The Role of Supplementation with Natural Compounds in Post-Stroke Patients
Abstract
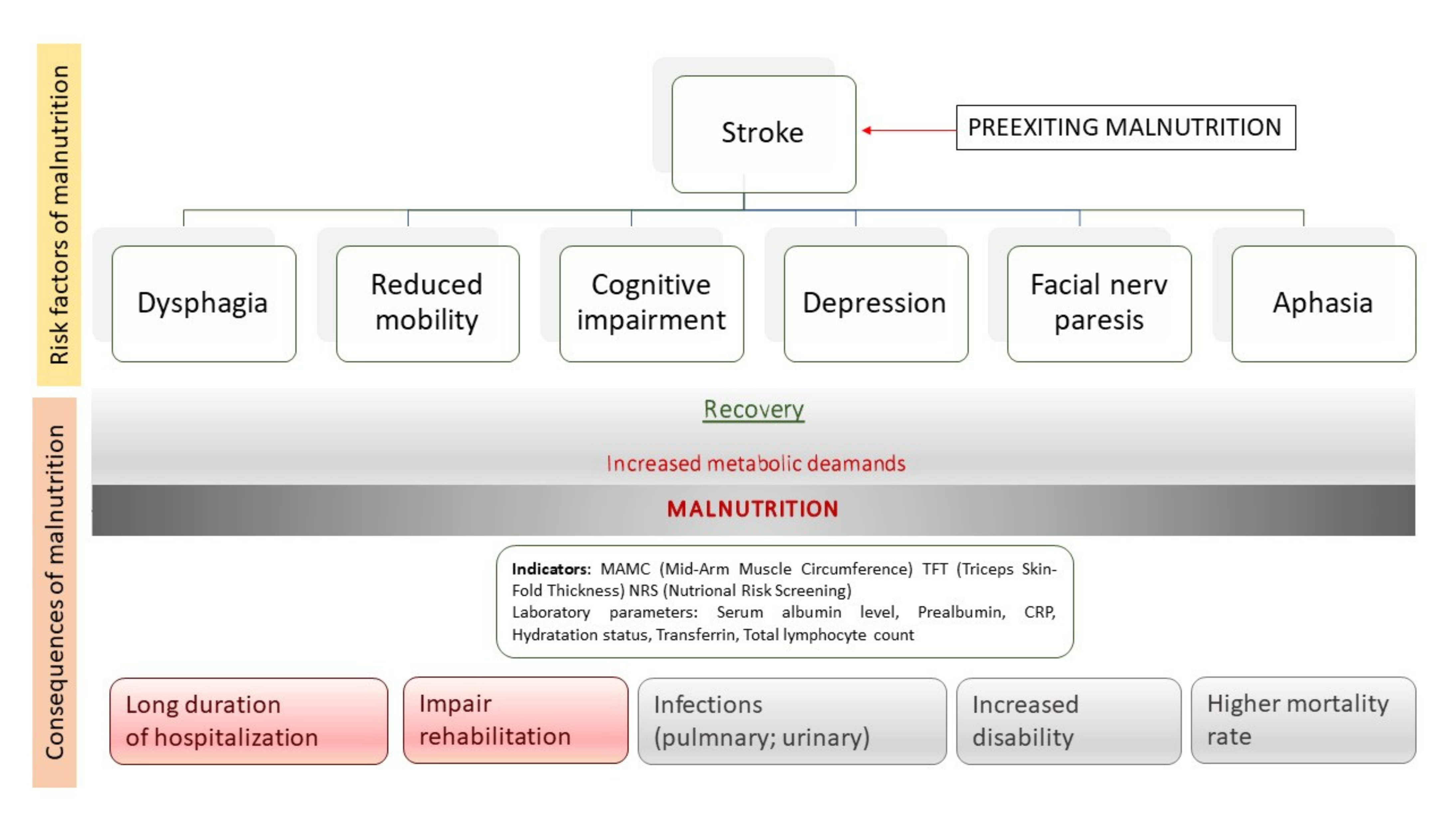
1. Introduction
2. Natural Compounds
2.1. Vitamins
2.2. Flavonoids
2.3. PUFA
2.4. Macroelements
2.5. Endogenous Substance
2.6. Other Bioactive Compounds
3. Conclusions
Funding
Conflicts of Interest
References
- Chauwa, L.; Appiah, C.A.; Nsiah, K.; Sarfo, F.S. Nutritional risk markers among stroke out-patients at the neurology clinic of a teaching hospital in Ghana. Pan Afr. Med. J. 2020, 37, 258. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N. Nutrition and hydration management among stroke patients in inpatient rehabilitation: A best practice implementation project. JBI Evid. Implement. 2021, 19, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshimura, Y.; Abe, T. Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients 2021, 13, 943. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, X.; Chen, J.; Wang, H.; Zhang, L.; Dong, B.; Yang, M. Predicting long-term mortality in hospitalized elderly patients using the new ESPEN definition. Sci. Rep. 2017, 7, 4067. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Chu, F.C.S.; Chow, T.W.; Shum, N.C. Prevalence of malnutrition and its risk factors in stroke patients residing in an infirmary. Singap. Med. J. 2008, 49, 290–296. [Google Scholar]
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocrit. Care 2017, 29, 374–384. [Google Scholar] [CrossRef]
- Foley, N.C.; Salter, K.L.; Robertson, J.; Teasell, R.W.; Woodbury, M.G. Which Reported Estimate of the Prevalence of Malnutrition After Stroke Is Valid? Stroke 2009, 40, e66–e74. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Fang, J.; Lu, Q.; He, L. Risk factors for malnutrition in stroke patients: A meta-analysis. Clin. Nutr. 2019, 38, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.S.; Lewis, S.C.; Warlow, C. FOOD Trial Collaboration Routine oral nutritional supplementation for stroke patients in hospital (FOOD): A multicentre randomised controlled trial. Lancet 2005, 365, 755–763. [Google Scholar] [CrossRef]
- Institute of Medicine; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Ali, A.A.; Mukhtar, M.M.; Shaheen, S.; Mohamed, A.O. Assessment of plasma BMP-2, BMP-7, BMP-10, vitamin D, and TGF β1 in simple fractures among Sudanese patients. PLoS ONE 2021, 16, e0247472. [Google Scholar] [CrossRef]
- Miller, E.D.; Dziedzic, A.; Saluk-Bijak, J.; Bijak, M. A Review of Various Antioxidant Compounds and their Potential Utility as Complementary Therapy in Multiple Sclerosis. Nutrient 2019, 11, 1528. [Google Scholar] [CrossRef]
- Apostolakis, M.; Armeni, E.; Bakas, P.; Lambrinoudaki, I. Vitamin D and cardiovascular disease. Maturitas 2018, 115, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, A.; Goddeau, R.P.; Henninger, N. Low Serum Vitamin D Is Independently Associated with Larger Lesion Volumes after Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Zhong, Y.; Liao, S.; Lu, Z. Serum 25-hydroxyvitamin D deficiency predicts poor outcome among acute ischemic stroke patients without hypertension. Neurochem. Int. 2018, 118, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wang, M.; Mi, D.; Zhao, J.; Tu, W.; Liu, Q. Vitamin D status and the risk of recurrent stroke and mortality in ischemic stroke patients: Data from a 24-month follow-up study in China. J. Nutr. Health Aging 2017, 21, 766–771. [Google Scholar] [CrossRef]
- Yalbuzdag, S.A.; Sarifakioglu, B.; Afsar, S.I.; Çelik, C.; Can, A.; Yegin, T.; Senturk, B.; Guzelant, A.Y. Is 25(OH)D Associated with Cognitive Impairment and Functional Improvement in Stroke? A Retrospective Clinical Study. J. Stroke Cerebrovasc. Dis. 2015, 24, 1479–1486. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; bin Riaz, I.; Khan, M.S.; Kaluski, E.; et al. Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes:An Umbrella Review and Evidence Map. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Chen, F.; Du, M.; Blumberg, J.B.; Chui, K.K.H.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association Among Dietary Supplement Use, Nutrient Intake, and Mortality Among U.S. Adults: A Cohort Study. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef]
- Bolland, M.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.; Reid, I.R. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010, 341, c3691. [Google Scholar] [CrossRef]
- Sayeed, I.; Turan, N.; Stein, D.G.; Wali, B. Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp. Neurol. 2019, 312, 63–71. [Google Scholar] [CrossRef]
- Bus, C.; Zizmare, L.; Feldkaemper, M.; Geisler, S.; Zarani, M.; Schaedler, A.; Klose, F.; Admard, J.; Mageean, C.J.; Arena, G.; et al. Human Dopaminergic Neurons Lacking PINK1 Exhibit Disrupted Dopamine Metabolism Related to Vitamin B6 Co-Factors. iScience 2020, 23, 101797. [Google Scholar] [CrossRef]
- Strand, T.A.; Ulak, M.; Hysing, M.; Ranjitkar, S.; Kvestad, I.; Shrestha, M.; Ueland, P.M.; McCann, A.; Shrestha, P.S.; Shrestha, L.S.; et al. Effects of vitamin B12 supplementation on neurodevelopment and growth in Nepalese Infants: A randomized controlled trial. PLoS Med. 2020, 17, e1003430. [Google Scholar] [CrossRef]
- Burgess, K.; Bennett, C.; Mosnier, H.; Kwatra, N.; Bethel, F.; Jadavji, N.M. The Antioxidant Role of One-Carbon Metabolism on Stroke. Antioxidants 2020, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wu, G.; Li, Y.; Wang, X.; Hou, F.F.; Xu, X.; Qin, X.; Cai, Y. Meta-analysis of folic acid efficacy trials in stroke prevention: Insight into effect modifiers. Neurology 2017, 88, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Yi, Q.; Hankey, G. B vitamins in stroke prevention: Time to reconsider. Lancet Neurol. 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Juola, F.A.; McGraw, K.; Dearborn, D.C. Carotenoids and throat pouch coloration in the great frigatebird (Fregata minor). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 370–377. [Google Scholar] [CrossRef]
