Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident
Abstract
:1. Introduction
2. Results
2.1. Patient Demographics and Information
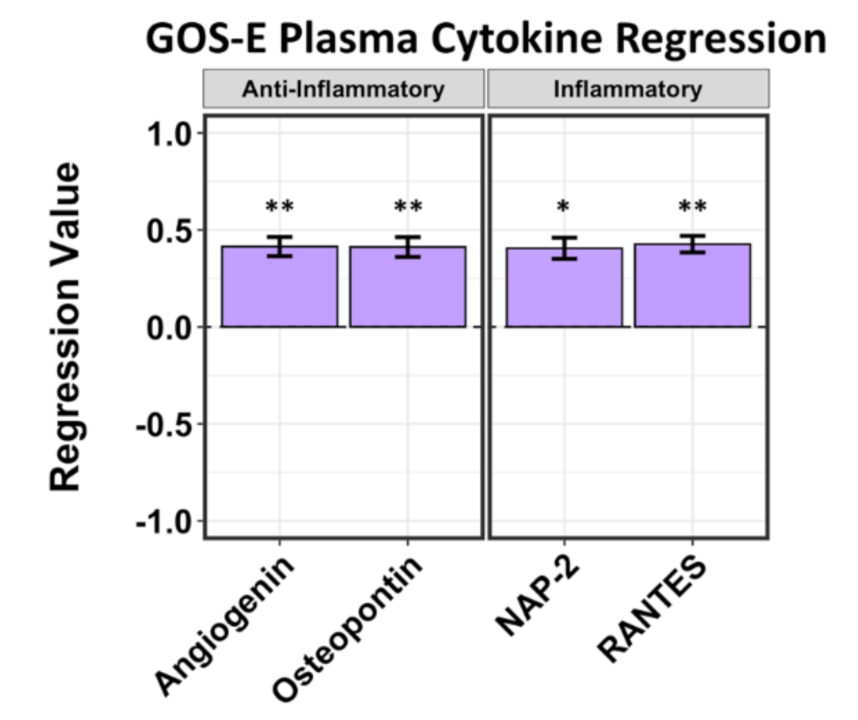
2.2. A Limited Number of Plasma Cytokines Are Associated with Positive and Negative GCS Scores and Positive GOS-E Scores in Hemorrhagic Stroke Patients
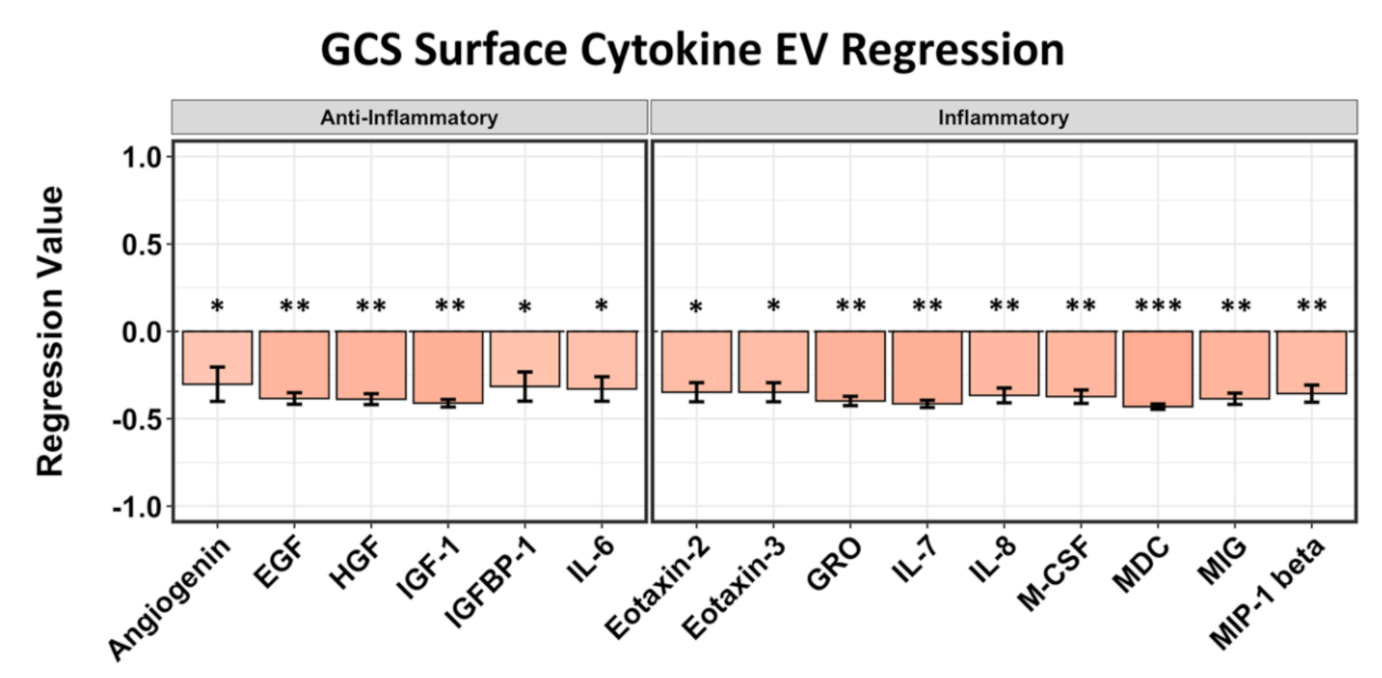
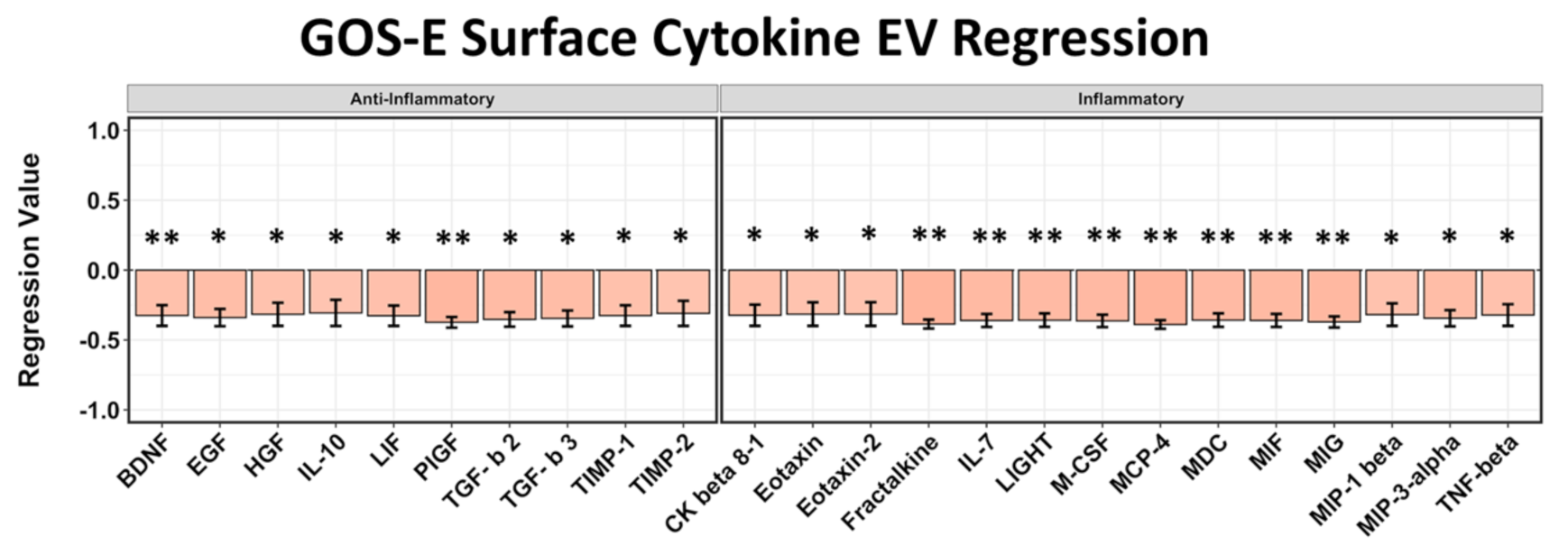
2.3. EV Surface-Bound Cytokines Correlated to Negative GCS and GOS-E Scores
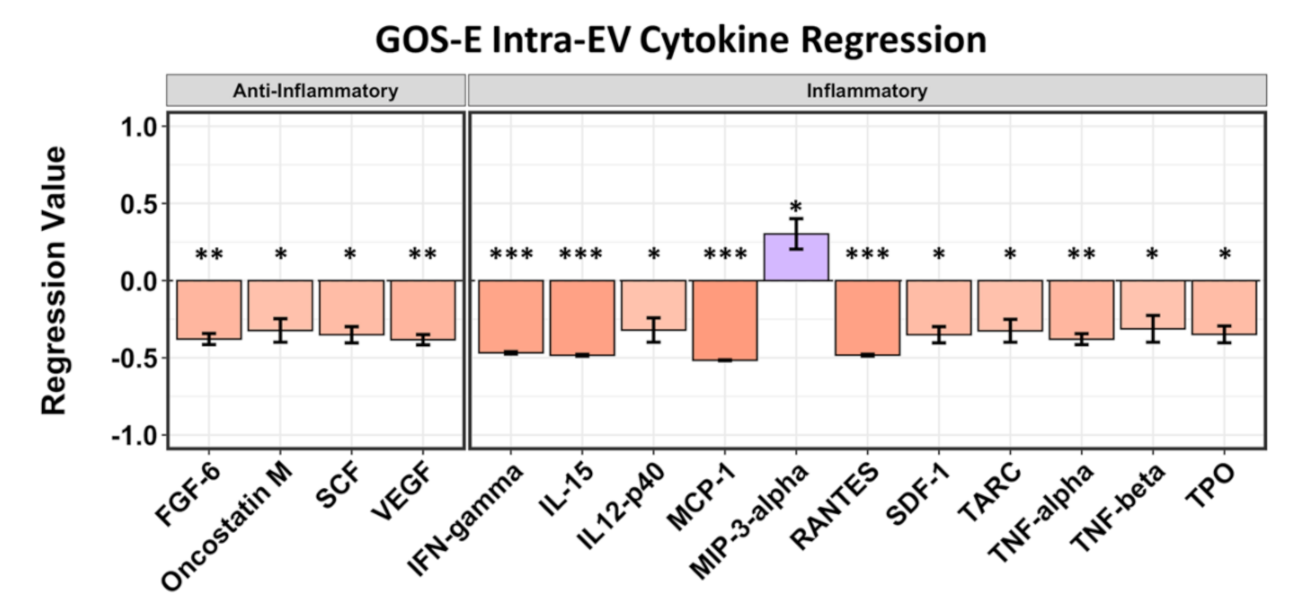
2.4. Intra-EV Cytokines Are Mostly Correlated with Negative GOS-E Scores
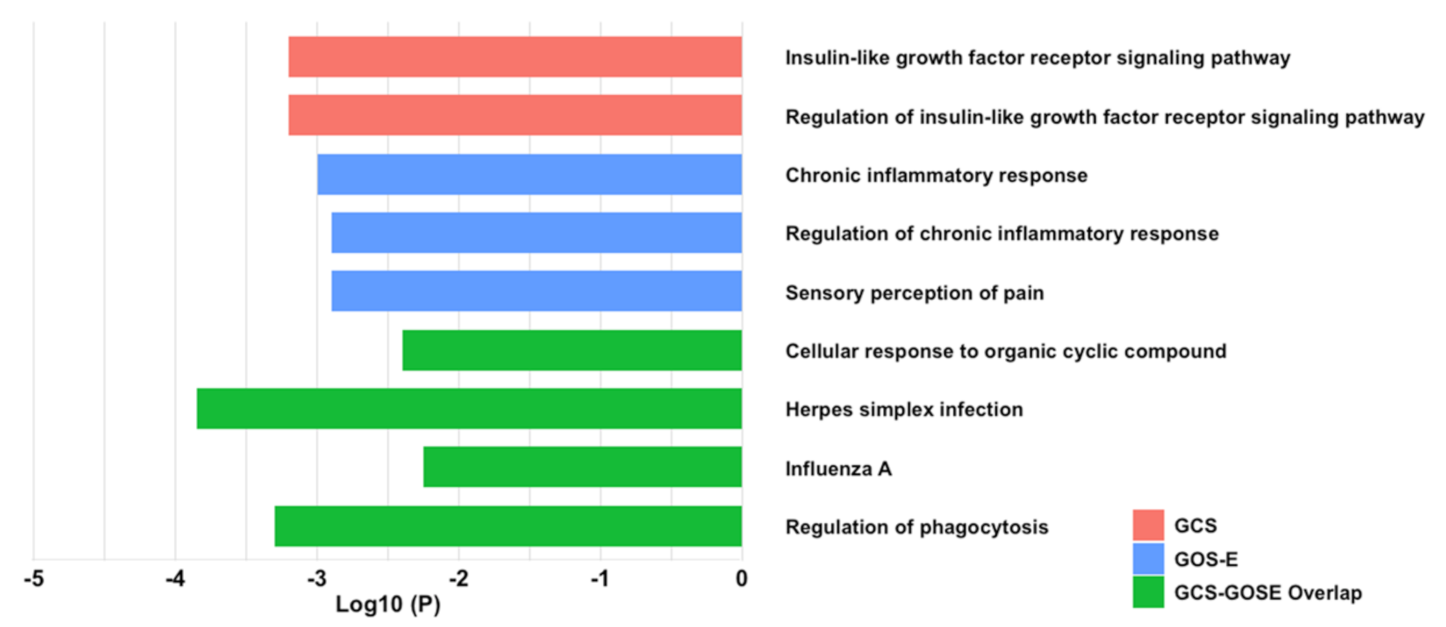
2.5. EV-Associated Cytokines Share Protein–Protein Inflammatory Pathways
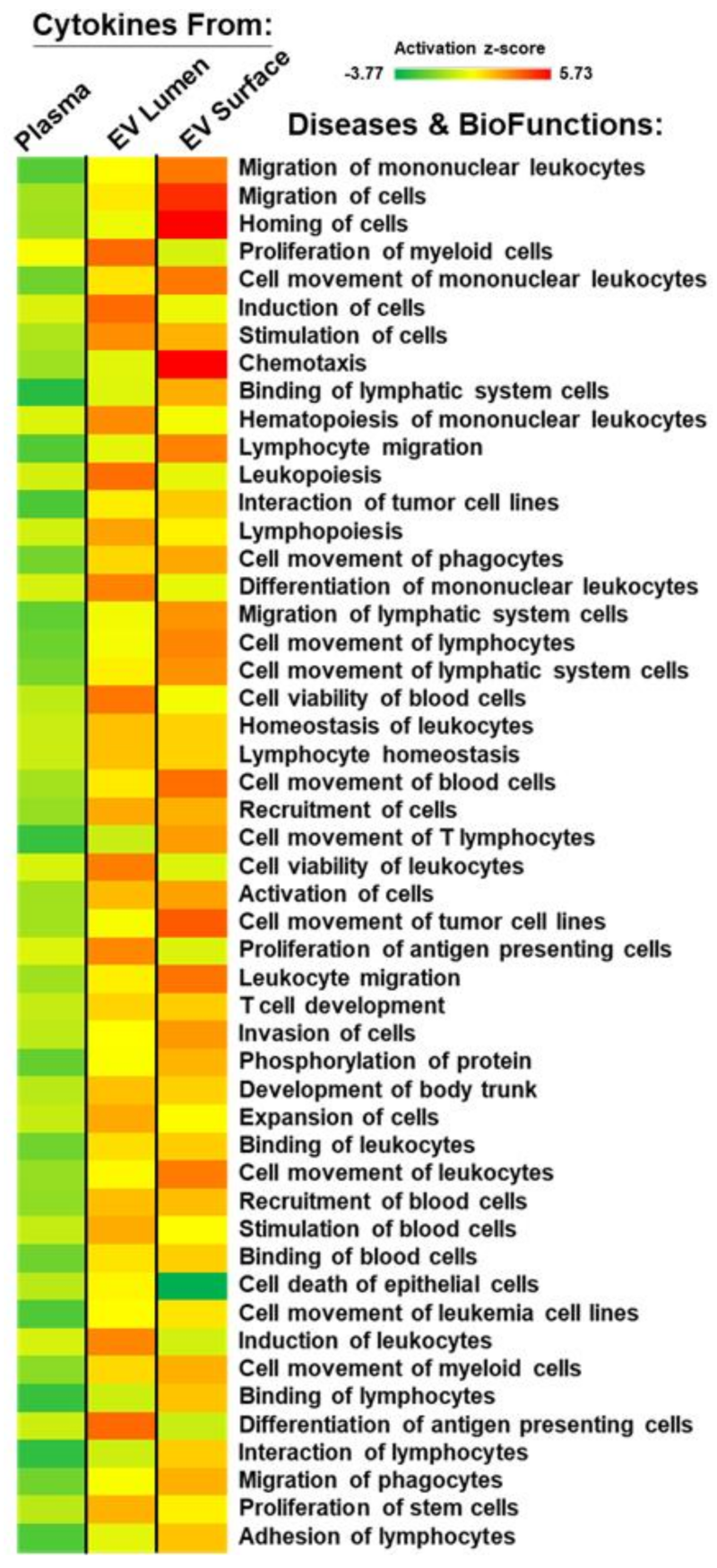
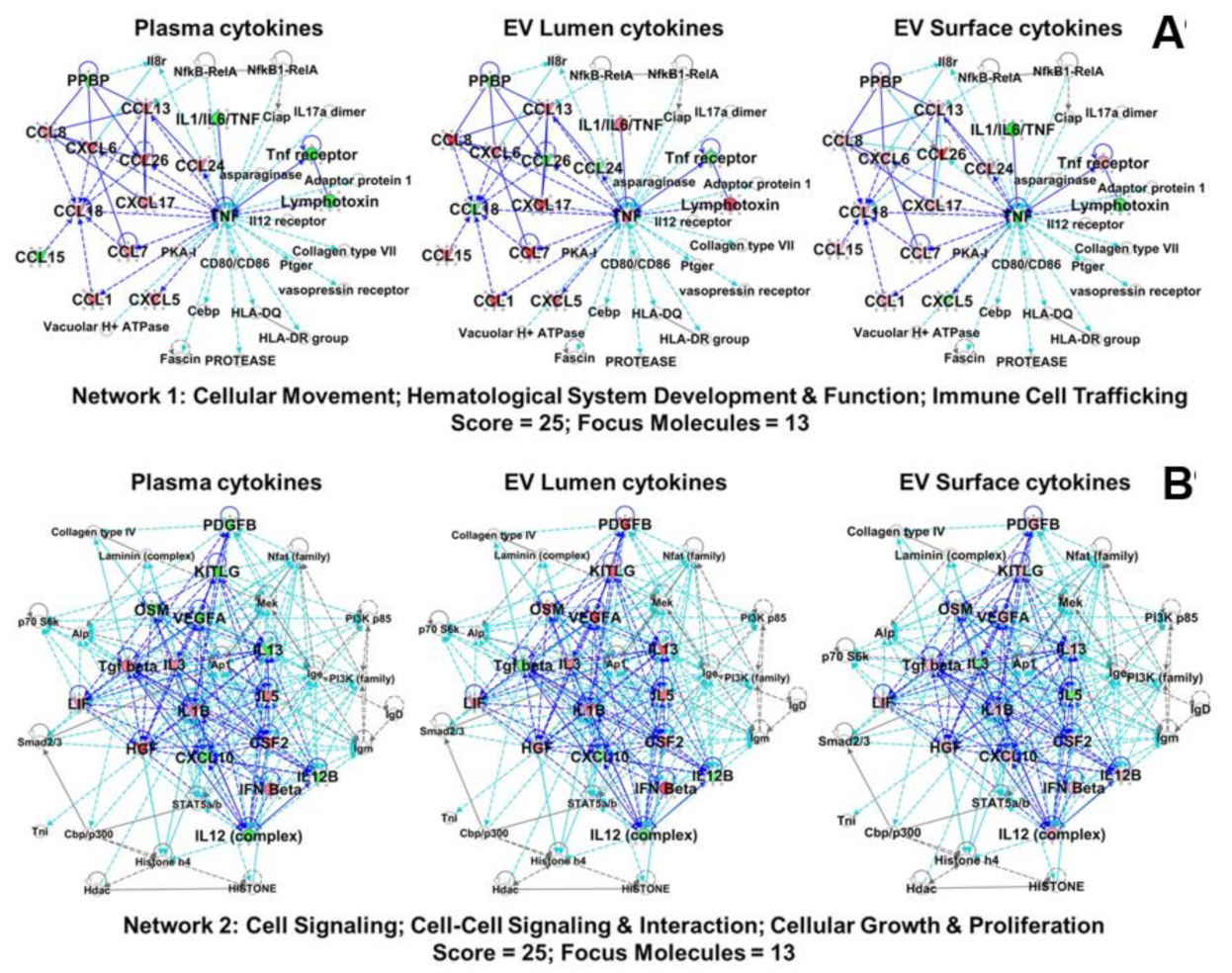
2.6. Plasma and EV-Associated Cytokines Predict Differential Changes in Immune Cell Movement and Function
3. Discussion
4. Materials and Methods
4.1. Inclusion Criteria
4.2. Peripheral Blood Collection
4.3. Volumetric Analysis of Brain Hemorrhagea
