrease in Z-ave with increasing pressure was more pronounced when number of HPH cycles applied was 5 and 10, while the change in Z-ave was less noticeable in case of 15 homogenization cycles and especially when PEG2000-DSPE was used as PEG-PL. Moreover, the process factors’ combination of 800 bar/10 cycles yielded NEs with the smallest Z-ave in case of NEs formulated with PEG5000-DPPE, whereas in case of NEs based on PEG2000-DSPE no significant difference in droplet size was observed after 10 and 15 cycles of homogenization at 800 bar. In the AD interaction plots (
Figure 2) it was observed that, regardless of the PEG-PL concentration, increase of homogenization cycles from 5 to 15, reduced the Z-ave of PEG2000-DSPE NEs; the decrease in Z-ave is more pronounced at 500 bar compared to 800 bar. The same scenario was observed with PEG5000-DPPE NEs produced at 500 bar—Z-ave decreased as the number of cycles increased. Slight deviation from this observation was seen when PEG5000-DPPE NEs were produced at 800 bar, where the smallest droplet size was obtained with 10 homogenization cycles.
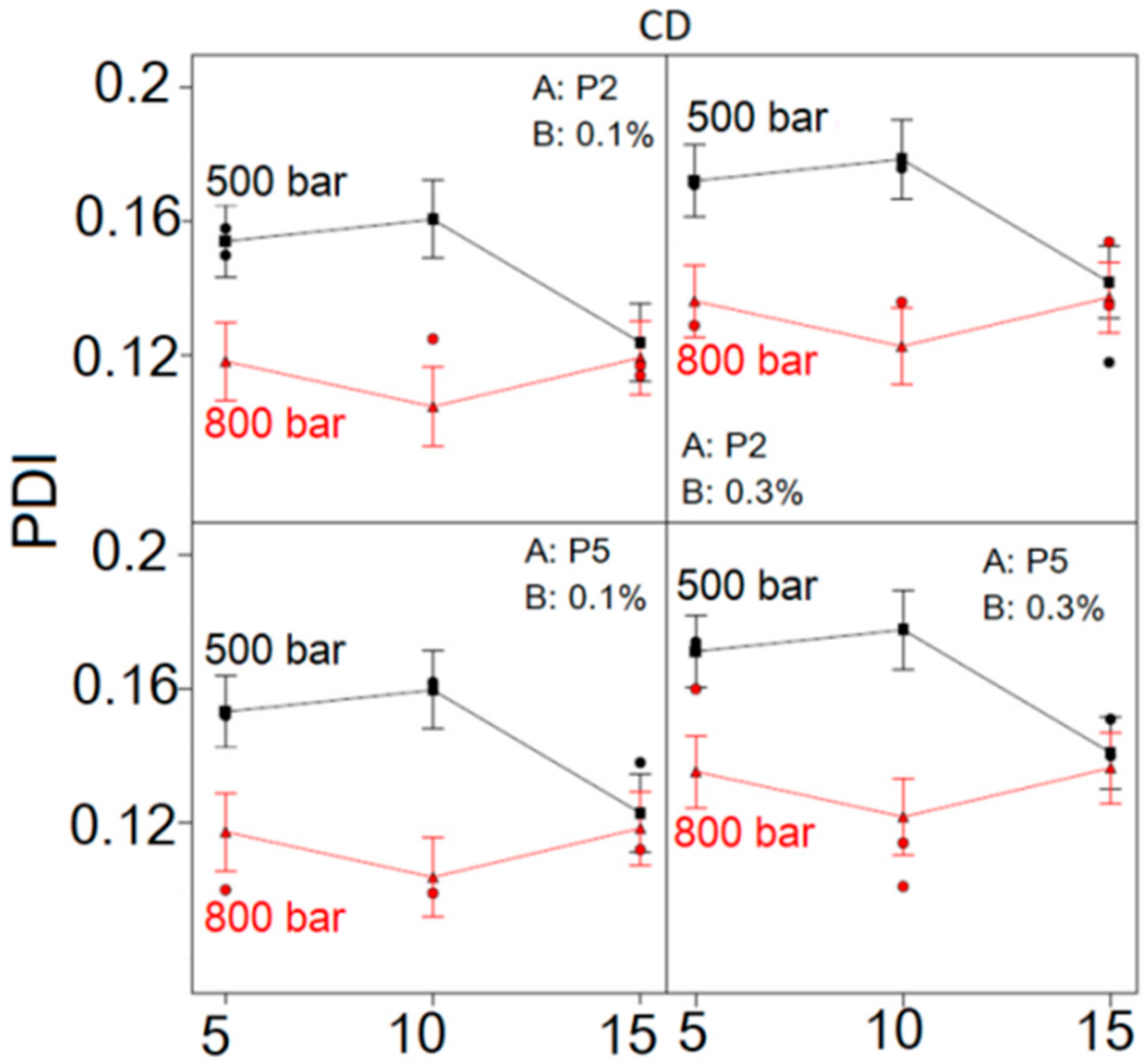
Considering the influence of CD interaction on NE droplet size distribution (
Figure 3), it could be observed that PDI was lower at HPH pressure of 800 bar, regardless of the number of HPH cycles, type and concentration of PEG-PL, compared to the PDI of formulations made at 500 bar. The difference in PDI between NEs produced at 500 and 800 bar was pronounced when the number of applied HPH cycles was 5 and 10, whereas with 15 homogenization cycles this difference became negligible. At the HPH pressure of 500 bar, the increase of HPH cycles from 5 to 10 led to increase of PDI, whereas further increase of HPH cycles from 10 to 15 caused the PDI of NEs to decrease. On the contrary, at HPH pressure of 800 bar, the initial decline of PDI between HPH cycles 5 and 10 was followed by an increase between cycles 10 and 15, irrespective of PEG-PL type and concentration. Likewise, the use of higher PEG-PL concentration led to increase in PDI, independent of PEG-PL type, HPH cycles and pressure. In general, the lowest PDI was observed at HPH pressure of 800 bar after 10 homogenization cycles, disregarding PEG-PL type or concentration.
