Ligand-Tuning of the Stability of Pd(II) Conjugates with Cyanocobalamin
Abstract
1. Introduction
2. Results and Discussion
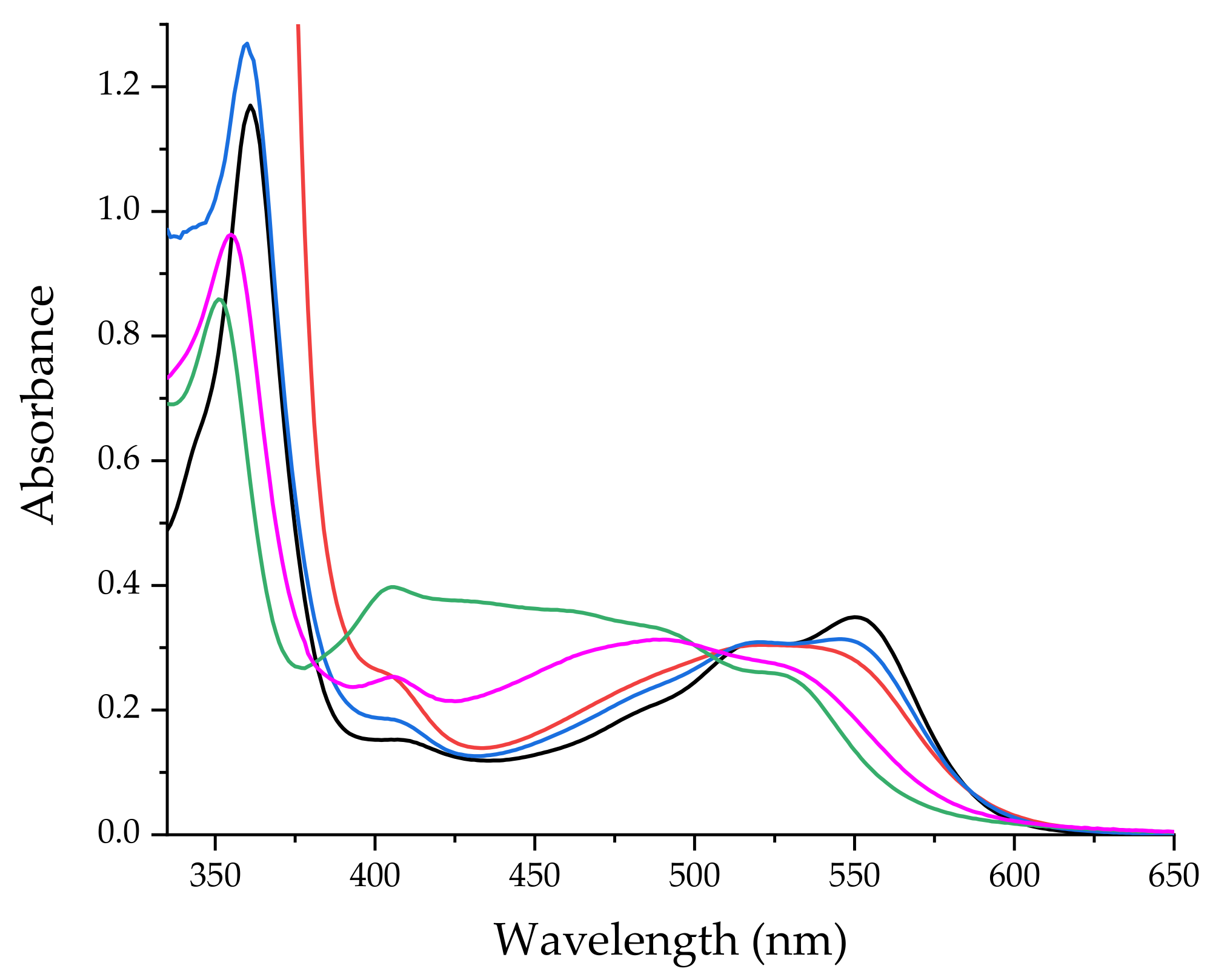
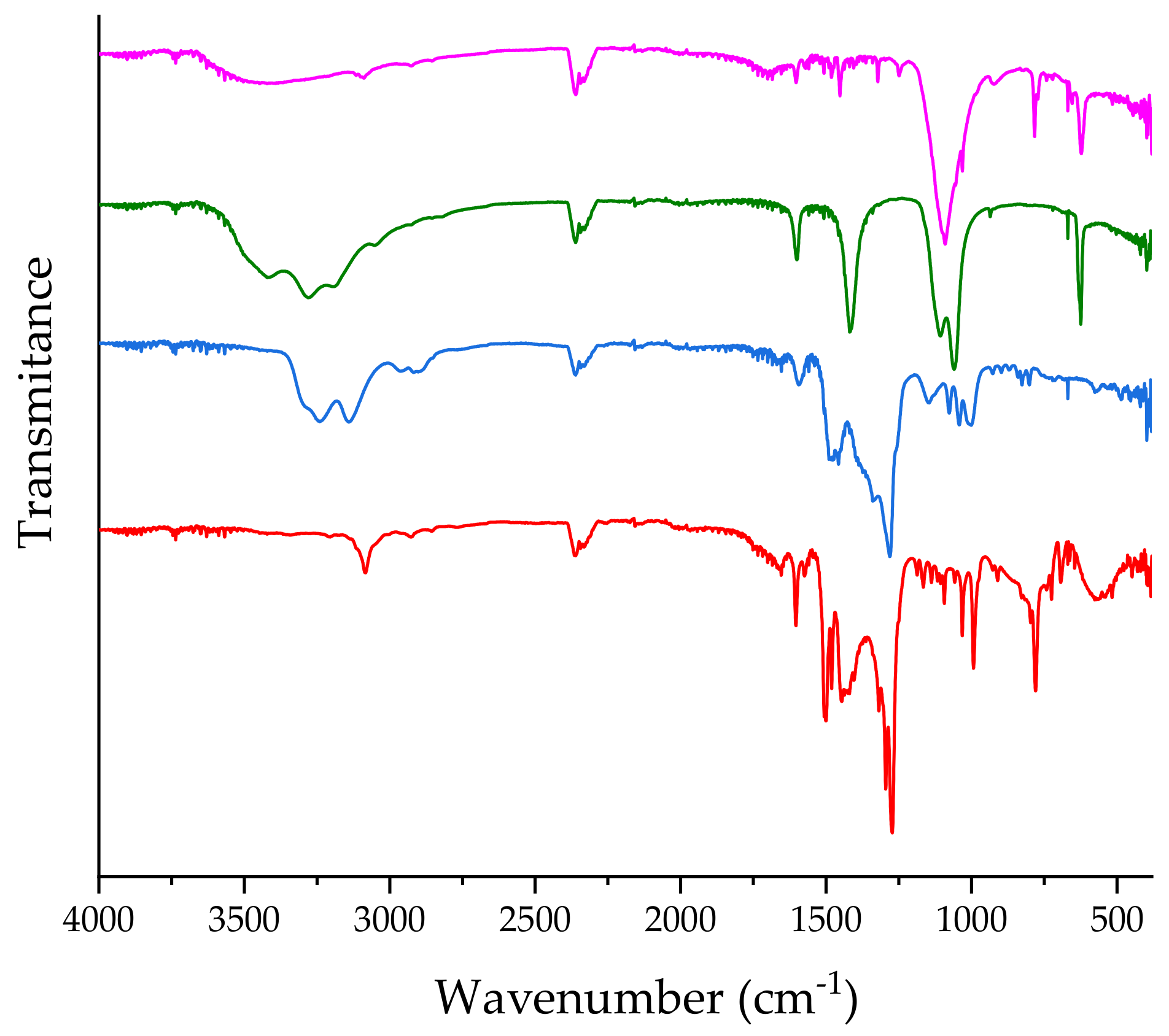
2.1. Formation of CblCN-Pd(II) Conjugates
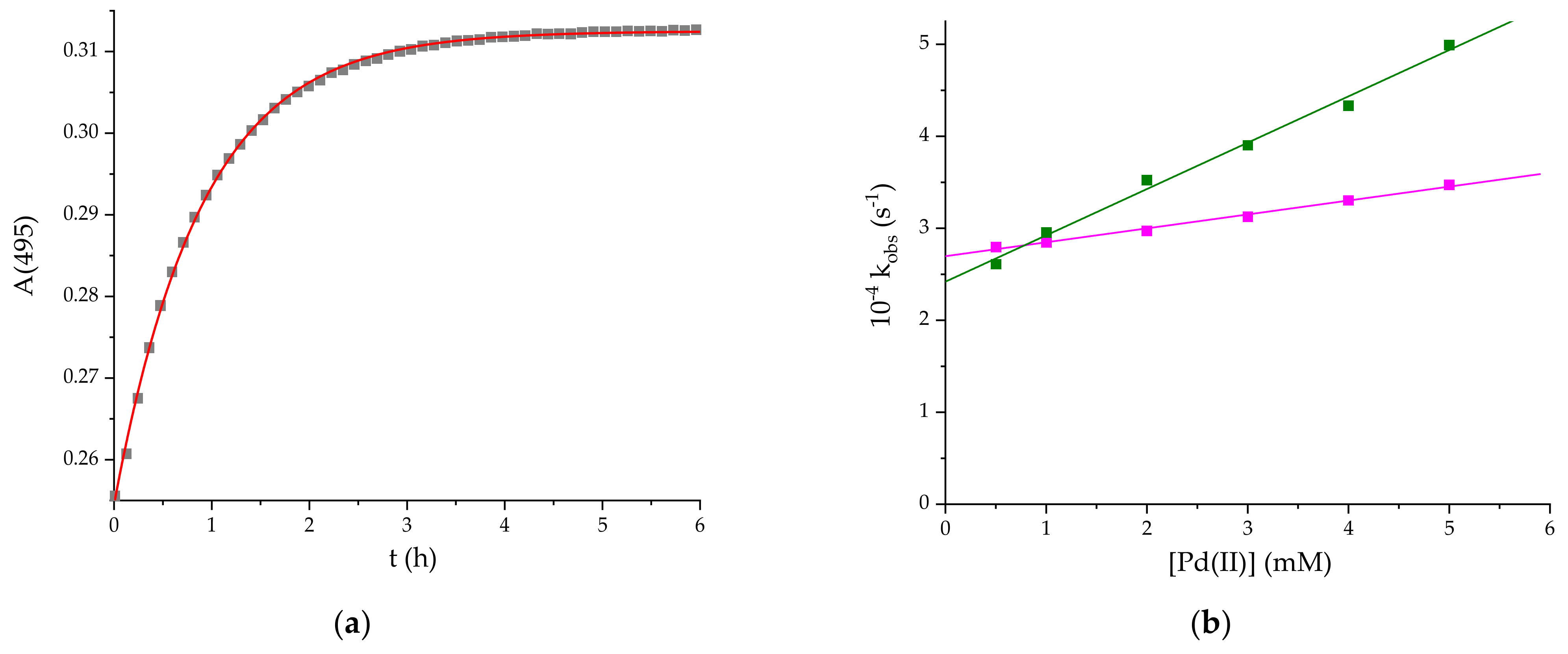
2.2. Mechanistic Considerations
2.3. DFT Calculations
3. Materials and Methods
3.1. Chemicals
3.2. General Procedure
3.3. Syntheses of the Palladium(II) Complexes
3.3.1. [PdCln(Solv)4−n]2−n
3.3.2. [Pd(dien)(H2O)]2+
3.3.3. [Pd(terpy)(H2O)]2+
3.3.4. Adducts with CblCN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, R. Chemistry and Biochemistry of B12; Wiley: New York, NY, USA, 1999; p. 952. [Google Scholar]
- Gruber, K.; Puffer, B.; Kraeutler, B. Vitamin B12-derivatives—Enzyme cofactors and ligands of proteins and nucleic acids. Chem. Soc. Rev. 2011, 40, 4346–4363. [Google Scholar] [CrossRef]
- Kräutler, B.; Arigoni, D.; Golding, B.T.; Eschenmoser, A. Vitamin B12 and B12-Proteins; Wiley: New York, NY, USA, 2008; p. 559. [Google Scholar]
- Zelder, F. Recent trends in the developement of vitamin B12 derivatives for medicinal applications. Chem. Commun. 2015, 51, 14004–14017. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef]
- Shepherd, G.; Velez, L.I. Role of hydroxocobalamin in acute cyanide poisoning. Ann. Pharmacother. 2008, 42, 661–669. [Google Scholar] [CrossRef]
- Zelder, F.; Zhou, K.; Sonnay, M. Peptide B12: Emerging trends at the interface of inorganic chemistry, chemical biology and medicine. Dalton Trans. 2013, 42, 854–862. [Google Scholar] [CrossRef]