- Polidori, M.C.; Cherubini, A.; Stahl, W.; Senin, U.; Sies, H.; Mecocci, P. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: Relationship to early outcome. Free Radic. Res. 2002, 36, 265–268. [Google Scholar] [CrossRef]
- Leinonen, J.S.; Ahonen, J.-P.; Lönnrot, K.; Jehkonen, M.; Dastidar, P.; Molnár, G.; Alho, H. Low Plasma Antioxidant Activity Is Associated with High Lesion Volume and Neurological Impairment in Stroke. Stroke 2000, 31, 33–39. [Google Scholar] [CrossRef]
- Iversen, P.O.; Ha, L.; Blomhoff, R.; Hauge, T.; Veierød, M.B. Baseline oxidative defense and survival after 5–7 years among elderly stroke patients at nutritional risk: Follow-up of a randomized, nutritional intervention trial. Clin. Nutr. 2015, 34, 775–778. [Google Scholar] [CrossRef]
- Yamagata, K.; Nakayama, C.; Suzuki, K. Dietary β-carotene regulates interleukin-1β-induced expression of apolipoprotein E in astrocytes isolated from stroke-prone spontaneously hypertensive rats. Neurochem. Int. 2013, 62, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Ma, J.; Powell, C.B.; Campos, H.; Gaziano, J.M.; Willett, W.C.; Stampfer, M.J. Prospective Study of Plasma Carotenoids and Tocopherols in Relation to Risk of Ischemic Stroke. Stroke 2004, 35, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Sansawa, H.; Takahashi, M.; Tsuchikura, S.; Endo, H. Effect of chlorella and its fractions on blood pressure, cerebral stroke lesions, and life-span in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2006, 52, 457–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leppälä, J.M.; Virtamo, J.; Fogelholm, R.; Huttunen, J.K.; Albanes, D.; Taylor, P.R.; Heinonen, O.P. Controlled Trial of α-Tocopherol and β-Carotene Supplements on Stroke Incidence and Mortality in Male Smokers. Arter. Thromb. Vasc. Biol. 2000, 20, 230–235. [Google Scholar] [CrossRef] [PubMed]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Date, C.; Kokubo, Y.; Yoshiike, N.; Matsumura, Y.; Tanaka, H. Serum Vitamin C Concentration Was Inversely Associated with Subsequent 20-Year Incidence of Stroke in a Japanese Rural Community. Stroke 2000, 31, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Luben, R.; Welch, A.A.; Bingham, S.A.; Wareham, N.J.; Khaw, K.-T. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer–Norfolk prospective population study. Am. J. Clin. Nutr. 2008, 87, 64–69. [Google Scholar] [CrossRef]
- Al-Khudairy, L.; Flowers, N.; Wheelhouse, R.; Ghannam, O.; Hartley, L.; Stranges, S.; Rees, K. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 3, CD011114. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Ye, Y.; Li, J.; Yuan, Z. Effect of Antioxidant Vitamin Supplementation on Cardiovascular Outcomes: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef]
- Huang, J.; Agus, D.B.; Winfree, C.J.; Kiss, S.; Mack, W.J.; McTaggart, R.A.; Choudhri, T.F.; Kim, L.J.; Mocco, J.; Pinsky, D.J.; et al. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. USA 2001, 98, 11720–11724. [Google Scholar] [CrossRef]
- Mack, W.J.; Mocco, J.; Ducruet, A.F.; Laufer, I.; King, R.G.; Zhang, Y.; Guo, W.; Pinsky, D.J.; Connolly, E.S. A Cerebroprotective Dose of Intravenous Citrate/Sorbitol-stabilized Dehydroascorbic Acid is Correlated with Increased Cerebral Ascorbic Acid and Inhibited Lipid Peroxidation after Murine Reperfused Stroke. Neurosurgery 2006, 59, 383–388. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Kim, J.H.; Choi, J.Y.; Lee, K.M.; Lee, J.E.; Kim, J.Y. Dehydroascorbic Acid Attenuates Ischemic Brain Edema and Neurotoxicity in Cerebral Ischemia: An in vivo Study. Exp. Neurobiol. 2015, 24, 41–54. [Google Scholar] [CrossRef]
- Henry, P.T.; Chandy, M.J. Effect of ascorbic acid on infarct size in experimental focal cerebral ischaemia and reperfusion in a primate model. Acta Neurochir. 1998, 140, 977–980. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.-I.; Josse, R.G.; Vieth, R.; Sahye-Pudaruth, S.; Paquette, M.; Patel, D.; Mejia, S.B.; et al. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Cheng, N.; Butler, L.T.; Lamport, D.J.; Williams, C.M. Flavonoid-Rich Mixed Berries Maintain and Improve Cognitive Function Over a 6 h Period in Young Healthy Adults. Nutrents 2019, 11, 2685. [Google Scholar] [CrossRef]
- Barfoot, K.L.; May, G.; Lamport, D.J.; Ricketts, J.; Riddell, P.M.; Williams, C.M. The effects of acute wild blueberry supplementation on the cognition of 7–10-year-old schoolchildren. Eur. J. Nutr. 2019, 58, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.-E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols From Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, Y.; Xu, Y.; Han, X.; Peng, J.; Liu, K.; Sun, C. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia–reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013, 63, 522–532. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Ravaioli, G.; Vafeiadou, K.; Rodriguez-Mateos, A.; Angeloni, C.; Spencer, J.P. Peroxynitrite induced formation of the neurotoxins 5-S-cysteinyl-dopamine and DHBT-1: Implications for Parkinson’s disease and protection by polyphenols. Arch. Biochem. Biophys. 2008, 476, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.; Bondonno, C.; Rich, L.; Mas, E.; Shinde, S.; Ward, N.; Hodgson, J.M.; Croft, K.D. Acute effects of quercetin-3-O-glucoside on endothelial function and blood pressure: A randomized dose-response study. Am. J. Clin. Nutr. 2016, 104, 97–103. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.; Kromhout, D.; Hollman, P.C.H. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Park, J.-W.; Jang, Y.-H.; Kim, J.-M.; Lee, H.; Park, W.-K.; Lim, M.-B.; Chu, Y.-K.; Lo, E.H.; Lee, S.-R. Green tea polyphenol (-)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J. Neurosci. Res. 2009, 87, 567–575. [Google Scholar] [CrossRef]