4.4. Isolation of Extracellular Vesicles
4.5. Validation of Extracellular Vesicle Isolation
4.6. Surface Protein Stripping and Lysis of Extracellular Vesicles
4.7. Plasma, EV Surface-Bound, and Intra-EV Cytokine Quantification
4.8. Detection of Apolipoproteins
4.9. Outcome Reporting
4.10. Metascape Analysis
4.11. Ingenuity Pathway Analysis (IPA)
4.12. Statistical Analysis and Graphics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| ANAX | annexin |
| AP1 | activator protein 1 |
| APO | apolipoprotein |
| ASA | American Stroke Association |
| BCA | bicinchoninic acid |
| BDNF | brain-derived neurotrophic factor |
| COMIRB | Colorado Multiple Institutional Review Board |
| CCL | chemokine (C-C motif) ligand |
| CCR | chemokine (C-C motif) receptor |
| CD | cluster of differentiation |
| CSF | cerebrospinal fluid |
| CSF1 | colony stimulating factor 1 |
| CT | computed tomography |
| CVA | cerebrovascular accident |
| CX3CL | chemokine (C-X3-C motif) ligand |
| CXCL | chemokine (C-X-C motif) ligand |
| DTT | dithiothreitol |
| EGF | epidermal growth factor |
| EV | extracellular vesicle |
| FGF | fibroblast growth factor |
| FLOT | flotillin |
| GCS | Glasgow Coma Scale |
| GCP | granulocyte chemotactic protein |
| GM | c-s-Golgi matrix protein |
| GM-CSF | granulocyte-macrophage colony stimulating factor |
| GOS-E | Glasgow Coma Scale-Extended |
| GRO | growth-activated oncogene |
| HGF | hepatocyte growth factor |
| HRP | horse radish peroxidase |
| ICAM | intracellular adhesion molecule |
| ICH | intracranial hemorrhage |
| IFN | interferon |
| Ig | immunoglobulin |
| IGF | insulin-like growth factor |
| IGFBP | insulin-like growth factor binding protein |
| IL | interleukin |
| IP-10 | interferon gamma-induced protein 10 |
| IPA | Ingenuity Pathway Analysis |
| IPH | intraparenchymal hemorrhage |
| IVH | intraventricular hemorrhage |
| JUN | homolog of viral oncoprotein v-jun |
| KITLG | KIT (proto-oncogene receptor tyrosine kinase) ligand |
| LIF | leukemia inhibitor factor |
| LT | lymphotoxin |
| M-CSF | macrophage-colony stimulating factor |