- Giedyk, M.; Proinsias, K.Ó.; Kurcoń, S.; Sharina, I.; Martin, E.; Gryko, D. Small alterations in cobinamide structure considerably influence sGC activation. ChemMedChem 2014, 9, 2344–2350. [Google Scholar] [CrossRef]
- Pettenuzzo, A.; Pigot, R.; Ronconi, L. Vitamin B12—Metal conjugates for targeted chemotherapy and diagnosis: Current status and future prospects. Eur. J. Inorg. Chem. 2017, 1625–1638. [Google Scholar] [CrossRef]
- Collins, D.A.; Hogenkamp, H.P.C.; O’Connor, M.K.; Naylor, S.; Benson, L.M.; Hardyman, T.J.; Thorson, L.M. Biodistribution of radiolabeled adenosylcobalamin in patients diagnosed with various malignancies. Mayo Clin. Proc. 2000, 75, 568–580. [Google Scholar] [CrossRef]
- Waibel, R.; Treichler, H.; Schaefer, N.G.; van Staveren, D.R.; Mundwiler, S.; Kunze, S.; Kuenzi, M.; Alberto, R.; Nuesch, J.; Knuth, A.; et al. New derivatives of vitamin B12 show preferential targeting of tumors. Cancer Res. 2008, 68, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Bunge, S.D.; van Eldik, R.; Jacobsen, D.W.; Kratky, C.; Gruber, K.; Brasch, N.E. X-ray structural characterization of imidazolylcobalamin and histidinylcobalamin: Cobalamin models for aquacobalamin bound to the B12 transporter protein transcobalamin. Inorg. Chem. 2007, 46, 3613–3618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wuerges, J.; Garau, G.; Geremia, S.; Fedosov, S.N.; Petersen, T.E.; Randaccio, L. Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc. Natl. Acad. Sci. USA 2006, 103, 4386–4391. [Google Scholar] [CrossRef]
- Tran, M.T.Q.; Stuerup, S.; Lambert, I.H.; Gammelgaard, B.; Furger, E.; Alberto, R. Cellular uptake of metallated cobalamins. Metallomics 2016, 8, 298–304. [Google Scholar] [CrossRef]