- Nan, W.; Zhonghang, X.; Keyan, C.; Tongtong, L.; Wanshu, G.; Zhongxin, X. Epigallocatechin-3-Gallate Reduces Neuronal Apoptosis in Rats after Middle Cerebral Artery Occlusion Injury via PI3K/AKT/eNOS Signaling Pathway. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Han, J.; Wang, M.; Jing, X.; Shi, H.; Ren, M.; Lou, H. (−)-Epigallocatechin Gallate Protects Against Cerebral Ischemia-Induced Oxidative Stress via Nrf2/ARE Signaling. Neurochem. Res. 2014, 39, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.-L.; Ma, H.; Man, Y.-G.; Lv, H.-Y. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can. J. Physiol. Pharmacol. 2017, 95, 1396–1405. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, X.; Sun, C.; Zheng, P.; Gao, J.; Wang, X.; Min, D.; Sun, H.; Xie, N.; Cai, J. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res. Bull. 2011, 85, 396–402. [Google Scholar] [CrossRef]
- Cheng, O.; Li, Z.; Han, Y.; Jiang, Q.; Yan, Y.; Cheng, K. Baicalin improved the spatial learning ability of global ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain Res. 2012, 1470, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Wang, Y.; Si, C.; Wang, X.; Wang, R.-T.; Lv, Z. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging 2020, 12, 3791–3806. [Google Scholar] [CrossRef]
- Tu, X.-K.; Yang, W.-Z.; Shi, S.-S.; Chen, Y.; Wang, C.-H.; Chen, C.-M.; Chen, Z. Baicalin Inhibits TLR2/4 Signaling Pathway in Rat Brain Following Permanent Cerebral Ischemia. Inflammation 2011, 34, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Qu, X.-J.; Yang, Y.; Sheng, X.-H.; Cheng, F.; Jiang, E.-N.; Wang, J.-H.; Bu, W.; Liu, Z.-P. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochem. Biophys. Res. Commun. 2010, 403, 398–404. [Google Scholar] [CrossRef]
- Oh, S.B.; Park, H.R.; Jang, Y.J.; Choi, S.Y.; Son, T.G.; Lee, J. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br. J. Pharmacol. 2012, 168, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Qin, X.; Ren, T.; Cao, J. Baicalin reduces blood lipids and inflammation in patients with coronary artery disease and rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Zhang, L.; Shi, D.-L.; Song, X.-H.; Shen, Y.-L.; Zheng, M.-Z.; Wang, L.-L. Resveratrol Attenuates Subacute Systemic Inflammation-Induced Spatial Memory Impairment via Inhibition of Astrocyte Activation and Enhancement of Synaptophysin Expression in the Hippocampus. Ann. Clin. Lab. Sci. 2017, 47, 17–24. [Google Scholar]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef]
- Tang, F.; Guo, S.; Liao, H.; Yu, P.; Wang, L.; Song, X.; Chen, J.; Yang, Q. Resveratrol Enhances Neurite Outgrowth and Synaptogenesis Via Sonic Hedgehog Signaling Following Oxygen-Glucose Deprivation/Reoxygenation Injury. Cell. Physiol. Biochem. 2017, 43, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis After Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transplant. 2018, 27, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Kalani, K.; Tyagi, N. Epigenetic impact of curcumin on stroke prevention. Metab. Brain Dis. 2014, 30, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, N.; Lin, L. Curcumin Suppresses Apoptosis and Inflammation in Hypoxia/Reperfusion-Exposed Neurons via Wnt Signaling Pathway. Med. Sci. Monit. 2020, 26, e920445-1. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Mol. Med. Rep. 2017, 16, 4710–4720. [Google Scholar] [CrossRef]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.I.; Gobernado, R.G.; Montaner, J. Neuroprotective diets for stroke. Neurochem. Int. 2017, 107, 4–10. [Google Scholar] [CrossRef]
- Saber, H.; Yakoob, M.Y.; Shi, P.; Longstreth, W.; Lemaitre, R.N.; Siscovick, D.; Rexrode, K.; Willett, W.C.; Mozaffarian, D. Omega-3 Fatty Acids and Incident Ischemic Stroke and Its Atherothrombotic and Cardioembolic Subtypes in 3 US Cohorts. Stroke 2017, 48, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Suenaga, J.; Pu, H.; Wei, Z.; Smith, A.D.; Hu, X.; Shi, Y.; Chen, J. Post-stroke administration of omega-3 polyunsaturated fatty acids promotes neurovascular restoration after ischemic stroke in mice: Efficacy declines with aging. Neurobiol. Dis. 2019, 126, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.-Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, J.; Hu, X.; Li, P.; Leak, R.; Gao, Y.; Chen, J. n -3 Polyunsaturated Fatty Acids Reduce Neonatal Hypoxic/Ischemic Brain Injury by Promoting Phosphatidylserine Formation and Akt Signaling. Stroke 2015, 46, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Khoutorova, L.; Atkins, K.D.; Eady, T.N.; Hong, S.; Lu, Y.; Obenaus, A.; Bazan, N.G. Docosahexaenoic Acid Therapy of Experimental Ischemic Stroke. Transl. Stroke Res. 2010, 2, 33–41. [Google Scholar] [CrossRef]
- Eady, T.N.; Belayev, L.; Khoutorova, L.; Atkins, K.D.; Zhang, C.; Bazan, N.G. Docosahexaenoic Acid Signaling Modulates Cell Survival in Experimental Ischemic Stroke Penumbra and Initiates Long-Term Repair in Young and Aged Rats. PLoS ONE 2012, 7, e46151. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Kuan, Y.-H.; Li, J.-R.; Chen, W.-Y.; Ou, Y.-C.; Pan, H.-C.; Liao, S.-L.; Raung, S.-L.; Chang, C.-J.; Chen, C.-J. Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J. Nutr. Biochem. 2013, 24, 2127–2137. [Google Scholar] [CrossRef]