Taking into consideration all the individual factors, as well as their interactions, numerical optimization process was used in order to choose the formulations with smallest Z-ave and PDI. In that vein, the program chose the top 10 formulations (out of 25 possibilities, including repeated runs, see
Table 1) and assigned each one a desirability value. Higher desirability value indicated better accordance with set goals—minimized Z-ave and PDI (
Table 2). According to obtained results, both PEG2000-DSPE and PEG5000-DPPE could be used in both proposed concentrations (0.1% and 0.3%), while HPH pressure of 800 bar and 10 homogenization cycles were chosen as optimal process parameters. These were the templates for curcumin loaded formulations.
To verify validity of the optimal parameters and calculated predicated responses, three of the proposed optimized nanoemulsion formulations (solutions 1, 2, and 5;
Table 2) were selected. Three batches of each formulation were prepared, and obtained experimental data for Z-ave and PDI were compared with predicted ones. The observed experimental values from the confirmation experiment were within the calculated prediction interval, that is, in agreement with the predicted values (
Supplementary Materials,
Table S1), suggesting that the developed experimental design could be successfully used for evaluation and optimization of nanoemulsion formulation and manufacturing method, within the actual design space.
2.3. Curcumin-Loaded Nanoemulsions—Characterization and Stability Evaluation
Based on the results of experimental design, PEGylated nanoemulsions loaded with curcumin were prepared with the addition of 0.1%/0.3% of PEG2000-DSPE (CP21 and CP23, respectively) in oil phase or 0.1%/0.3% of PEG5000-DPPE (CP51 and CP53, respectively) in aqueous phase (
Table 3), employing the homogenization at 800 bar for 10 cycles. The comprehensive evaluation of obtained nanoemulsions, together with the control non-PEGylated formulation (CNPEG), regarding their physicochemical properties, morphology and two-year stability during storage at room temperature, was performed. Upon preparation, all curcumin-loaded NEs were yellow in color and homogeneous in appearance. Average droplet size was 101–108 nm, with narrow distribution (PDI < 0.15), indicating suitability for parenteral application [
33]. Absolute values of ZP were above 30 mV (
Table 4), pH values between 6.76 and 6.89 and viscosity was in the range 23–46 mPa*s (
Table 5). Additional LD measurement confirmed the absence of droplets bigger than 1 µm with d100 below 0.258 µm (
Supplementary Materials,
Figure S1). Osmolality values for the CNPEG, CP21, CP23, CP51 and CP53 were 556, 555, 531, 562 and 543 mOsm/kg, respectively. This would indicate good tolerability upon parenteral administration, as osmolality values higher than 600 mOSm/kg have been reported to cause shriveling up of red blood cells and significant pain, while osmolality values less than 150 mOsm/kg may cause hemolysis [
34].