- Petrus, A.K.; Fairchild, T.J.; Doyle, R.P. Traveling the vitamin B12 pathway: Oral delivery of protein and peptide drugs. Angew. Chem. Int. Ed. 2009, 48, 1022–1028. [Google Scholar] [CrossRef]
- Kunze, S.; Zobi, F.; Kurz, P.; Spingler, B.; Alberto, R. Vitamin B12 as a ligand for technetium and rhenium complexes. Angew. Chem. Int. Ed. 2004, 43, 5025–5029. [Google Scholar] [CrossRef]
- Spingler, B.; Mundwiler, S.; Ruiz-Sanchez, P.; van Staveren, D.R.; Alberto, R. Structures of the b- and d-acid derivatives of vitamin B12 and their complexes with [M(CO)3]+ (M = Tc-99m, Re). Eur. J. Inorg. Chem. 2007, 2641–2647. [Google Scholar] [CrossRef]
- Van Staveren, D.R.; Waibel, R.; Mundwiler, S.; Schubiger, P.A.; Alberto, R. Conjugates of vitamin B12 with N-epsilon-functionalized histidine for labeling with [Tc-99m(OH2)3(CO)3]+: Synthesis and biodistribution studies in tumor bearing mice. J. Organomet. Chem. 2004, 689, 4803–4810. [Google Scholar] [CrossRef]
- Siega, P.; Wuerges, J.; Arena, F.; Gianolio, E.; Fedosov, S.N.; Dreos, R.; Geremia, S.; Aime, S.; Randaccio, L. Release of toxic Gd3+ ions to tumour cells by vitamin B12 bioconjugates. Chem. Eur. J. 2009, 15, 7980–7989. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, C.C.; Cannon, M.J.; Pinson, P.R.; Munger, J.D., Jr.; West, F.G.; Grissom, C.B. Synthesis and characterization of fluorescent cobalamin (CobalaFluor) derivatives for imaging. Org. Lett. 2001, 3, 799–801. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Grissom, C.B.; Fedosova, N.U.; Moestrup, S.K.; Nexø, E.; Petersen, T.E. Application of a fluorescent cobalamin analogue for analysis of the binding kinetics. A study employing recombinant human transcobalamin and intrinsic factor. FEBS J. 2006, 273, 4742–4753. [Google Scholar] [CrossRef]
