Supramolecular Zn(II)-Dipicolylamine-Azobenzene-Aminocyclodextrin-ATP Complex: Design and ATP Recognition in Water
Abstract
1. Introduction
2. Results and Discussion
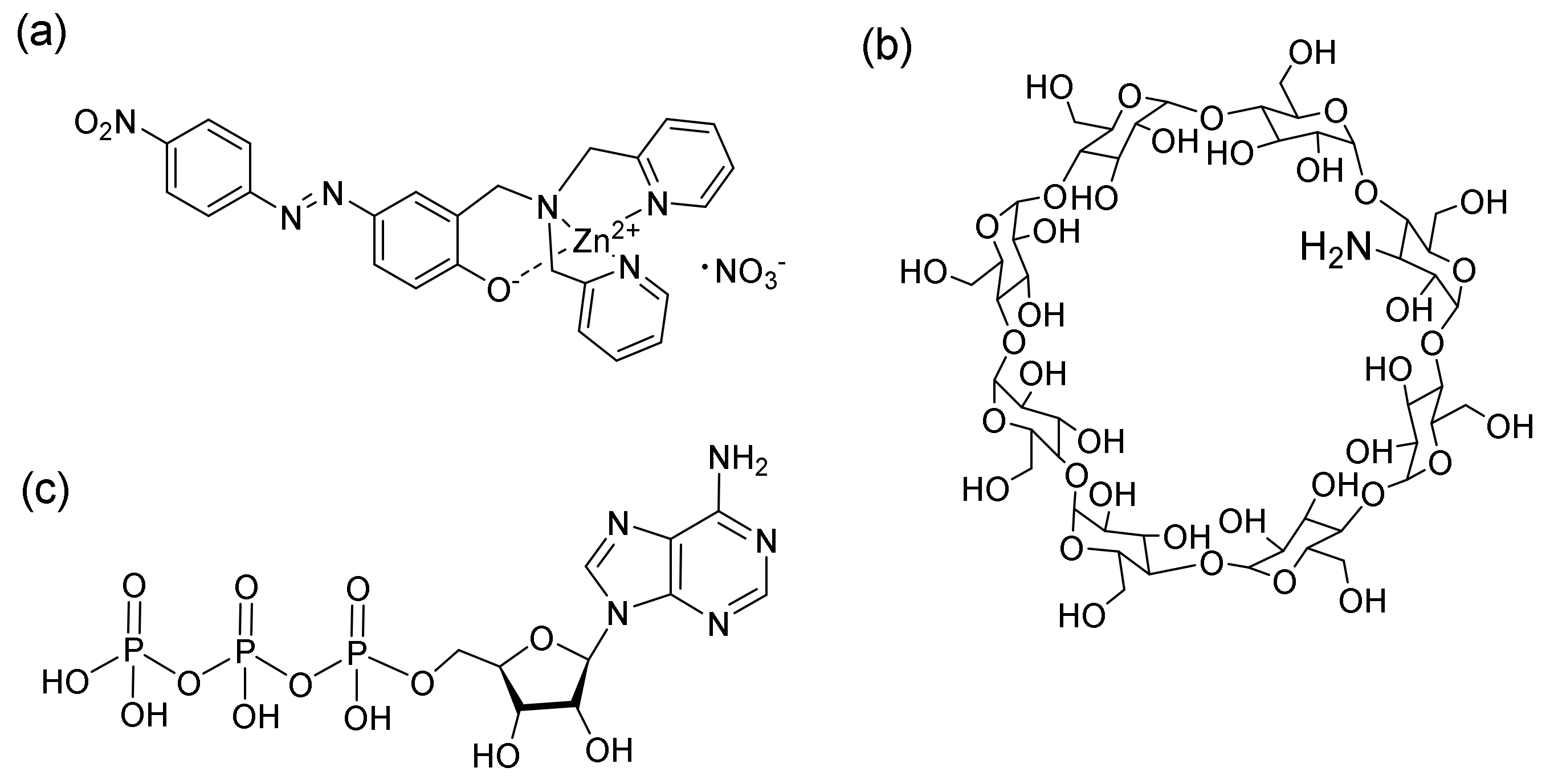
2.1. Design of 1-Zn Complex
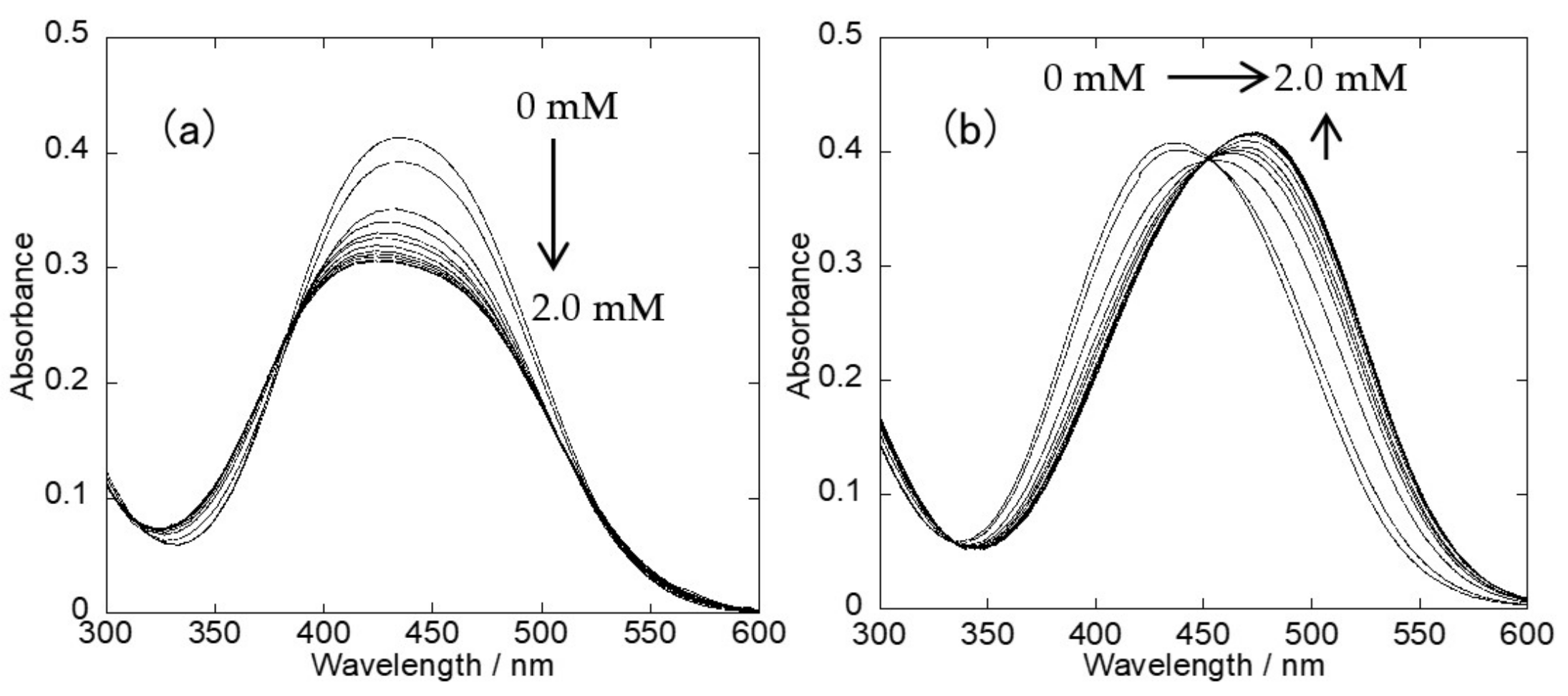
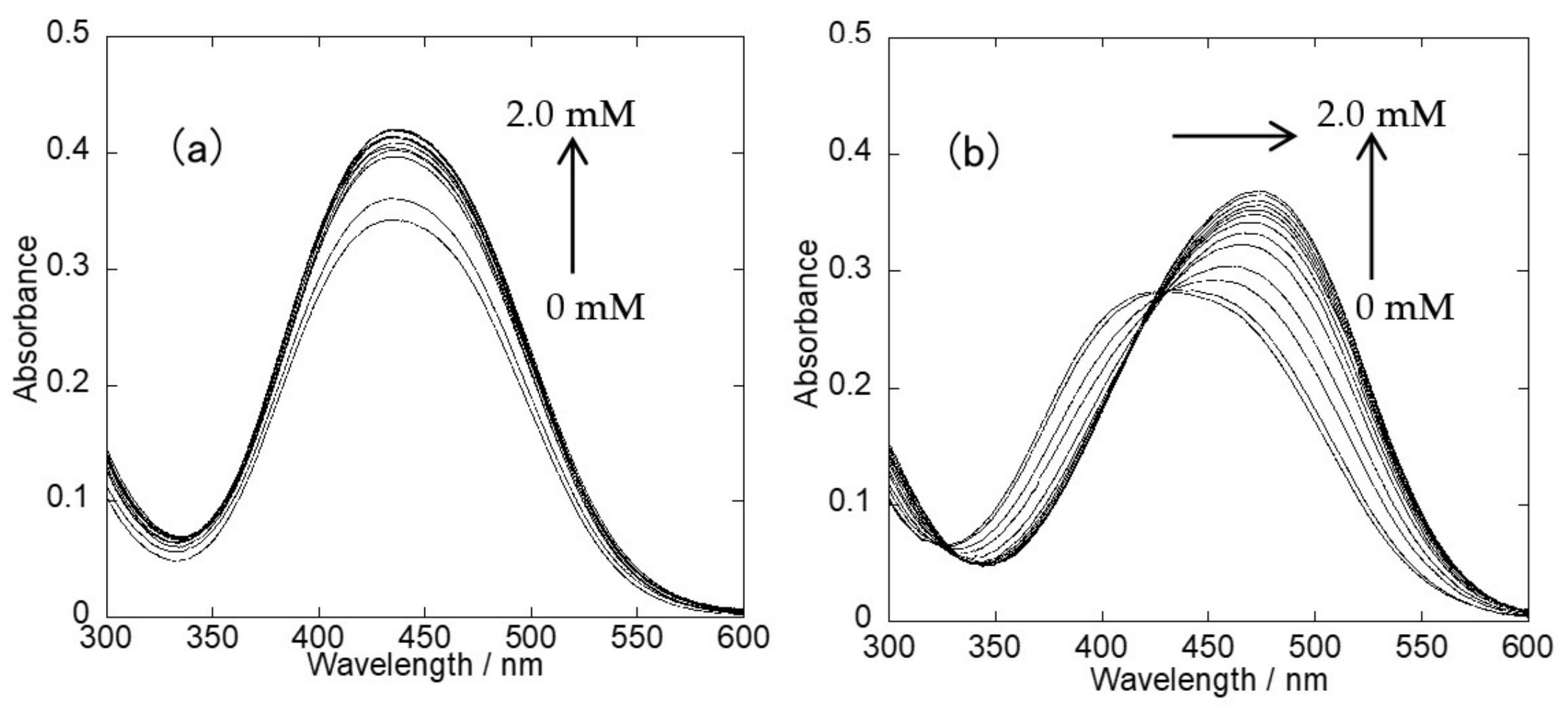
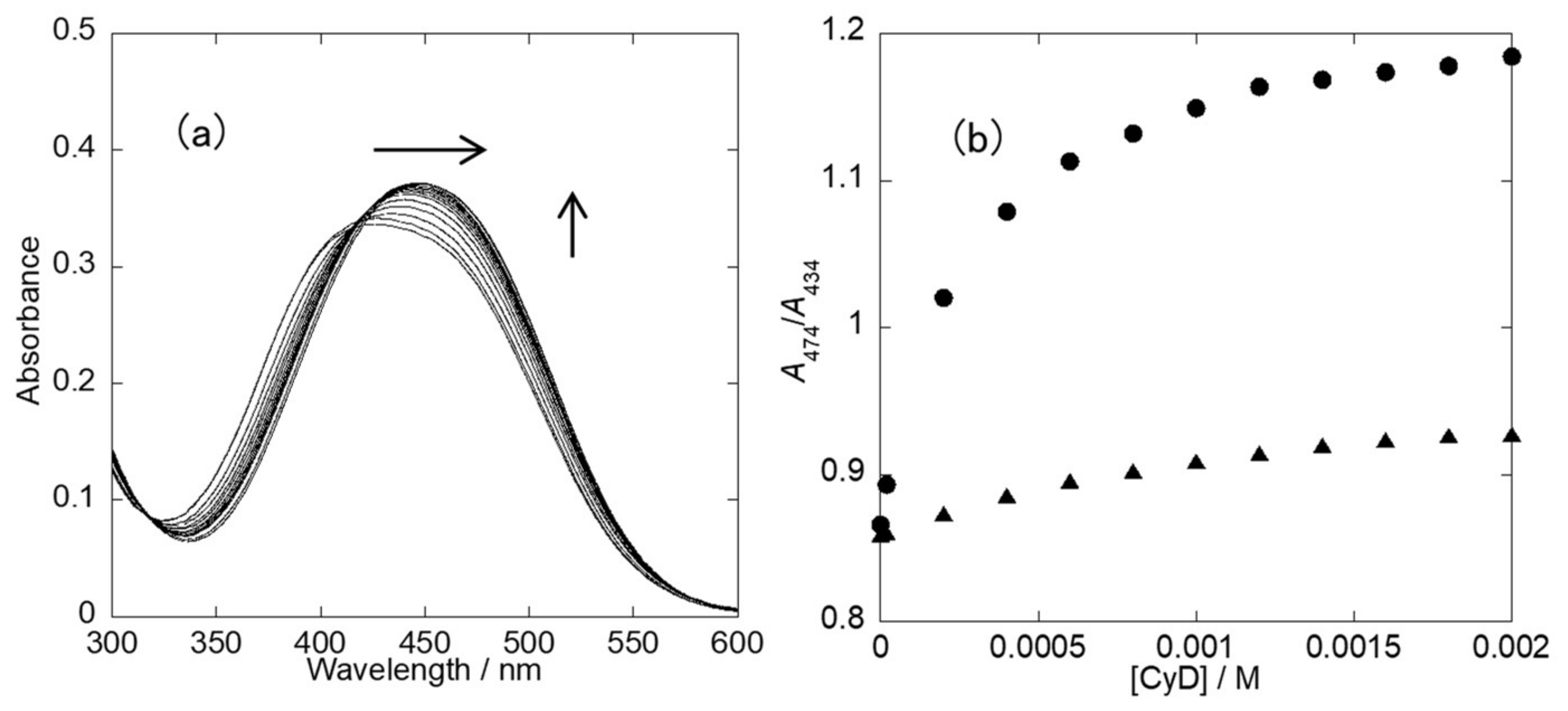
2.2. UV-Vis Spectra
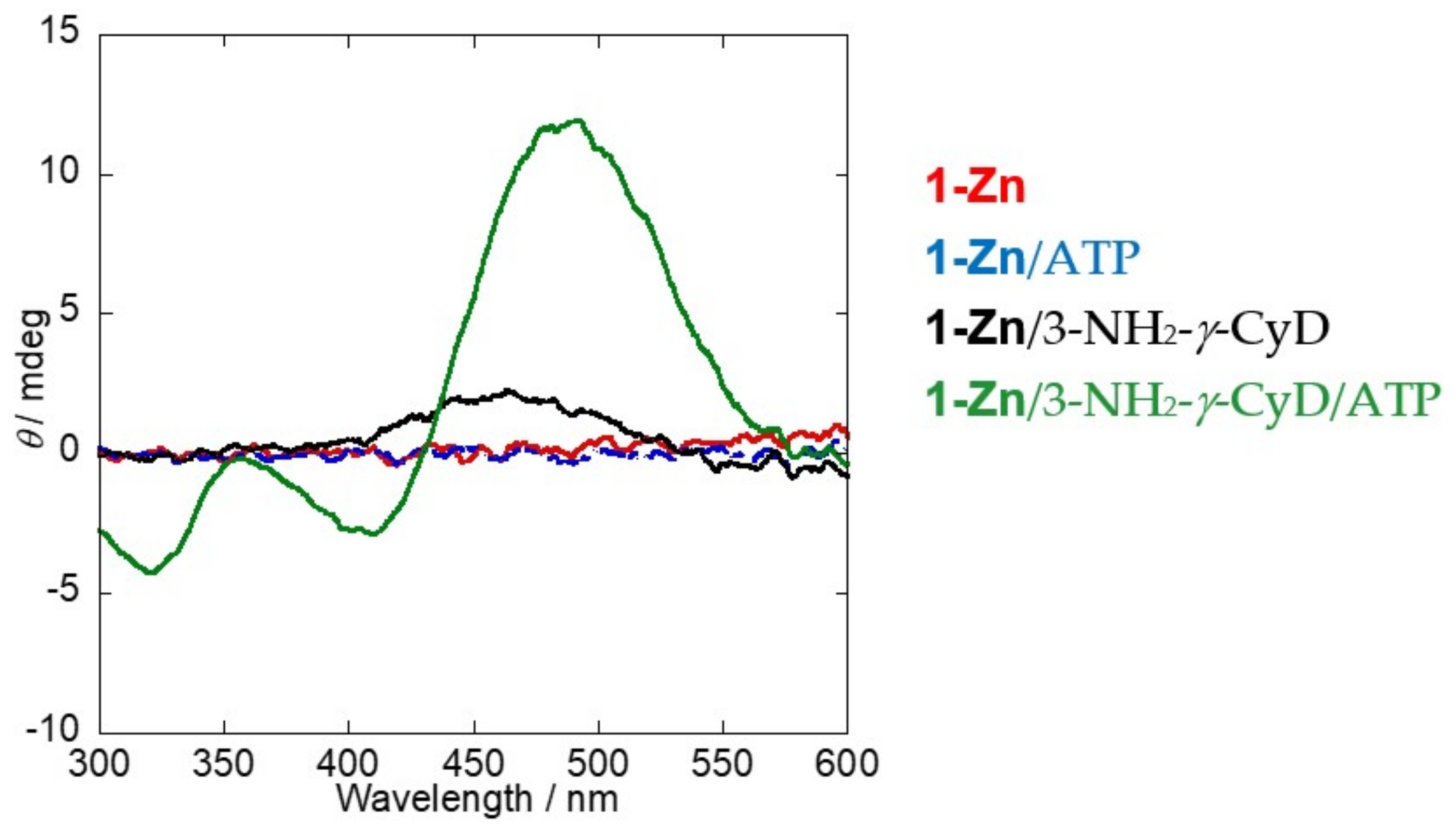
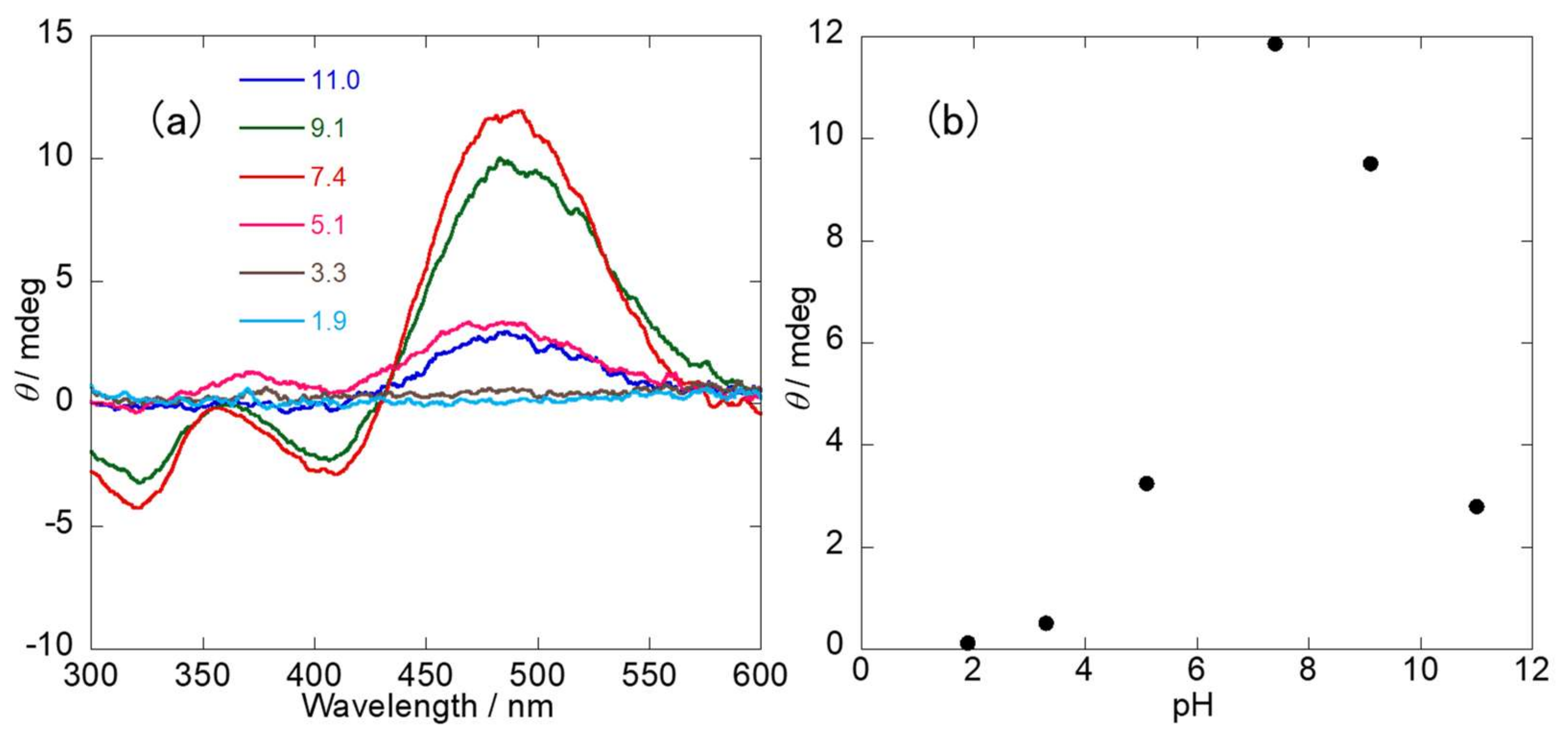
2.3. Induced Circular Dichroism (ICD) Spectra
2.4. NMR Spectra
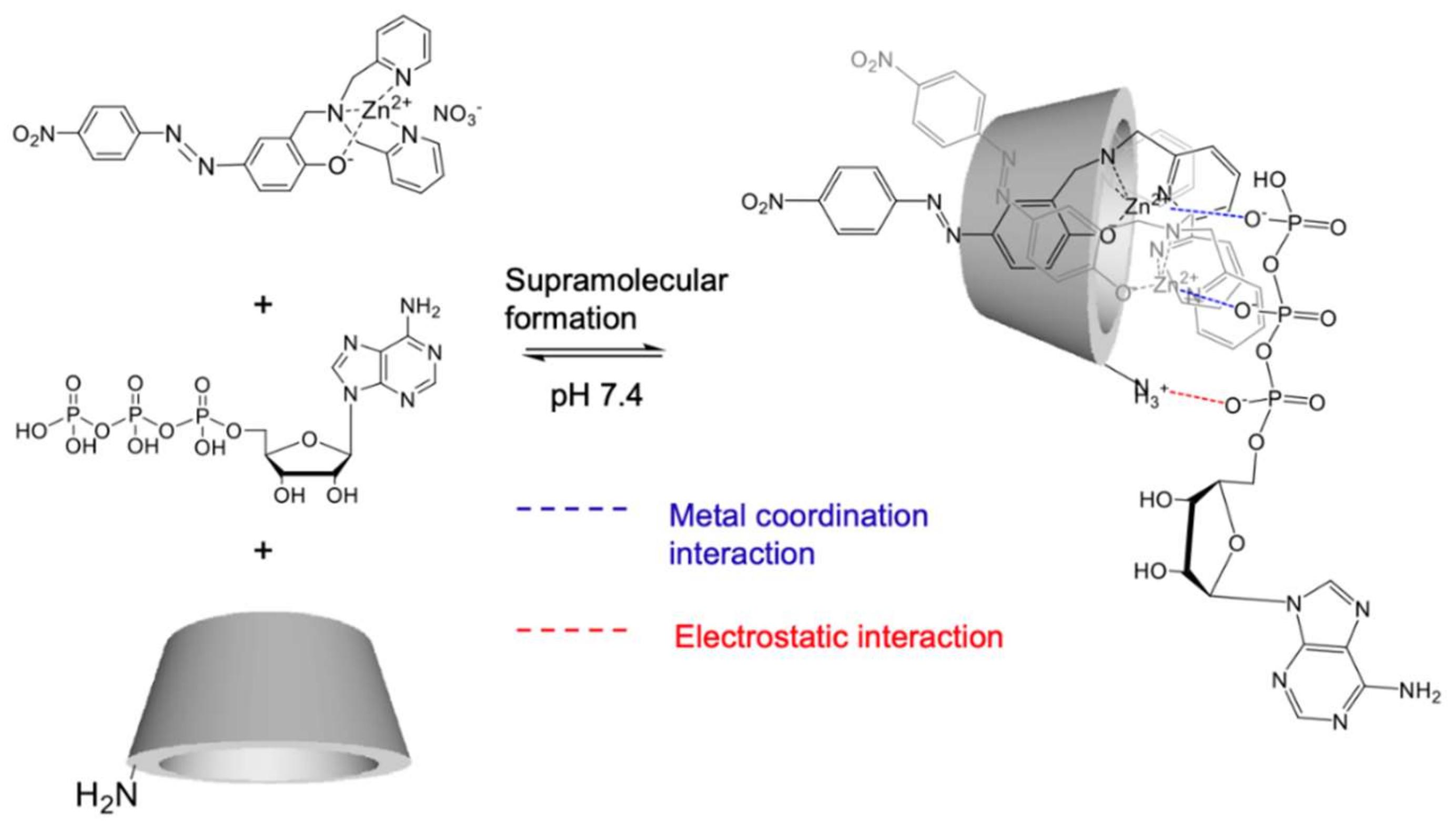
2.5. Supramolecular Structure
2.6. Effect of Other Phosphate Derivatives
3. Experimental Section
3.1. Reagents
3.2. Synthesis of 1 (2-Dipicolylaminomethyl-4-(4-nitrophenylazo)phenol)
3.3. Measurement Instruments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Fischer, E. Einfluss der configuration auf die wirkung der enzyme. II. Ber. Dtsch. Chem. Ges. 1894, 27, 2984–2993. [Google Scholar] [CrossRef]
- Matsushita, A.; Kuwabara, T.; Nakamura, A.; Ikeda, H.; Ueno, A. Guest-induced color change and molecule-sensing ability of p-nitrophenol-modified cyclodextrins. J. Chem. Soc. Perkin Trans. 2 1997, 1705–1710. [Google Scholar] [CrossRef]