When comparing CNPEG to C-PEG-NEs (
Table 4), a slight (less than 10 nm), but statistically significant increase in average droplet size was observed (
t-test,
p < 0.001). This could indicate that the maximal decrease in droplet size has already been achieved and further addition of stabilizers (PEG-PLs) cannot additionally reduce droplet size. On the contrary, due to the hydrophilic nature of the PEG chains covering the droplets surface, the hydrodynamic radius measured by DLS technique and expressed as Z-ave, was enlarged [
14,
35]. There were no statistically significant differences in Z-ave between placebo and curcumin-loaded formulations, except when 0.3% of PEG-PLs were used (
t-test,
p < 0.001, CP23 vs. P23;
p < 0.01, CP53 vs. P53), where curcumin-loading caused decrease in average droplet size. This could indicate interactions between curcumin and stabilizing layer of nanoemulsions for CP23 and CP53 [
36]. Absolute values of ZP about 40 mV indicated a good chance for the long term stability. The highest absolute value of zeta potential in curcumin-loaded NEs was observed in CNPEG, while the addition of PEG-PLs caused decrease in zeta potential values in C-PEG-NEs (
Table 4). This could be explained by the fact that PEG chains provide a steric coverage of the droplet surface, thus reducing the zeta potential value compared to the CNPEG, which should not be taken as a sign of decreased stability [
35]. In NEs stabilized with 0.1% of PEG2000-DSPE/PEG5000-DPPE, the addition of curcumin led to changes in ZP values, a decrease of absolute ZP values for CP21, and an increase for CP51 (
t-test,
p < 0.05), while the differences in ZP between other placebo and corresponding curcumin-loaded NEs were not statistically significant (
Table 4).
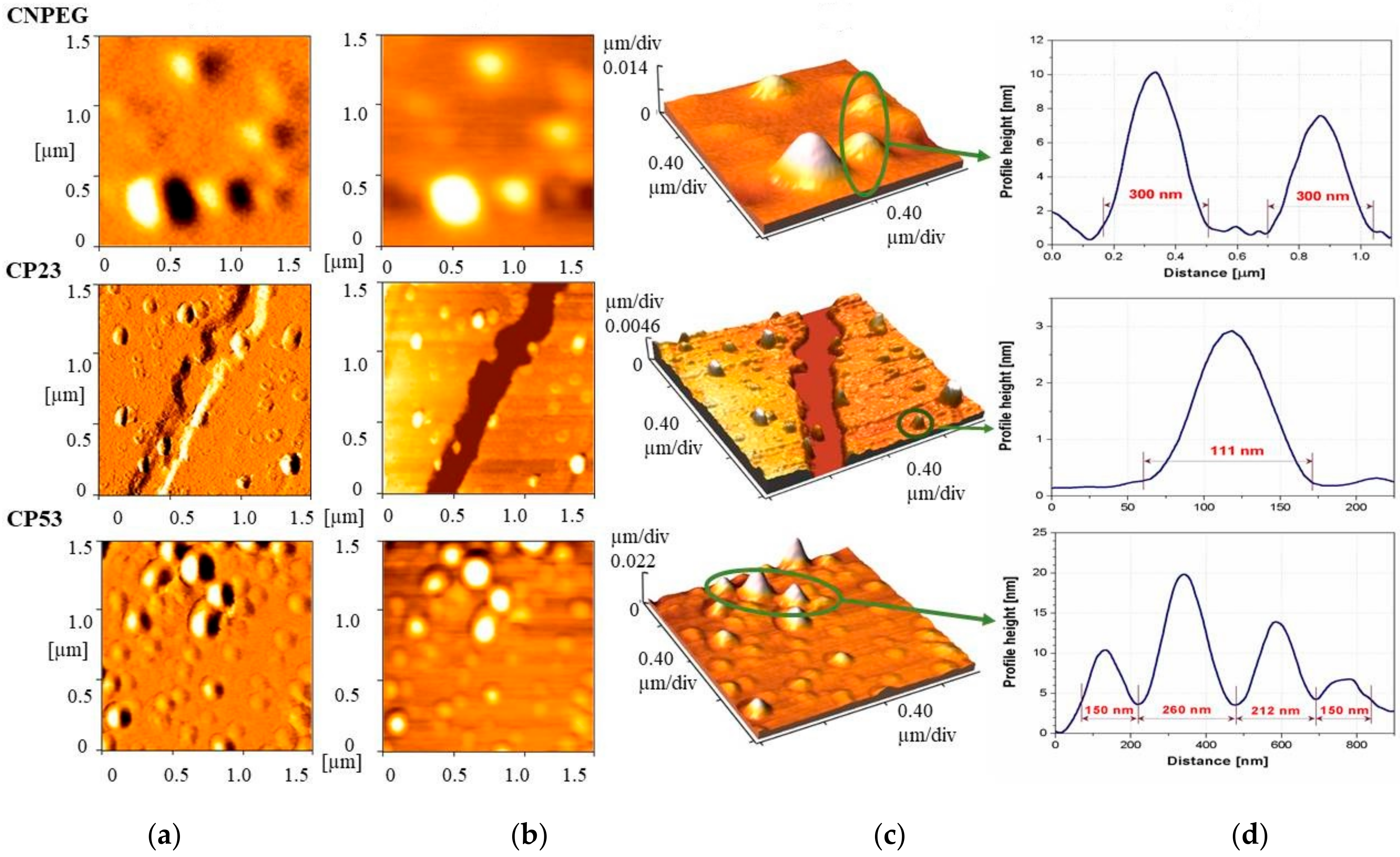
In order to provide additional information regarding droplet size, size distribution, morphology and potential aggregation of curcumin loaded samples, AFM analysis was performed. The AFM images depicting error signal, 2D and 3D topography from the selected samples—the non-PEGylated CNPEG and two PEGylated NEs—CP23 and CP53 are shown on
Figure 4a,
4b and
4c, respectively. Height profiles for the selected droplets are presented in
Figure 4d. AFM photomicrographs showed slightly larger droplet diameter of CNPEG NE than data from DLS and LD analyses suggested, possibly due to the drying process used for sample preparation in AFM that might cause the droplet merger. On the other hand, the photomicrographs of the PEGylated samples showed droplet sizes concordant with the DLS and LD measurements. It appeared that droplets of CP23 sample were more spherical in shape, while the droplets of CP53 were more elliptical. Additionally, it would appear that the droplets of CP23 were better defined compared to the other two samples, possibly indicating a more compact interlayer formed by PEG2000-DSPE compared to PEG5000-DPPE. However, such assumption should be further investigated by other techniques, such as electron paramagnetic resonance spectroscopy. No undissolved drug crystals were detected in any of the formulations.