- McGreevy, J.M.; Cannon, M.J.; Grissom, C.B. Minimally invasive lymphatic mapping using fluorescently labeled vitamin B12. J. Surg. Res. 2003, 111, 38–44. [Google Scholar] [CrossRef]
- Mundwiler, S.; Spingler, B.; Kurz, P.; Kunze, S.; Alberto, R. Cyanide-bridged vitamin B12-cisplatin conjugates. Chem. Eur. J. 2005, 11, 4089–4095. [Google Scholar] [CrossRef]
- Ventura, G.; Arnesano, F.; Calvano, C.D.; Palmisano, F.; Cataldi, T.R.I. Cyanocobalamin conjugates of cisplatin and diaminocyclohexane-platinum(II): Matrix-assisted laser desorption ionization mass spectrometry characterization using 4-chloro-a-cyanocinnamic acid as the matrix. RSC Adv. 2017, 7, 53658–53666. [Google Scholar] [CrossRef]
- Ventura, G.; Nardella, M.I.; Panella, A.; Arnesano, F.; Calvano, C.D.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Structural elucidation of cisplatin and hydrated cis-diammineplatinum(II) complex conjugated with cyanocobalamin by liquid chromatography with electrospray ionization—Mass spectrometry and multistage mass spectrometry. ACS Omega 2018, 3, 12914–12922. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchez, P.; Mundwiler, S.; Medina-Molner, A.; Spingler, B.; Alberto, R. Iodination of cisplatin adduct of Vitamin B12 [{B12}-CN-{cis-PtCl(NH3)2}]+. J. Organomet. Chem. 2007, 692, 1358–1362. [Google Scholar] [CrossRef]
- Tran, M.T.; Furger, E.; Alberto, R. Two-step activation prodrugs: Transplatin mediated binding of chemotherapeutic agents to vitamin B12. Org. Biomol. Chem. 2013, 11, 3247–3254. [Google Scholar] [CrossRef]