- Dyall, S.; Mandhair, H.; Fincham, R.; Kerr, D.; Roche, M.; Molina-Holgado, F. Distinctive effects of eicosapentaenoic and docosahexaenoic acids in regulating neural stem cell fate are mediated via endocannabinoid signalling pathways. Neuropharmacology 2016, 107, 387–395. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Hernán, M.A.; Giovannucci, E.L.; Kawachi, I.; Stampfer, M.J.; Willett, W.C. Intake of Potassium, Magnesium, Calcium, and Fiber and Risk of Stroke Among US Men. Circulation 1998, 98, 1198–1204. [Google Scholar] [CrossRef]
- Khaw, K.T.; Barrett-Connor, E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N. Engl. J. Med. 1987, 316, 235–240. [Google Scholar] [CrossRef]
- Weisbrod, D. Small and Intermediate Calcium Activated Potassium Channels in the Heart: Role and Strategies in the Treatment of Cardiovascular Diseases. Front. Physiol. 2020, 11, 590534. [Google Scholar] [CrossRef]
- Pan, W.-H.; Lai, Y.-H.; Yeh, W.-T.; Chen, J.-R.; Jeng, J.-S.; Bai, C.-H.; Lin, R.-T.; Lee, T.-H.; Chang, K.-C.; Lin, H.-J.; et al. Intake of potassium- and magnesium-enriched salt improves functional outcome after stroke: A randomized, multicenter, double-blind controlled trial. Am. J. Clin. Nutr. 2017, 106, ajcn148536. [Google Scholar] [CrossRef] [PubMed]
- Sahota, P.; Savitz, S.I. Investigational Therapies for Ischemic Stroke: Neuroprotection and Neurorecovery. Neurotherapeutics 2011, 8, 434–451. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Ahmad, F.; Shuaib, A. Survival and histological evaluation of therapeutic window of post-ischemia treatment with magnesium sulfate in embolic stroke model of rat. Neurosci. Lett. 2000, 285, 119–122. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; He, Y.; Ran, Y.; Lu, B.; Gao, J.; Shu, C.; Li, J.; Zhao, Y.; Zhang, X.; et al. Protective effect of melatonin entrapped PLGA nanoparticles on radiation-induced lung injury through the miR-21/TGF-β1/Smad3 pathway. Int. J. Pharm. 2021, 602, 120584. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Woo, Y.S.; Park, S.W.; Seog, D.-H.; Seo, M.K.; Bahk, W.-M. The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease. Brain Sci. 2019, 9, 285. [Google Scholar] [CrossRef]
- Pei, Z.; Pang, S.; Cheung, R. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2002, 32, 168–172. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Pandey, A.K.; Paul, S.; Patnaik, R. Melatonin renders neuroprotection by protein kinase C mediated aquaporin-4 inhibition in animal model of focal cerebral ischemia. Life Sci. 2014, 100, 97–109. [Google Scholar] [CrossRef]
- Lee, E.-J.; Wu, T.-S.; Lee, M.-Y.; Chen, T.-Y.; Tsai, Y.-Y.; Chuang, J.-I.; Chang, G.-L. Delayed treatment with melatonin enhances electrophysiological recovery following transient focal cerebral ischemia in rats. J. Pineal Res. 2004, 36, 33–42. [Google Scholar] [CrossRef]
- De Butte, M.; Gieseking, B. Efficacy of a low-dose melatonin pretreatment in protecting against the neurobehavioral consequences of chronic hypoperfusion in middle-aged female rats. Behav. Brain Res. 2020, 377, 112257. [Google Scholar] [CrossRef]
- Bin-Jaliah, I.; Sakr, H.F. Melatonin ameliorates brain oxidative stress and upregulates senescence marker protein-30 and osteopontin in a rat model of vascular dementia. Physiol. Int. 2018, 105, 38–52. [Google Scholar] [CrossRef]
- Al Dera, H.; Alassiri, M.; Eleawa, S.M.; Alkhateeb, M.A.; Hussein, A.; Dallak, M.; Sakr, H.F.; Alqahtani, S.; Khalil, M.A. Melatonin Improves Memory Deficits in Rats with Cerebral Hypoperfusion, Possibly, Through Decreasing the Expression of Small-Conductance Ca2+-Activated K+ Channels. Neurochem. Res. 2019, 44, 1851–1868. [Google Scholar] [CrossRef]
- Wongprayoon, P. Melatonin Receptor as a Drug Target for Neuroprotection. Curr. Mol. Pharmacol. 2020, 14, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Wu, Q.; Li, W.; Tu, Y.; Sirianni, A.C.; Chen, Y.; Jiang, J.; Zhang, X.; Chen, W.; Zhou, S.; et al. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT1 receptor. J. Pineal Res. 2017, 64, e12443. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ma, M.; Wu, Y.; Deng, M.-F.; Hu, F.; Almansoub, H.; Huang, H.-Z.; Wang, D.-Q.; Zhou, L.-T.; Wei, N.; et al. Activation of MT2 receptor ameliorates dendritic abnormalities in Alzheimer’s disease via C/EBPα/miR-125b pathway. Aging Cell 2019, 18, e12902. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Lu, S.-S.; Liu, M.-R.; Tang, W.-D.; Chen, J.-Z.; Zheng, Y.-R.; Ahsan, A.; Cao, M.; Jiang, L.; Hu, W.-W.; et al. Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharmacol. Sin. 2020, 41, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef]
- Kim, W.; Hahn, K.R.; Jung, H.Y.; Kwon, H.J.; Nam, S.M.; Kim, J.W.; Park, J.H.; Yoo, D.-Y.; Kim, D.W.; Won, M.; et al. Melatonin ameliorates cuprizone-induced reduction of hippocampal neurogenesis, brain-derived neurotrophic factor, and phosphorylation of cyclic AMP response element-binding protein in the mouse dentate gyrus. Brain Behav. 2019, 9, e01388. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Paine, T.A.; Slipp, L.E.; Carlezon, W.A. Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology 2011, 36, 1703–1713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gajcy, K.; Lochynski, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347. [Google Scholar] [CrossRef]
- Zareian, M.; Ebrahimpour, A.; Abu Bakar, F.; Mohamed, A.K.S.; Forghani, B.; Ab-Kadir, M.S.B.; Saari, M. A Glutamic Acid-Producing Lactic Acid Bacteria Isolated from Malaysian Fermented Foods. Int. J. Mol. Sci. 2012, 13, 5482–5497. [Google Scholar] [CrossRef]