- Kuwabara, T.; Shiba, K.; Nakajima, H.; Ozawa, M.; Miyajima, N.; Hosoda, M.; Kuramoto, N.; Suzuki, Y. Host-guest complexation affected by pH and length of spacer for hydroxyazobenzene-modified cyclodextrins. J. Phys. Chem. A 2006, 110, 13521–13529. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Sugiyama, K. Hyperchromisity and molecular recognition of a novel modified β-cyclodextrin tethering with phenylaminoazobenzene. Anal. Sci. 2013, 29, 905–909. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Shi, Y.; Wanga, W.; Yuan, Z. Study on the association between CTD peptides and zinc(ii)-dipicolylamine appended β-cyclodextrin. RSC Adv. 2015, 5, 80434–80440. [Google Scholar] [CrossRef]
- Yadav, V.B.; Rai, P.; Sagir, H.; Kumar, A.; Siddiqui, I.R. A green route for the synthesis of pyrrolo[2,3-d]pyrimidine derivatives catalyzed by β-cyclodextrin. New. J. Chem. 2018, 42, 628–633. [Google Scholar] [CrossRef]
- Breslowong, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998, 98, 1997–2012. [Google Scholar] [CrossRef]
- Shimpuku, C.; Ozawa, R.; Sasaki, A.; Sato, F.; Hashimoto, T.; Yamauchi, A.; Suzuki, I.; Hayashita, T. Selective glucose recognition by boronic acid azoprobe/γ-cyclodextrin complexes in water. Chem. Commun. 2009, 13, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Kumai, M.; Kozuka, S.; Hashimoto, T.; Hayashita, T. Design of boronic acid fluorophore/aminated cyclodextrin complexes for sugar sensing in water. J. Ion Exch. 2010, 21, 249–254. [Google Scholar]
- Kumai, M.; Kozuka, S.; Samizo, M.; Hashimoto, T.; Suzuki, I.; Hayashita, T. Glucose recognition by a supramolecular complex of boronic acid fluorophore with boronic acid-modified cyclodextrin in water. Anal. Sci. 2012, 28, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Tsuchido, Y.; Kasahara, C.; Casulli, M.A.; Fujiwara, S.; Hashimoto, T.; Hayashita, T. Selective sugar recognition by anthracene-type boronic acid fluorophore/cyclodextrin supramolecular complex under physiological pH condition. Front. Chem. 2019, 7, 806. [Google Scholar] [CrossRef]
- Azath, I.A.; Suresh, P.; Pitchumani, K. Per-6-ammonium-β-cyclodextrin/p-nitrophenol complex as a colorimetric sensor for phosphate and pyrophosphate anions in water. Sens. Actuators B 2011, 155, 909–914. [Google Scholar] [CrossRef]