- Shell, T.A.; Lawrence, D.S. A new trick (hydroxyl radical generation) for an old vitamin (B12). J. Am. Chem. Soc. 2011, 133, 2148–2150. [Google Scholar] [CrossRef]
- Zobi, F.; Blacque, O.; Jacobs, R.A.; Schaub, M.C.; Bogdanova, A.Y. 17 e− rhenium dicarbonyl CO-releasing molecules on a cobalamin scaffold for biological application. Dalton Trans. 2012, 41, 370–378. [Google Scholar] [CrossRef]
- Zobi, F.; Quaroni, L.; Santoro, G.; Zlateva, T.; Blacque, O.; Sarafimov, B.; Schaub, M.C.; Bogdanova, A.Y. Live-fibroblast IR imaging of a cytoprotective photoCORM activated with visible light. J. Med. Chem. 2013, 56, 6719–6731. [Google Scholar] [CrossRef]
- Ventura, G.; Calvano, C.D.; Losito, I.; Viola, A.; Cinquepalmi, V.; Cataldi, T.R.I. In vitro reactions of a cyanocobalamin–cisplatin conjugate with nucleoside monophosphates. Rapid Commun. Mass Spectrom. 2020, 34, e8945. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, P.; Mundwiler, S.; Spingler, B.; Buan, N.R.; Escalante-Semerena, J.C.; Alberto, R. Syntheses and characterization of vitamin B12-Pt(II) conjugates and their adenosylation in an enzymatic assay. J. Biol. Inorg. Chem. 2008, 13, 335–347. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Lee, L.P.; Sibert, J.W. Alkylcobalamins and alkylcobaloximes. Electronic structure, spectra, and mechanism of photodealkylation. J. Am. Chem. Soc. 1970, 92, 2997–3005. [Google Scholar] [CrossRef]
- Machalska, E.; Zajac, G.; Halat, M.; Wierzba, A.J.; Gryko, D.; Baranska, M. Resonance raman optical activity spectroscopy in probing structural changes invisible to circular dichroism spectroscopy: A study on truncated vitamin B12 derivatives. Molecules 2020, 25, 4386. [Google Scholar] [CrossRef] [PubMed]