| MCP | monocyte chemoattractant protein |
| MDC | macrophage-derived chemokine |
| MIG | monokine induced by gamma interferon |
| MIP | macrophage inflammatory protein |
| mm | millimeter |
| mRS | modified Rankin Scale |
| NAP | neutrophil activating peptide |
| NEB | New England Biolabs |
| NIHSS | National Institute of Health Stroke Scale |
| PGF | placental growth factor |
| PlGF | placental growth factor |
| PPBP | platelet basic protein |
| RANTES | Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted |
| RIPA | radio-immune precipitation assay |
| RPM | revolutions per minute |
| SAH | subarachnoid hemorrhage |
| SCF | stem cell factor |
| SD | standard deviation |
| SDH | subdural hemorrhage |
| SF | scatter factor |
| SMAD | homologous to SMA (small) and MAD (mothers against decapentaplegic) families |
| SPP | secreted phosphoprotein |
| TBST | Tris-buffered saline with Tween |
| TGFB | transforming growth factor beta |
| TH | T-helper cell |
| TIMP | tissue inhibitor of metalloproteinases |
| TNF | tumor necrosis factor |
| TSG | tumor susceptibility gene |
| VEGF | vascular endothelial growth factor |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and stroke: An overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Doll, D.N.; Barr, T.L.; Simpkins, J.W. Cytokines: Their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 2014, 5, 294–306. [Google Scholar]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Derm. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Gao, L.; Cheng, H. Inflammatory profiles of the interleukin family and network in cerebral hemorrhage. Cell Mol. Neurobiol. 2018, 38, 1321–1333. [Google Scholar] [CrossRef]
- Donohue, M.M.; Cain, K.; Zierath, D.; Shibata, D.; Tanzi, P.M.; Becker, K.J. Higher plasma fractalkine is associated with better 6-month outcome from ischemic stroke. Stroke 2012, 43, 2300–2306. [Google Scholar] [CrossRef] [Green Version]