Considering the pore diameter for aseptic filtration is 0.22 µm, droplet size should be below 200 nm to avoid the loss of droplets and incorporated drug. Droplet size (Z-ave) was measured prior to and upon the filtration to find out the impact of the sterilization process on droplet size and PDI and results are shown in
Table 6. Although there were some differences in PDI observed between the formulations before and after filtration, additional LD measurements confirmed absence of larger droplets (
Supplementary Materials,
Figure S2), proving the aseptic filtration did not impact physicochemical characteristics of the formulation.
The stability of prepared curcumin-loaded NEs was followed for two years, which was particularly relevant considering the shelf life of some marketed formulations—such as Diazemuls
®, 5 mg/mL emulsion for injection (Accord Healthcare Limited, Harrow, UK) or Diprivan
®, 20 mg/mL emulsion for injection or infusion (Aspen Pharma Trading Limited, Dublin, Ireland) is two years, according to their summary of product characteristics (SmPC). Upon visual inspection there were no signs of NEs phase separation in the vials during the two-year storage period. Although the change in average droplet size (
Table 4) was statistically significant for all curcumin-loaded NEs (
t-test,
p < 0.05), except for CP21, the slight increase in Z-ave (up to 10 nm for C-PEG-NEs and about 30 nm for CNPEG) still deemed them acceptable for parenteral application. Such findings may indicate an efficient stabilizing role of the PEG-PLs during the long-term storage period. After two years, PDI values remained below 0.25 (
Table 4), indicating narrow droplet size distribution and the absence of coalescence of droplets. LD measurements, performed after one year of storage showed no significant change in droplet size (
Supplementary Materials,
Figure S1). More importantly, the absence of a population of larger droplets was confirmed. Some larger droplets could be observed under optical microscope at 1000× magnification (
Supplementary Materials,
Figure S3) after two years of storage, but this is somehow expected when preparing NEs with HPH technique and, taken together with results obtained from other characterization techniques, was not interpreted as a sign of instability. No signs of curcumin crystals were detected. Absolute ZP values of the curcumin-loaded NEs (
Table 4), changed slightly during storage (
t-test,
p < 0.05), while still remaining above 30 mV.
After two years storage, pH values of investigated NEs dropped by approximately 1 unit, from about 7 to about 6 (
Table 5). With the exception of CP53, the decrease of pH was more pronounced in non-PEGylated (CNPEG) compared to the PEGylated formulations, which was attributed to the fact that the PEG chains on the droplets surface may provide protection against the degradation of lecithin and the release of free fatty acids stabilizing pH in that way [
36]. Viscosity of the curcumin-loaded formulations and their conductivity also decreased during storage (
Table 5), which was not seen as a significant influence on the safety of nanoemulsions. After two years osmolality values for CNPEG, CP21, CP23, CP51 and CP53 were 538, 547, 539, 543 and 546 mOsm/kg, respectively, remaining in the same acceptable hypertonic range. As another stability indicator, concentration of curcumin in the formulations did not decrease during the two-year storage period (
Supplementary Materials,
Figure S4), additionally supporting the claim of stability for designed curcumin-NEs.
2.4. Antioxidant Assay
Due to the fact that most antioxidants are multifunctional, more than one type of assay should be performed in order to take into account the various mechanisms of antioxidant action [
37]. DPPH assay is based on the reduction of DPPH free radical in the presence of a hydrogen donating antioxidant, resulting in the formation of the non-radical form of DPPH, which is manifested by the loss of violet color [
38]. The degree of discoloration corresponds to the radical scavenging potential of the investigated antioxidant [
39]. The assessment of curcumin, tocopherol and BHT through this assay showed that that the antioxidant potential of curcumin and tocopherol were similar (
Supplementary Materials,
Figure S5), with IC50 values of 0.1195 ± 0.0015 and 0.1023 ± 0.0045 mg/mL, respectively, coinciding with previous research results [
23]. However, scavenging activity of BHT appeared to be much lower at the same concentrations (
Figure S5,
Supplementary Materials), potentially because BHT alone has shown less hydrogen donating ability compared to the tocopherol [
40], which is the main mechanism of antioxidant action in this assay.