- Boily, J.F.; Seward, T.M. Palladium(II) chloride complexation: Spectrophotometric investigation in aqueous solutions from 5 to 125 °C and theoretical insight into Pd-Cl and Pd-OH2 interactions. Geochim. Cosmochim. Acta 2005, 69, 3773–3789. [Google Scholar] [CrossRef]
- Elding, L.I. Palladium(II) halide complexes. I. Stabilities and spectra of palladium(II) chloro and bromo aqua complexes. Inorg. Chim. Acta 1972, 6, 647–651. [Google Scholar] [CrossRef]
- Serjeant, E.P.; Dempsey, B. Ionisation Constants of Organic Acids in Aqueous Solution; Pergamon Press, Inc.: New York, NY, USA, 1979; Volume 23, p. 989. [Google Scholar]
- Silverstein, T.P.; Heller, S.T. pKa values in the undergraduate curriculum: What is the real pKa of water? J. Chem. Educ. 2017, 94, 690–695. [Google Scholar] [CrossRef]
- Breett, E.L.J.; van Eldik, R.; Kelm, H. Kinetics and mechanism of some fast anation reactions of a series of substituted dien complexes of palladium(II). Temperature and pressure dependencies in weakly acidic aqueous solution. Polyhedron 1983, 2, 1181–1187. [Google Scholar] [CrossRef]
- Kotowski, M.; van Eldik, R. Spontaneous solvolysis reactions of some Pd(II)-dien complexes in aqueous solution: Equilibrium and kinetic data. Inorg. Chem. 1984, 23, 3310–3312. [Google Scholar] [CrossRef]
- Palmer, D.A.; Kelm, H. Effect of pressure on the substitution reactions of [Pt(dien)X]+ complexes in aqueous solution. Inorg. Chim. Acta 1976, 19, 117–128. [Google Scholar] [CrossRef]
- Cregan, A.G.; Brasch, N.E.; van Eldik, R. Thermodynamic and kinetic studies on the reaction between the vitamin B12 derivative β-(N-methylimidazolyl)cobalamin and N-methylimidazole: Ligand displacement at the α–axial site of cobalamins. Inorg. Chem. 2001, 40, 1430–1438. [Google Scholar] [CrossRef]