- Mahato, P.; Ghosh, A.; Mishra, S.K.; Shrivastav, A.; Mishra, S.; Das, A. Zn(II)-cyclam based chromogenic sensors for recognition of ATP in aqueous solution under physiological conditions and their application as viable staining agents for microorganism. Inorg. Chem. 2011, 50, 4162–4170. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wei, Y.; Kang, X.; Su, Z. Selective recognition and sensing for adenosine triphosphate by label-free electrochemistry based on its inclusion with per-6-ammonium-β-cyclodextrin. Anal. Methods 2015, 7, 10129–10135. [Google Scholar] [CrossRef]
- Hu, T.; Na, W.; Yan, X.; Su, X. Sensitive fluorescence detection of ATP based on host-guest recognition between near-infrared β-cyclodextrin-CuInS2 QDs and aptamer. Talanta 2017, 165, 194–200. [Google Scholar] [CrossRef]
- Song, C.; Xiao, Y.; Li, K.; Zhang, X.; Lu, Y. β-Cyclodextrin polymer based fluorescence enhancement method for sensitive adenosine triphosphate detection. Chin. Chem. Lett. 2019, 30, 1249–1252. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Xiao, C.; Rao, M.; Fan, C.; Borovkov, V.; Cheng, G.; Zhou, D.; Zhong, Z.; Su, D.; Yu, X.; et al. A quinoline-appended cyclodextrin derivative as a highly selective receptor and colorimetric probe for nucleotides. iScience 2020, 23, 100927. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.R.; Singh, P.K. A supramolecule based fluorescence turn-on and ratiometric sensor for ATP in aqueous solution. J. Mater. Chem. B 2020, 8, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Fujiwara, S.; Yamada, T.; Tsuchido, Y.; Hashimoto, T.; Hayashita, T. Design and Function of supramolecular recognition systems based on guest-targeting probe-modified cyclodextrin receptors for ATP. J. Org. Chem. 2017, 82, 976–981. [Google Scholar] [CrossRef]
- Yamada, T.; Fujita, K.; Fujiwara, S.; Tsuchido, Y.; Hashimoto, T.; Hayashita, T. Development of dipicolylamine-modified cyclodextrins for the design of selective guest-responsive receptors for ATP. Molecules 2018, 23, 635. [Google Scholar] [CrossRef]
- Ngo, H.T.; Liu, X.; Jolliffe, K.A. Anion recognition and sensing with Zn(II)–dipicolylamine complexes. Chem. Soc. Rev. 2012, 41, 4928–4965. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Im, J.H.; Son, S.U.; Chung, Y.K.; Hong, J.I. An azophenol-based chromogenic pyrophosphate sensor in water. J. Am. Chem. Soc. 2003, 125, 7752–7753. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, S.Y.; Hong, J.I. A fluorescent pyrophosphate sensor with high selectivity over ATP in water. Angew. Chem. Int. Ed. 2004, 43, 4777–4780. [Google Scholar] [CrossRef]
- Roy, B.; Rao, A.S.; Ahn, K.H. Mononuclear Zn(II)- and Cu(II)-complexes of a hydroxynaphthalene-derived dipicolylamine: Fluorescent sensing behaviors toward pyrophosphate ions. Org. Biomol. Chem. 2011, 9, 7774–7779. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, G.; Williams, N.H. Highly selective colorimetric sensing pyrophosphate in water by a NBD-phenoxo-bridged dinuclear Zn(II) complex. Org. Biomol. Chem. 2012, 10, 5606–5612. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, A.E.; Zhong, Z.; Sessler, J.L.; Anslyn, E.V. Algorithms for the determination of binding constants and enantiomeric excess in complex host: Guest equilibria using optical measurements. New J. Chem. 2010, 34, 348–354. [Google Scholar] [CrossRef]
- Mayer, B.; Zhang, X.; Nau, W.M.; Marconi, G. Co-conformational variability of cyclodextrin complexes studied by induced circular dichroism of azoalkanes. J. Am. Chem. Soc. 2001, 123, 5240–5248. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, M. A general rule for circular dichromism induced by a chiral macrocycle. J. Am. Chem. Soc. 1993, 115, 3702–3705. [Google Scholar] [CrossRef]
- Tinoco, I., Jr. Theoretical aspects of optical activity. Part two: Polymers. Adv. Chem. Phys. 1962, 4, 113–160. [Google Scholar]
- Dean, J.A. Lange’s Handbook of Chemistry, 13th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1985; pp. 5–19. [Google Scholar]
- Takahashi, K.; Andou, K.; Fujiwara, S. Characterization and structural determination of 3A-amino-3A-deoxy-(2AS, 3AS)-cyclodextrins by NMR spectroscopy. Polym. J. 2012, 44, 850–854. [Google Scholar] [CrossRef][Green Version]
- Huang, C.; Lv, J.; Tian, X.; Wang, Y.; Yu, Y.; Liu, J. Miniaturized swimming soft robot with complex movement actuated and controlled by remote light signals. Sci. Rep. 2015, 5, 17414. [Google Scholar] [CrossRef] [PubMed]
- Glasoe, P.K.; Long, F.A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 1960, 64, 188–190. [Google Scholar] [CrossRef]


| K1/M−1 | K2/M−1 | K1K2/M−2 | |
|---|---|---|---|
| γ-CyD | 1.95 × 103 | 1.02 × 103 | 1.99 × 106 |