Antioxidant potential of curcumin-loaded nanoemulsions (CNPEG, CP21, CP51) assessed through DPPH assay indicated that curcumin maintained its antioxidant potential, as evidenced by the IC50 values obtained after testing freshly prepared formulations and the ones kept for two years at room temperature (
Table 7), which is in line with the curcumin chemical stability in the formulations (
Supplementary Materials,
Figure S4). It can also be noted the scavenging activity of the selected formulations was higher compared to the curcumin alone (lower IC50 values), which could be explained by the presence of BHT in the formulations.
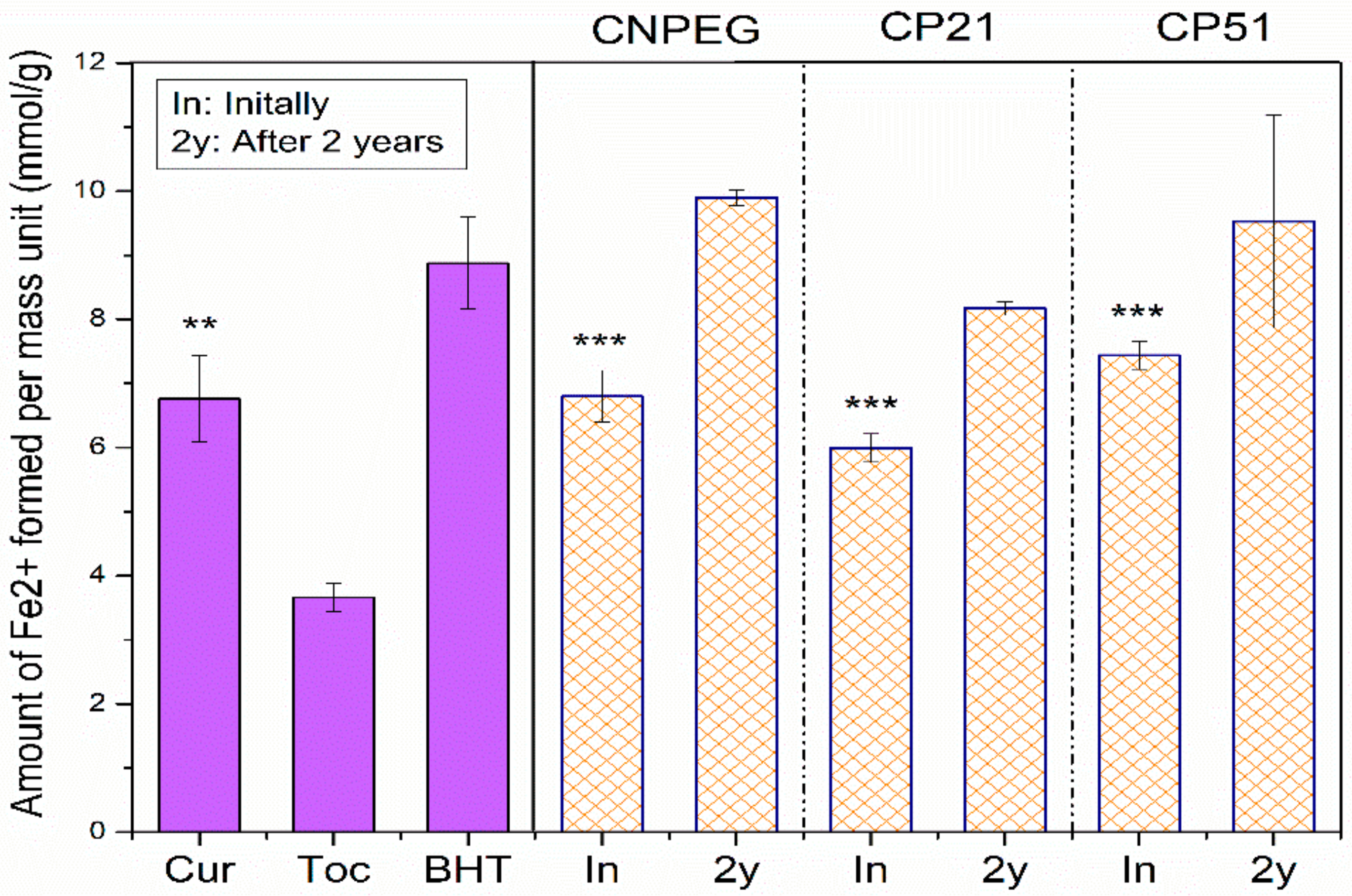
On the other hand, FRAP assay is based on the antioxidant induced reduction of ferric tripyridyltriazine complex to the ferrous tripyridyltriazine, at low pH [
23]. Another point of distinction between these two assays lies in the fact that FRAP assay is performed in aqueous surroundings where the droplet structure is preserved. FRAP values for tocopherol were significantly lower compared to curcumin and nanoemulsion formulations (CNPEG, CP21 and CP51)—3.66 ± 0.22 mmol Fe
2+/g versus 6.80 ± 0.40, 5.99 ± 0.22 and 7.44 ± 0.22 mmol Fe
2+/g, respectively (
p < 0.05, Student’s
t-test), probably due to the phenolic structure of curcumin which is known to impact FRAP values [
37]. On the other hand, there were differences between FRAP values of curcumin and its nanoemulsions (CNPEG, CP21, CP51), but these distinctions were deemed statistically insignificant (
p = 0.937;
p = 0.135 and
p = 0.175 for curcumin versus CNPEG, CP21 and CP51, Student’s
t-test). FRAP values of the formulations did not decrease after two years of storage, confirming the preservation of antioxidant potential during storage, with retention percentage values of 145.56%, 136.36% and 128.09% for CNPEG, CP21 and CP51, respectively, compared to the initial results (
Figure 5), which was found acceptable compared to the values obtained in other FRAP assays assessing the antioxidant potential of different fruits during storage [