- Tian, J.; Song, M.; Kaufman, D.L. Homotaurine limits the spreading of T cell autoreactivity within the CNS and ameliorates disease in a model of multiple sclerosis. Sci. Rep. 2021, 11, 5402. [Google Scholar] [CrossRef]
- Kakee, A.; Takanaga, H.; Terasaki, T.; Naito, M.; Tsuruo, T.; Sugiyama, Y. Efflux of a suppressive neurotransmitter, GABA, across the blood-brain barrier. J. Neurochem. 2008, 79, 110–118. [Google Scholar] [CrossRef]
- Leonte, A.; Colzato, L.; Steenbergen, L.; Hommel, B.; Akyürek, E.G. Supplementation of gamma-aminobutyric acid (GABA) affects temporal, but not spatial visual attention. Brain Cogn. 2018, 120, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; De Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- Kanehira, T.; Nakamura, Y.; Nakamura, K.; Horie, K.; Horie, N.; Furugori, K.; Sauchi, Y.; Yokogoshi, H. Relieving Occupational Fatigue by Consumption of a Beverage Containing γ-Amino Butyric Acid. J. Nutr. Sci. Vitaminol. 2011, 57, 9–15. [Google Scholar] [CrossRef]
- Wilby, M.J.; Hutchinson, P.J. The Pharmacology of Chlormethiazole: A Potential Neuroprotective Agent? CNS Drug Rev. 2006, 10, 281–294. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Sciacca, R.; Di Raimondo, D.; Arnao, V.; Renda, C.; Pinto, A.; Licata, G. Neuron protection as a therapeutic target in acute ischemic stroke. Curr. Top. Med. Chem. 2009, 9, 1317–1334. [Google Scholar] [CrossRef] [PubMed]
- Alicke, B.; Schwartz-Bloom, R.D. Rapid Down-Regulation of GABAA Receptors in the Gerbil Hippocampus Following Transient Cerebral Ischemia. J. Neurochem. 2002, 65, 2808–2811. [Google Scholar] [CrossRef] [PubMed]
- Chi, O.Z.; Hunter, C.; Liu, X.; Chi, Y.; Weiss, H.R. Effects of GABAA receptor blockade on regional cerebral blood flow and blood–brain barrier disruption in focal cerebral ischemia. J. Neurol. Sci. 2011, 301, 66–70. [Google Scholar] [CrossRef]
- Zubcevic, J.; Potts, J.T. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp. Physiol. 2010, 95, 909–918. [Google Scholar] [CrossRef]
- Visser, S.A.; Pozarek, S.; Martinsson, S.; Forsberg, T.; Ross, S.B.; Gabrielsson, J. Rapid and long-lasting tolerance to clomethiazole-induced hypothermia in the rat. Eur. J. Pharmacol. 2005, 512, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Sydserff, S.G.; Cross, A.J.; Green, A. The neuroprotective effect of chlormethiazole on ischaemic neuronal damage following permanent middle cerebral artery ischaemia in the rat. Neurodegeneration 1995, 4, 323–328. [Google Scholar] [CrossRef]
- Marshall, J.; Cross, A.; Ridley, R. Functional Benefit from Clomethiazole Treatment after Focal Cerebral Ischemia in a Nonhuman Primate Species. Exp. Neurol. 1999, 156, 121–129. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Wang, L.-N. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database Syst. Rev. 2018, 10, CD009622. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- Evans, C.E.L. Dietary fibre and cardiovascular health: A review of current evidence and policy. Proc. Nutr. Soc. 2020, 79, 61–67. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Dietary Fiber Intake Is Inversely Associated with Stroke Incidence in Healthy Swedish Adults. J. Nutr. 2014, 144, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; Katzke, V.; Kühn, T.; Boeing, H.; et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: A prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fiber intake and risk of first stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Beta-glucans from oats and/or barley in a ready-to-eat cereal manufactured. EFSA J. 2021, 19, e06493. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.F.; Dos Santos, O.; Barbosa, A.M.; Vasconcelos, A.F.D.; Aranda-Selverio, G.; Monteiro, N.K.; Dekker, R.F.; Pereira, M.S.; Tovar, A.M.F.; Mourão, P.A.D.S.; et al. Sulfonation and anticoagulant activity of botryosphaeran from Botryosphaeria rhodina MAMB-05 grown on fructose. Int. J. Biol. Macromol. 2009, 45, 305–309. [Google Scholar] [CrossRef]
- Wouk, J.; Dekker, R.F.; Queiroz, E.A.; Barbosa-Dekker, A.M. β-Glucans as a panacea for a healthy heart? Their roles in preventing and treating cardiovascular diseases. Int. J. Biol. Macromol. 2021, 177, 176–203. [Google Scholar] [CrossRef] [PubMed]
- Park, O.K.; Choi, J.H.; Park, J.H.; Kim, I.H.; Yan, B.C.; Ahn, J.H.; Kwon, S.-H.; Lee, J.-C.; Kim, Y.S.; Kim, M.; et al. Comparison of neuroprotective effects of five major lipophilic diterpenoids from Danshen extract against experimentally induced transient cerebral ischemic damage. Fitoterapia 2012, 83, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.A.; Ramadan, A.A.; Abdelmonsif, D.A.; El-Kamel, A.H. Enhanced oral bioavailability of Tanshinone IIA using lipid nanocapsules: Formulation, in-vitro appraisal and pharmacokinetics. Int. J. Pharm. 2020, 586, 119598. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Wang, C.; Sun, Q. Tanshinone inhibits neuronal cell apoptosis and inflammatory response in cerebral infarction rat model. Int. J. Immunopathol. Pharmacol. 2017, 30, 123–129. [Google Scholar] [CrossRef]
- Cai, M.; Guo, Y.; Wang, S.; Wei, H.; Sun, S.; Zhao, G.; Dong, H. Tanshinone IIA Elicits Neuroprotective Effect Through Activating the Nuclear Factor Erythroid 2-Related Factor-Dependent Antioxidant Response. Rejuvenation Res. 2017, 20, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.-Y.; Li, J.; Lu, B.-L.; Liu, J.; Yang, F.-Z.; Zhou, L.; Luo, H.; Li, W.-W.; Zhou, J. Tanshinone IIA increases levels of NeuN, protein disulfide isomerase, and Na+/K+-ATPase and decreases evidence of microglial activation after cerebral ischemic injury. NeuroReport 2016, 27, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, X.; Zhang, X.; Ye, Y.; Jian, Z.; Gao, W.; Gu, L. Sodium Tanshinone IIA Sulfonate Protects Against Cerebral Ischemia–reperfusion Injury by Inhibiting Autophagy and Inflammation. Neuroscience 2020, 441, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, Z.-L.; Gao, H.; Ma, W.-W.; Dai, Y.; Wang, Y.; Zhong, Y.; Yao, X.-S. Bioactive Iridoid Glucosides from the Fruit ofGardenia jasminoides. J. Nat. Prod. 2009, 72, 1459–1464. [Google Scholar] [CrossRef]