- Pratt, J.M. Inorganic Chemistry of Vitamin B12; Academic Press: New York, NY, USA, 1972; p. 348. [Google Scholar]
- Reenstra, W.W.; Jencks, W.P. Reactions of cyanide with cobalamins. J. Am. Chem. Soc. 1979, 101, 5780–5791. [Google Scholar] [CrossRef]
- Rutkowska-Zbik, D.; Jaworska, M.; Witko, M. Application of the DFT theory to study cobalamin complexes. Struct. Chem. 2004, 15, 431–435. [Google Scholar] [CrossRef]
- Rutkowska-Zbik, D.; Witko, M.; Stochel, G. Ligand binding properties of cobalamins. Theor. Chem. Acc. 2008, 120, 411–419. [Google Scholar] [CrossRef]
- Rutkowska-Zbik, D.; Mazur, G.; Drzewiecka-Matuszek, A.; Orzel, L.; Stochel, G. Exploring novel modified vitamin B12 as a drug carrier: Forecast from density functional theory modeling. J. Phys. Chem. B 2013, 117, 9655–9661. [Google Scholar] [CrossRef]
- Al-Allaf, T.A.K.; Rashan, L.J.; Abu-Surrah, A.S.; Fawzi, R.; Steimann, M. Chemical properties and cytotoxic activity of complexes of platinum(II) and palladium(II) containing dmso and various anions; synthesis and structural characterization of [Pt(dmso)2{O2(CO)2CCH2CH2CH2}]. Transit. Met. Chem. 1998, 23, 403–406. [Google Scholar] [CrossRef]
- Annibale, G.; Brandolisio, M.; Pitteri, B. New routes for the synthesis of chloro(diethylenetriamine) platinum(II) chloride and chloro(2,2′: 6′,2″-terpyridine) platinum(II) chloride dihydrate. Polyhedron 1995, 14, 451–453. [Google Scholar] [CrossRef]
- McDermott, J.X.; White, J.F.; Whitesides, G.M. Thermal decomposition of bis(phosphine)platinum(II) metallocycles. J. Am. Chem. Soc. 1976, 98, 6521–6528. [Google Scholar] [CrossRef]