| 3-NH2-γ-CyD | 2.64 × 103 | 1.37 × 103 | 3.62 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minagawa, S.; Fujiwara, S.; Hashimoto, T.; Hayashita, T. Supramolecular Zn(II)-Dipicolylamine-Azobenzene-Aminocyclodextrin-ATP Complex: Design and ATP Recognition in Water. Int. J. Mol. Sci. 2021, 22, 4683. https://doi.org/10.3390/ijms22094683
Minagawa S, Fujiwara S, Hashimoto T, Hayashita T. Supramolecular Zn(II)-Dipicolylamine-Azobenzene-Aminocyclodextrin-ATP Complex: Design and ATP Recognition in Water. International Journal of Molecular Sciences. 2021; 22(9):4683. https://doi.org/10.3390/ijms22094683
Chicago/Turabian StyleMinagawa, Shohei, Shoji Fujiwara, Takeshi Hashimoto, and Takashi Hayashita. 2021. "Supramolecular Zn(II)-Dipicolylamine-Azobenzene-Aminocyclodextrin-ATP Complex: Design and ATP Recognition in Water" International Journal of Molecular Sciences 22, no. 9: 4683. https://doi.org/10.3390/ijms22094683
APA StyleMinagawa, S., Fujiwara, S., Hashimoto, T., & Hayashita, T. (2021). Supramolecular Zn(II)-Dipicolylamine-Azobenzene-Aminocyclodextrin-ATP Complex: Design and ATP Recognition in Water. International Journal of Molecular Sciences, 22(9), 4683. https://doi.org/10.3390/ijms22094683