- Hua, J.; Qi, J.; Yu, B.-Y. Iridoid and phenylpropanoid glycosides from Scrophularia ningpoensis Hemsl. and their α-Glucosidase inhibitory activities. Fitoterapia 2014, 93, 67–73. [Google Scholar] [CrossRef]
- Gao, B.-B.; She, G.-M.; She, D.-M. Chemical Constituents and Biological Activities of Plants from the GenusLigustrum. Chem. Biodivers. 2013, 10, 96–128. [Google Scholar] [CrossRef]
- Habtemariam, S. Iridoids and Other Monoterpenes in the Alzheimer’s Brain: Recent Development and Future Prospects. Molecules 2018, 23, 117. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, B.; Dai, M.; Sun, Y.; Sun, Q.; Yang, G.; Bian, L. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci. Lett. 2013, 555, 24–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, F.; Liu, J.; Liu, Z.; Guo, L.; Xia, Z.; Zidichouski, J. Geniposide attenuates insulin-deficiency-induced acceleration of β-amyloidosis in an APP/PS1 transgenic model of Alzheimer’s disease. Neurochem. Int. 2015, 89, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.W.; Yang, W.T.; Chen, S.; Xu, Q.Q.; Shan, C.S.; Zheng, G.Q.; Ruan, J.C. Neuroprotection of Catalpol for Experimental Acute Focal Ischemic Stroke: Preclinical Evidence and Possible Mechanisms of Antioxidation, Anti-Inflammation, and Antiapoptosis. Oxid. Med. Cell. Longev. 2017, 2017, 5058609. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Gao, X.; Talalay, P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc. Natl. Acad. Sci. USA 2004, 101, 10446–10451. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear Factor E2-Related Factor 2-Dependent Antioxidant Response Element Activation by tert-Butylhydroquinone and Sulforaphane Occurring Preferentially in Astrocytes Conditions Neurons against Oxidative Insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, J.; Yu, S.; Chen, Y.; Wu, J.; Zhao, Y. Sulforaphane protects primary cultures of cortical neurons against injury induced by oxygen-glucose deprivation/reoxygenation via antiapoptosis. Neurosci. Bull. 2012, 28, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Xing, G.-P.; Yu, Y.; Liang, H.; Yu, T.-X.; Zheng, W.-H.; Lai, T.-B. Sulforaphane exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Int. J. Clin. Exp. Med. 2015, 8, 17811–17817. [Google Scholar]
- Yu, C.; He, Q.; Zheng, J.; Li, L.Y.; Hou, Y.H.; Song, F.Z. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017, 45, 74–78. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Yang, G.-P.; Jiang, D.-J.; Wang, F.; Song, T.; Tan, X.-H.; Sun, Z.-Q. Protection of capsaicin against hypoxia–reoxygenation-induced apoptosis of rat hippocampal neurons. Can. J. Physiol. Pharmacol. 2008, 86, 785–792. [Google Scholar] [CrossRef]
- Huang, M.; Cheng, G.; Tan, H.; Qin, R.; Zou, Y.; Wang, Y.; Zhang, Y. Capsaicin protects cortical neurons against ischemia/reperfusion injury via down-regulating NMDA receptors. Exp. Neurol. 2017, 295, 66–76. [Google Scholar] [CrossRef]
- Pegorini, S.; Braida, D.; Verzoni, C.; Guerini-Rocco, C.; Consalez, G.G.; Croci, L.; Sala, M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br. J. Pharmacol. 2005, 144, 727–735. [Google Scholar] [CrossRef]
- Cao, W.; Xiao, X.; Zhang, L.; Liu, Y.; Wang, L.; Zou, Z.; Cao, Y.; Li, C.; Zheng, Q.; Zhou, S.; et al. Compound glycyrrhizin combined with antihistamines for chronic urticaria: A protocol for systematic review and meta analysis. Medicine 2020, 99, e21624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-H.; Li, X.; He, L.-F.; Cai, H.-F.; Ye, B.; Wu, Z.-M. Glycyrrhizic acid, as an inhibitor of HMGB1, alleviates bleomycin-induced pulmonary toxicity in mice through the MAPK and Smad3 pathways. Immunopharmacol. Immunotoxicol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The Inflammatory Response to Cell Death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Weng, Z.; Zhou, T.; Feng, T.; Lin, Y. Glycyrrhizin protects brain against ischemia–reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain Res. 2014, 1582, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Gu, L.; Wang, Y.; Luo, Y.; Zhang, H.; Lee, J.; Krams, S.; Zhu, S.; Zhao, H. Glycyrrhizin protects against focal cerebral ischemia via inhibition of T cell activity and HMGB1-mediated mechanisms. J. Neuroinflamm. 2016, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Jin, Y.; Shin, J.-H.; Kim, I.-D.; Lee, H.-K.; Park, S.; Han, P.-L.; Lee, J.-K. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol. Dis. 2012, 46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Xiang, L.; Yuan, L.; Hu, L.; Wu, W.; Cai, L.; Yin, L.; Dong, H. Protective Effect of Glycyrrhizin, a Direct HMGB1 Inhibitor, on Focal Cerebral Ischemia/Reperfusion-Induced Inflammation, Oxidative Stress, and Apoptosis in Rats. PLoS ONE 2014, 9, e89450. [Google Scholar] [CrossRef]
- Yan, S.; Fang, C.; Cao, L.; Wang, L.; Du, J.; Sun, Y.; Tong, X.; Lu, Y.; Wu, X. Protective effect of glycyrrhizic acid on cerebral ischemia/reperfusion injury via inhibiting HMGB1-mediated TLR4/NF-κB pathway. Biotechnol. Appl. Biochem. 2019, 66, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, C.; Yang, J.; Yao, Y. Glycyrrhizic Acid Ameliorates Cognitive Impairment in a Rat Model of Vascular Dementia Associated with Oxidative Damage and Inhibition of Voltage-Gated Sodium Channels. CNS Neurol. Disord. Drug Targets 2016, 15, 1001–1008. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Son, D.; Chung, T.-H.; Lee, Y.-J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef]