41].
2.5. In Vitro Drug Release
In vitro release of curcumin from the selected NEs was studied via reverse dialysis bag technique, which is considered to mimic in vivo situation for NEs and other colloidal systems upon their intravenous administration [
42,
43,
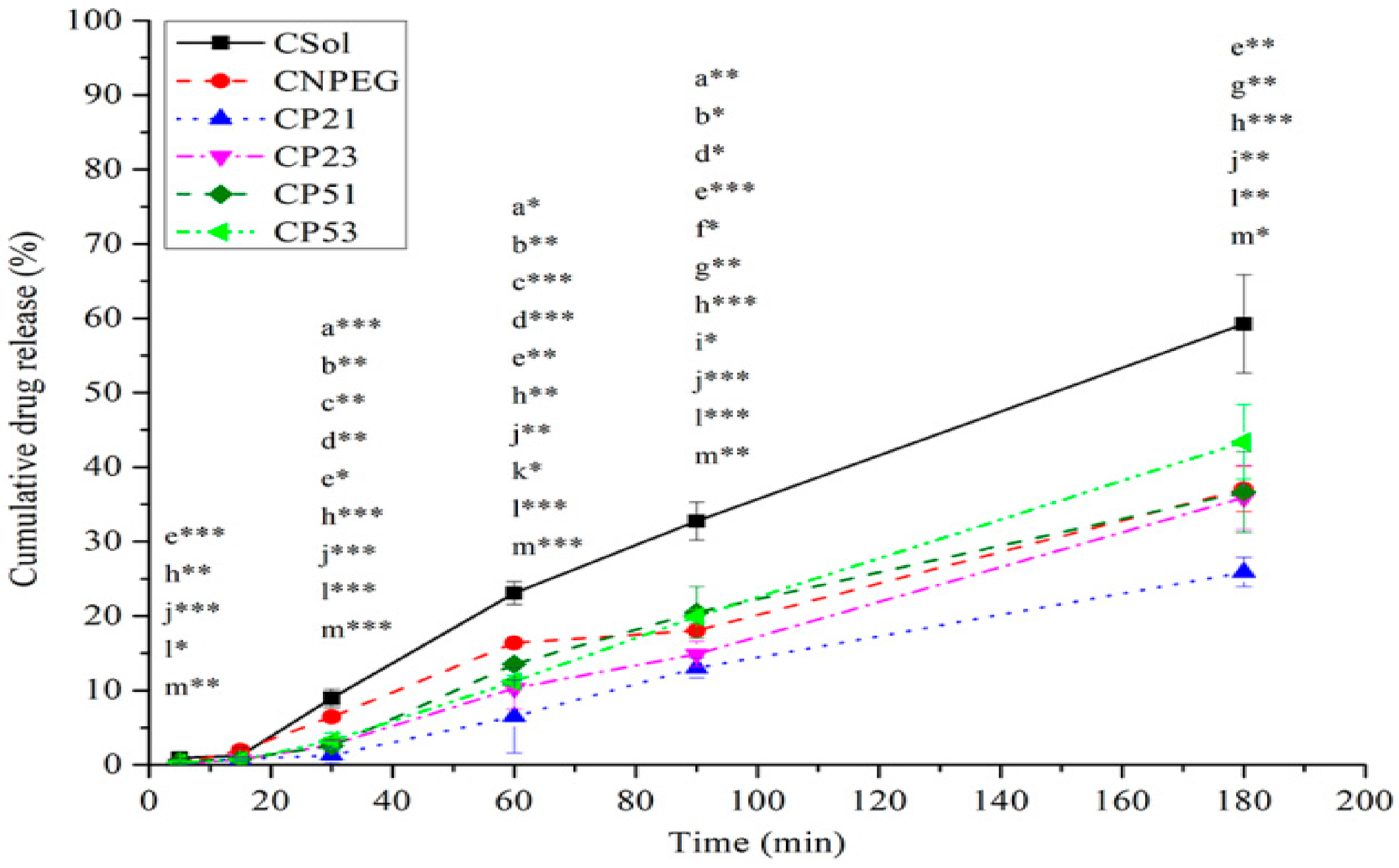
44]. It could be observed from the release profiles shown in
Figure 6 that after 60 min the highest fraction of curcumin was released from the non-PEGylated formulation (CNPEG—16.38% ± 0.23%, compared to 6.49% ± 4.93%, 10.28% ± 2.79%, 13.51% ± 0.48% and 11.20% ± 0.8% released from CP21, CP23, CP51 and CP53, respectively). This was expected, given that the PEG chains provide steric coating around the droplet, slowing down the release of curcumin from the PEGylated NE droplets [
14]. Interestingly, after 90 min, the fraction of curcumin released from CP53 was bigger compared to CNPEG (19.92% ± 0.89% vs. 18.04% ± 0.17%,
p < 0.05), but after 180 min there were no statistically significant differences in fractions of curcumin released from CNPEG and C-PEG-NEs. The only statistically significant difference in the fraction of released curcumin after 180 min was between CP21 and CP53 (25.88% ± 1.97% vs. 43.38% ± 4.99%,
p < 0.05). It should be noted that the fraction of curcumin released from all of the formulations was significantly lower (
p < 0.05) compared to the curcumin solution (CSol) at every time point, except after 15 min when there were no statistically significant differences in the fractions of curcumin released between any of the formulations (
Figure 6). This could be expected, given that curcumin is encapsulated inside the nanoemulsions, and it has to, firstly be released from the oil droplets and then pass the dialysis bag membrane, whereas with CSol the only limiting step is the dialysis bag itself. The difference between the release of curcumin between the CSol and nanoemulsions provides a better insight as to the encapsulation efficacy of the carrier itself. Interestingly, the