- Kapdi, A.R.; Fairlamb, J.S. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef] [PubMed]
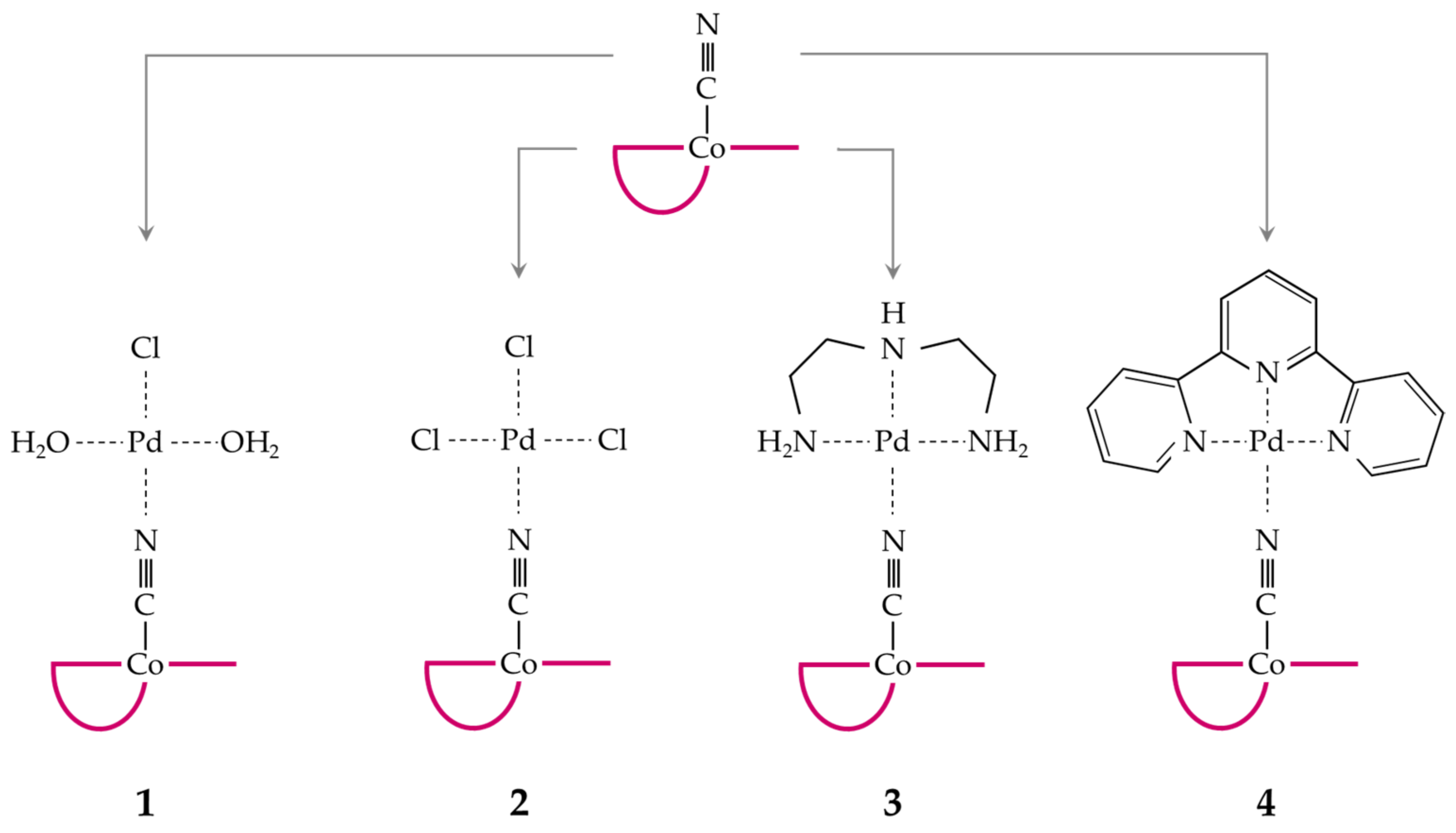
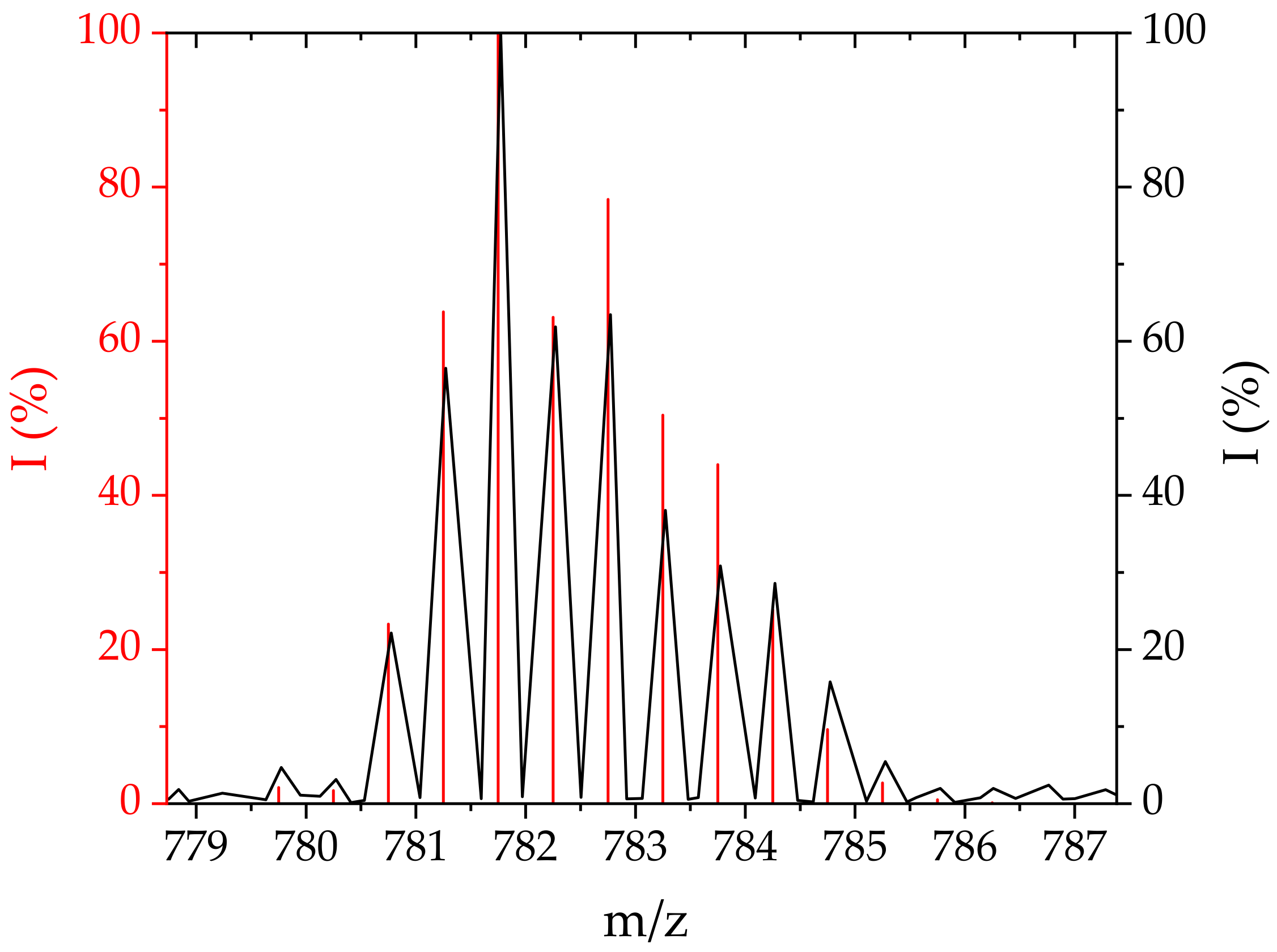
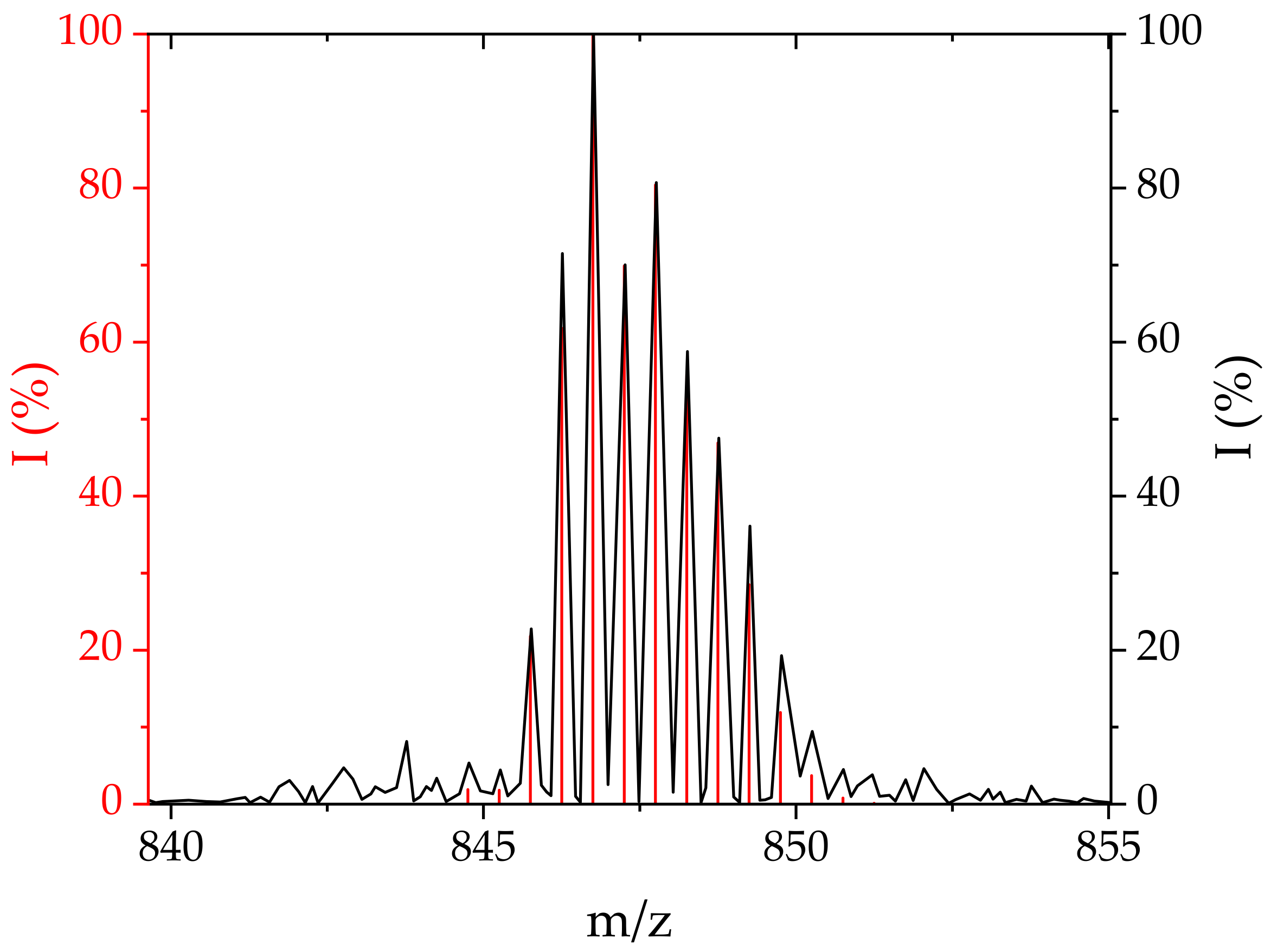

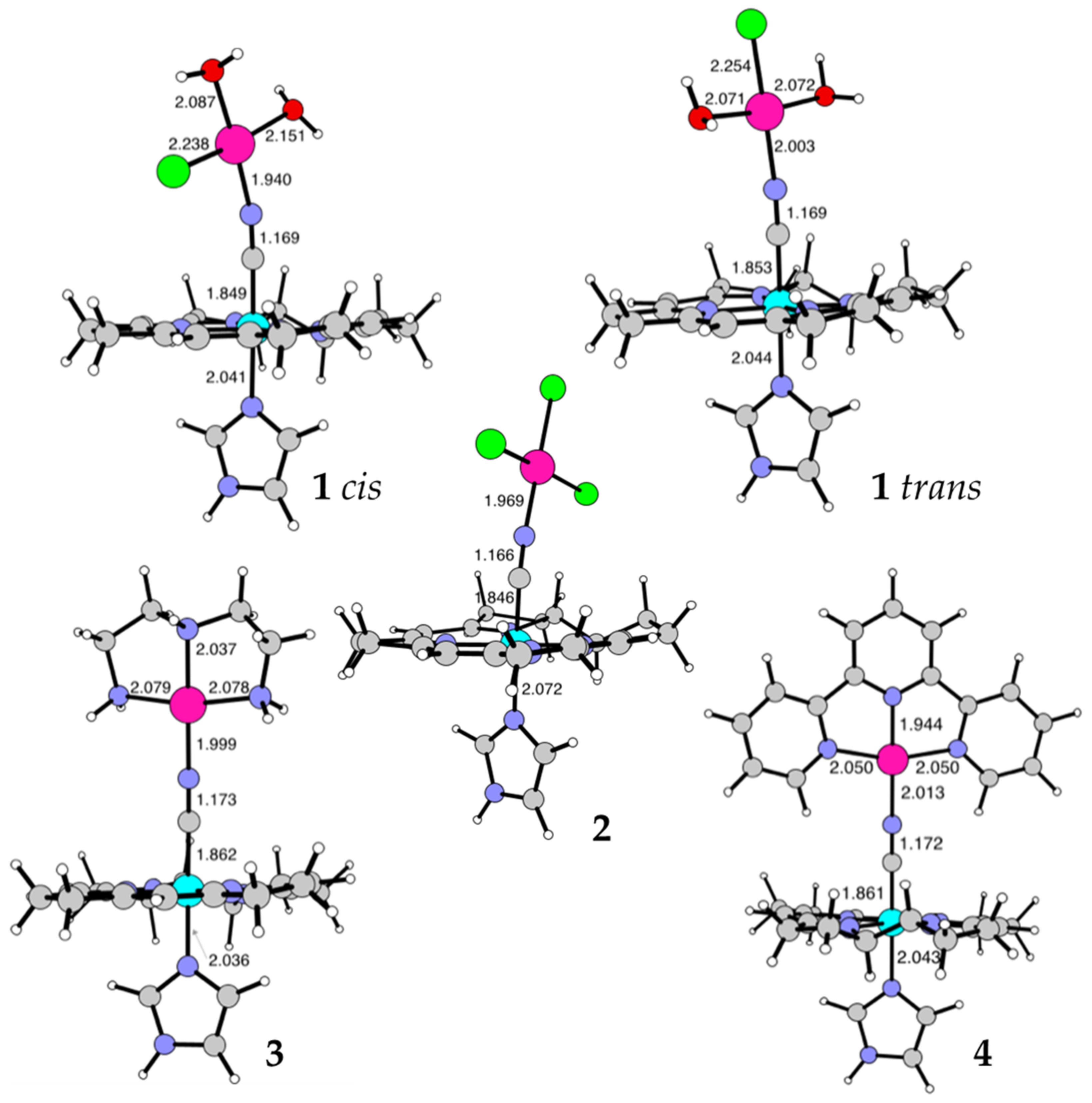
| Conjugate | Pd-N [Å] | N-C [Å] | C-Co [Å] |
|---|---|---|---|
| 1cis | 1.940 | 1.169 | 1.849 |
| 1trans | 2.003 | 1.169 | 1.853 |
| 2 | 1.969 | 1.166 | 1.846 |
| 3 | 1.999 | 1.173 | 1.862 |
| 4 | 2.013 | 1.172 | 1.861 |
| Conjugate | Ebin [kcal/mol] |
|---|---|
| 1cis | 3.4 |
| 1trans | 5.3 |
| 2 | −7.5 |
| 3 | −11.7 |
| 4 | −10.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porębska, D.; Orzeł, Ł.; Rutkowska-Zbik, D.; Stochel, G.; van Eldik, R. Ligand-Tuning of the Stability of Pd(II) Conjugates with Cyanocobalamin. Int. J. Mol. Sci. 2021, 22, 7973. https://doi.org/10.3390/ijms22157973
Porębska D, Orzeł Ł, Rutkowska-Zbik D, Stochel G, van Eldik R. Ligand-Tuning of the Stability of Pd(II) Conjugates with Cyanocobalamin. International Journal of Molecular Sciences. 2021; 22(15):7973. https://doi.org/10.3390/ijms22157973
Chicago/Turabian StylePorębska, Dominika, Łukasz Orzeł, Dorota Rutkowska-Zbik, Grażyna Stochel, and Rudi van Eldik. 2021. "Ligand-Tuning of the Stability of Pd(II) Conjugates with Cyanocobalamin" International Journal of Molecular Sciences 22, no. 15: 7973. https://doi.org/10.3390/ijms22157973
APA StylePorębska, D., Orzeł, Ł., Rutkowska-Zbik, D., Stochel, G., & van Eldik, R. (2021). Ligand-Tuning of the Stability of Pd(II) Conjugates with Cyanocobalamin. International Journal of Molecular Sciences, 22(15), 7973. https://doi.org/10.3390/ijms22157973