- Ingles, D.P.; Cruz Rodriguez, J.B.; Garcia, H. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Curr. Cardiol. Rep. 2020, 22, 22. [Google Scholar] [CrossRef]
- Bordelon, P.; Ghetu, M.V.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician 2009, 80, 841–846. [Google Scholar]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-van Straaten, H.M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 2018, 22, 70. [Google Scholar] [CrossRef]
- Pang, H.; Xue, W.; Shi, A.; Li, M.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Xiao, W.; He, G.; et al. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin. Drug Investig. 2016, 36, 713–724. [Google Scholar] [CrossRef]
| Natural Compounds | Biological Activity in Central Nervous System | Literature | |
|---|---|---|---|
| Primary stroke prevention | B vitamins (B6, B12, folic acid) | Inhibition of atherosclerotic processes by involvement in homocysteine methylation | [25,26,27,46] |
| Carotenoids | Reduction of fibroblast growth factor-1 (FGF1)-mediated gliosis of astrocytes by increasing the expression of genes related to cholesterol regulation: Abcg2, Abca1, Hmgcr, and Apoe Reducing the risk of death from stroke | [31,32,33] | |
| Polyunsaturated fatty acids (PUFAs) | Reduction of lower total stroke risk and decreased risk of atherothrombotic stroke Alleviation of post-stroke brain damage and reduction of sensorimotor disorders | [82,83] | |
| Potassium | Anti-hypotensive effect Reduction of free radical production, smooth muscle proliferation, and inhibition of macrophage adhesion to the vascular wall Reduction of the stroke risk | [91,93] | |
| Dietary fiber | Reduction of stroke risk | [133,134,135] | |
| β-glucans | Strong stimulants and modulators of the immune system Anti-viral, antibacterial, anti-hypertensive and anticancer properties Regulation of the body’s carbohydrate and lipid metabolism, lowering the level of triglycerides, cholesterol and glucose | [136] | |
| Vitamin C | Antioxidative and anti-inflammatory properties Dose-dependently reduction of infract volume, mortality, edema, and neurological disorders Improvement of neurological outcomes as well as blood flow | ||
| Neuroprotection | Vitamin D | Improvement of cerebral blood flow, reduction of blood pressure, and vasodilation by increasing the activity of nitric oxide synthase (NOS) Enhancement of the expression of neurotrophic factors (vascular endothelial growth factor—VEGF, stromal cell-derived factor 1α—SDF1α, and insulin-like growth factor 1—IGF-1) Reduction of neuronal degeneration Prevention of blood–brain barrier (BBB) disturbance by inhibiting oxidative stress and regulation of tight-junction protein occludin and claudin-5 expression | [16,19,22] |
| Flavonoid-rich food (FRF) | Improving cognitive function, regardless of age and medical history Reducing neuronal apoptosis and scavenging free radicals, as well as inhibiting neuroinflammation Reduction of proinflammatory biomarker expression (IL-1β, IL-6, IL-4, TNF-α, inducible nitric oxide synthase—iNOS, nuclear factor kap-pa-light-chain-enhancer of activated B cells NFκB, matrix metalloproteinase-9—MMP-9, and cyclooxygenase-2—COX-2) Decrease in the level of protein kinase RNA-like kinase endoplasmic reticulum (p-ERK), N-terminal c-jun kinase (p-JNK), and members of mitogen-activated protein kinase (MAPK) pathway The molecular neuroprotective mechanism associated with the activation of the cAMP response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF)/tropomyosin-related kinase B receptor (TrkB)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and/or ERK 1/2 pathways | [47,48,49,50,51,52] | |
| Quercetin | Anti-oxidant, -inflammatory, -platelet, -atherosclerotic, -obesity, -hypercholesterolemic, -cancer, and -allergic properties inhibiting cellular toxicity Reduction of both systolic diastolic blood pressure | [53,59] | |
| Baicalin | Enhancement of cognitive, behavioral and motor functions Improvement of neurological deficit Decrease in the infarct volume Enhancement of synaptic plasticity | [65,66,67,68,69,176] | |
| Epigallocatechin gallate | The activation of CREB/BDNF/TrkB-PI3K/Akt signaling Increases in Akt, phospho-Akt, mTORc1 and phospho-glycogen synthase kinase 3 (pGSK3b), as well as growth in BDNF and TrkB expression Decreases in neurological deficits Reduction of the level of brain injury and oxidative stress biomarkers Inhibition of neuronal apoptosis Promoting neuron survival | [61,63] | |
| Resveratrol | Anti-aging, -inflammatory, -apoptotic, -oxidative, -cancerous, -diabetic, hepato- and cardioprotective properties Reducing post-traumatic axonal degeneration and promoting neurite growth and synaptogenesis by activating the sonic hedgehog homolog (Shh) after oxygen–glucose depriva-tion/reoxygenation (OGD/R) neuronal injury The inhibition of oxidative stress, neuroinflammation and apoptosis Beneficial effect on blood pressure, lipid profile and body mass index in post-stroke patients | [72,73,74,75,76] | |
| Curcumin | Anti-lipidemic, -inflammatory and -aggregating properties Epigenetic modulator and neuroprotective agent Promoting neuronal viability Inhibition of apoptosis Reduction of the expression of IL-6, Wnt5a, TNFα, the level of JNK1 phosphorylation, and the NFκB nu-clear translocation Reduction of brain edema, disruption of the BBB, and infarct volume Upregulation of Nrf2 expression Decrease in expression of NFκB, as well as MMP9, intercellular adhesion molecule 1 (ICAM1), and caspase 3 expression | [77,78,79,80] | |