increase of the PEG2000-DSPE concentration (from 0.1% to 0.3%) did not further slowdown the release of curcumin from the nanoemulsions (CP21 and CP23, respectively). In fact, the release of curcumin was lower from the CP21 formulation compared to the CP23, while the similar trend did not apply to the CP51 and CP53 NEs, where there were no statistically significant differences between the fractions of curcumin released, except at 60 min time point (
t-test,
p < 0.05). Therefore, it could be noted that, while both types of PEG-PLs contributed to lower release of curcumin from the C-PEG-NEs compared to the CNPEG, PEG2000-DSPE had a bigger impact compared to the PEG5000-DPPE. The influence of the concentration of the PEG-PLs on the curcumin release was not that clear, given its low influence on the CP51 and CP53, and that an increase of PEG2000-DSPE concentration did not further lower the fraction of curcumin released, but had an opposite effect. Given the increase of PEGylated phospholipid concentration did not further slowdown the release of curcumin from the PEGylated formulations, the CP23 and CP53 formulations were excluded, leaving CP21 and CP51 as better candidates for further research.
In order to gain deeper insight into curcumin release kinetics from investigated NEs, the experimental data obtained from the release assay were fitted into different mathematical models—zero-order, first-order, Higuchi, Hixson–Crowell, Baker–Lonsdale, Korsmeyer–Peppas [
45,
46], and the parameters calculated are reported in
Table S2,
Supplementary Materials. Based on the highest values of the coefficient of determination (R
2) and the adjusted coefficient of determination (R
2 adjusted), and the lowest Akaike information criterion (AIC) [
45,
46], the best fitting of the experimental data was obtained with the Hixson–Crowell model for CNPEG and zero-order for the PEG-NEs (please see
Table S2,
Supplementary Materials). These models were chosen as optimal to interpret the release kinetics of curcumin from investigated nanoemulsions.
By analyzing the release rate constant of PEG-NEs (
Supplementary Materials,
Table S1, zero-order model), it could be noticed that the release rates of curcumin from CP21 and CP23 were significantly lower compared to the CP51 (
t-test,
p < 0.05, CP21 vs. CP51) and CP53 (
t-test,
p < 0.05, CP21 vs. CP53 and CP23 vs. CP53). This was probably due to the fact that DSPE provides a more rigid droplet interface compared to the DPPE [
14], resulting in retarded release, which should be corroborated in further investigations. PEG-NEs are best described by zero-order kinetic model used to describe dosage forms that do not disaggregate, indicating that the nanoemulsion droplets remained intact during testing, thus providing controlled release [
45,
46]. On the other hand, the non-PEGylated formulation is best described by Hixson–Crowel model, suggesting that nanoemulsion droplets were destructed during the release testing, and the release rate was limited by the curcumin’s dissolution rate, but not by the diffusion through the stabilizing layer. This furthermore corroborates the stabilizing effects of PEG-PLs on nanoemulsions.
2.6. Cytotoxic Assay and Hemolysis
In vitro cell viability assay was conducted in order to estimate the biological impact of selected nanoemulsion formulations. Firstly, the effect of the formulations was assessed towards the MRC5 cell line in order to check their safety profile. It could be observed from
Figure 7a that neither of the tested curcumin-loaded (CNPEG, CP21, CP51) nor their corresponding placebo formulations (NPEG, P21, P51) had an effect on this cell line in the investigated concentration range, indicating good safety profile. An increase of the formulation concentration in the cell medium led to slightly diminished effect on cell viability, which could probably be explained by the presence of benzyl alcohol in the formulations, which can act as preservative and has been demonstrated to have a concentration dependent cytotoxic effect [
47].
Safety of the curcumin-loaded formulations was further corroborated with hemolysis test results that found them to be nonhemolytic, with the percentage of hemolysis 7.17 ± 0.73%, 2.65 ± 0.39% and 2.96 ± 3.44% for CNPEG, CP21 and CP51, respectively [
48]. It should be noted that, even though all formulations were nonhemolytic, the non-PEGylated formulation (CNPEG) had more than two-fold increase in hemolytic activity compared to the PEGylated nanoemulsions (CP21 and CP51), probably due to the PEG chains hindering the interactions with erytrocytes. Additionally, this test was significant in confirming the safety of the formulations chosen for in vivo testing, given that it was performed using rat blood and undiluted formulations, thus simulating conditions upon intravenous nanoemulsion administration.
In addition to the MRC5 cell line, the effectiveness of the formulations was assessed on colon carcinoma cell line (LS). It was found that the curcumin-loaded formulations had an impact on cell viability, while their counterpart placebo prepared in the same dilution had not (
Figure 7b). Higher impact on cell viability coming from curcumin itself, compared to the formulations could be explained by the encapsulation of curcumin into the droplets and strong steric protection, which could slow down the release and cellular uptake of curcumin, giving rise to lower activity [
49]. Such conclusion is also in line with the in vitro release study.
2.7. A Pharmacokinetic Pilot Study
Inherent physicochemical instability of curcumin makes any computational predictions of its bioavailability very unreliable [
9]. This is further complicated when investigating the advanced drug delivery systems, such as PEGylated nanoemulsions. In vivo fate of curcumin had been followed for only 60 min [
50] when formulated as nanosuspensions, or up to 24 h, when incorporated into zein-polysulfobetaine conjugate-based nanocarriers [
51]. This is why the pilot study of pharmacokinetics was warranted—in order to elucidate whether or not there is a difference in curcumin concentration in different tissues and organs 20 min or 24 h after the treatment administration and to further help establishing the time intervals for curcumin detection in selected tissues and organs. It could be noticed that the concentration of curcumin in plasma 20 min after administration was three-fold higher for CP21 and CP51 compared to the curcumin solution and almost two-fold higher compared to CNPEG (
Table 8). This could be ascribed to the presence of PEGylated phospholipids in CP21 and CP51 which provide protection against mononuclear phagocytic system (MPS) and allow prolonged circulation of the nanoemulsion droplets [
14,
52]. On the other hand, 24 h upon administration no significant changes in curcumin plasma concentrations were found between the formulations, probably due to distribution to the extravascular tissues and organs (
Table 8). Distribution of curcumin into the brain was studied because it makes for a possible target site for its action [
53]. It could be noted that the concentration of curcumin did not differ both 20 min and 24 h upon application and between the formulations (
Table 8). This could potentially be explained by the Cmax concentration being reached sometime between these two time points, so it could not be detected at the chosen time points. When it comes to kidneys, it could be observed that after 24 h the concentration was at least two times lower compared to the values obtained after 20 min (
Table 8), indicating that curcumin had been eliminated from the kidneys sometime between, probably after metabolization through reduction and conjugation [
2,
54]. This study shows that, while the nanoemulsion itself, as well as its PEGylated forms prolong circulation time of curcumin, it is almost completely eliminated from all the investigated matrices. Therefore, even though PEGylated NEs seem to be promising carriers for curcumin delivery, a full scale study should be performed, with final time point set at under 24 h, in order to gain a more complete insight into the formulation’s impact on in vivo fate of curcumin upon application.