| Docosahexaenoic acid (DHA) | Promotion of translocation and PIP3-depended phosphorylation of Akt and activation of GSK-3β The induction of signaling pathways responsible for neuronal survival: protein kinase C (PKC) and Raf-1 kinase The activation of antioxidant mechanisms and modulation of neuroinflammation Reduction of infraction volume, edema, BBB disruption, infarct volume, and improved neurobehavior Promoting immunosuppression: decreased activation of macrophag-es/microglia and peripheral leukocytes, as well as the expression of proinflammatory cytokines Phosphorylation of JNK, c-Jun, activated activator protein 1 (AP-1), and increased the expression of Nrf2 and HO-1 | [84,85,86,87,88] | |
| Eicosapentaenoic acid (EPA) | Interaction with immune and endocannabinoid system promotes neurorepair Augmenting proliferation of neural stem cells (NSC) what is associated with enhancing levels of the endocannabinoid 2-arachidonylglycerol (2-AG) and p-p38 MAPK | [89,90] | |
| Magnesium | Augmentation of regional blood flow to ischemic regions Non-competitive inhibition of glutamate and voltage-sensitive calcium Enhancement of adenosine actionInhibition of glutamate release Increased regeneration of cellular energy metabolism Reduction of infarct volume and enhancing neurological outcomes | [94,95] | |
| Melatonin | Reducing the infarct volumeImprovement of behavioral deficits and reduction of damage to brain tissue Inhibition of oxidative stress Enhancement of neuronal viability Promoting neuronal survival and proliferation Increase in endogenous antioxidant levels via the Akt/ERK/CREB pathway Inhibition of apoptosis, memory loss, neurodegeneration, and neuroinflammation Promoting hippocampal neurogenesis by enhancing CREB phosphorylation and increasing BDNF levels | [101,102,103,104,105,110] | |
| γ-Aminobutyric acid (GABA) | Positive effect on such functions as temporal attention, reducing acrophobia, lessening psychological fatigue after completion of the task | [119,120,121] | |
| Tanshinones | Inhibition of the ischemia progression by reducing neuronal apoptosis Reducing oxidative damage and microglia activation Increase in the expression of both the gene and the Nrf2 protein Enhancing the activity of antioxidant enzymes Inhibition of neuroinflammation (reduction of the number of B lymphocytes, T lymphocytes and macrophages in the ischemic brain), as well as autophagy (decreased up-regulation of LC3-II, Sirt 6 and Beklin-1 proteins) | [141,142,143,144] | |
| Carvacrol | Improvement of neurological deficits Reducing cerebral edema and Evans blue leakage Decrease in AQP4 mRNA in a dose-dependent manner Reduction of AQP4 protein expression in the perihematomal area Reducing the oxidative stress in the cerebral cortexRegulation of the activities and concentration of SOD, glutathione peroxidase and catalase Reducing the levels of soluble Aβ40 and Aβ42 in the cerebral cortex Improvement of learning and memory Up-regulation of the protein levels of β-site APP cleaving enzyme (BACE1) and IDE Decrease in the protein levels of ADAM10 | [150,151] | |
| Glycyrrhizin | Anti-inflammatory, antioxidative, antiapoptotic and anti-excitotoxic properties Improvement of locomotor deficits Reduction of infarct volume and cerebral edema | [163,166,167,168,169] | |
| Sulforaphane | Anti-inflammatory, antioxidant and chemoprotective properties Increase in cell viability, Bcl-2 expression, and increased caspase 3 levels via the P13/Akt pathway Suppressing the inflammatory response induced by ischemia Reducing brain edema, BBB disruption, and the level of pro-inflammatory cytokines: IL-1β and TNF-α Suppressing the activity and expression of iNOS and COX-2 and NO concentration by inhibiting the NF-κB pathway Reduction of the stroke volume and improvement of neurological outcomes Suppressing the activation of the NLRP3 inflammasome and the down-regulation of caspase-1, and decrease in the expression of pro-inflammatory cytokines: IL-1β and IL-18 | [153,157,158,159] | |
| Capsaicin | Reduction of calcium ion influx and inhibition of excitotoxicity, oxidative stress and neuroinflammation, leading to increased survival of neurons Inhibition of caspase-3 and the production of ROS by activating the PI3K/Akt pathway leading to reduction of apoptosis and oxidative stress Decreased in hyperlocomotion, memory impairment Increase in the survival of pyramidal cells in the CA1 subfield Reduction of stroke volume and improvement of motor coordination and behavioral evaluation | [160,161,162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichon, N.; Saluk-Bijak, J.; Miller, E.; Gorniak, L.; Redlicka, J.; Niwald, M.; Bijak, M. The Role of Supplementation with Natural Compounds in Post-Stroke Patients. Int. J. Mol. Sci. 2021, 22, 7893. https://doi.org/10.3390/ijms22157893
Cichon N, Saluk-Bijak J, Miller E, Gorniak L, Redlicka J, Niwald M, Bijak M. The Role of Supplementation with Natural Compounds in Post-Stroke Patients. International Journal of Molecular Sciences. 2021; 22(15):7893. https://doi.org/10.3390/ijms22157893
Chicago/Turabian StyleCichon, Natalia, Joanna Saluk-Bijak, Elzbieta Miller, Leslaw Gorniak, Justyna Redlicka, Marta Niwald, and Michal Bijak. 2021. "The Role of Supplementation with Natural Compounds in Post-Stroke Patients" International Journal of Molecular Sciences 22, no. 15: 7893. https://doi.org/10.3390/ijms22157893
APA StyleCichon, N., Saluk-Bijak, J., Miller, E., Gorniak, L., Redlicka, J., Niwald, M., & Bijak, M. (2021). The Role of Supplementation with Natural Compounds in Post-Stroke Patients. International Journal of Molecular Sciences, 22(15), 7893. https://doi.org/10.3390/ijms22157893