- Landreneau, M.J.; Mullen, M.T.; Messe, S.R.; Cucchiara, B.; Sheth, K.N.; McCullough, L.D.; Kasner, S.E.; Sansing, L.H.; the Serum Markers After Spontaneous Cerebral Hemorrhage (SMASCH) Investigators. Ccl2 and cxcl10 are associated with poor outcome after intracerebral hemorrhage. Ann. Clin. Transl. Neurol. 2018, 5, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [Green Version]
- Sobowale, O.A.; Parry-Jones, A.R.; Smith, C.J.; Tyrrell, P.J.; Rothwell, N.J.; Allan, S.M. Interleukin-1 in stroke: From bench to bedside. Stroke 2016, 47, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Emsley, H.C.; Smith, C.J.; Georgiou, R.F.; Vail, A.; Hopkins, S.J.; Rothwell, N.J.; Tyrrell, P.J.; Acute Stroke, I. A randomised phase ii study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1366–1372. [Google Scholar] [CrossRef] [Green Version]
- Sykova, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [Green Version]
- Thurley, K.; Gerecht, D.; Friedmann, E.; Hofer, T. Three-dimensional gradients of cytokine signaling between t cells. PLoS Comput. Biol. 2015, 11, e1004206. [Google Scholar] [CrossRef] [Green Version]
- Oyler-Yaniv, A.; Oyler-Yaniv, J.; Whitlock, B.M.; Liu, Z.; Germain, R.N.; Huse, M.; Altan-Bonnet, G.; Krichevsky, O. A tunable diffusion-consumption mechanism of cytokine propagation enables plasticity in cell-to-cell communication in the immune system. Immunity 2017, 46, 609–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graner, M.W. Roles of extracellular vesicles in high-grade gliomas: Tiny particles with outsized influence. Annu. Rev. Genom. Hum. Genet. 2019, 20, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.H.; Madsen, H.J.; Hellwinkel, J.E.; Lencioni, A.M.; Graner, M.W. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014, 105, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graner, M.W.; Schnell, S.; Olin, M.R. Tumor-derived exosomes, microRNAs, and cancer immune suppression. Semin. Immunopathol. 2018, 40, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A system of cytokines encapsulated in extracellular vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [Green Version]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Wilson, L.; Boase, K.; Nelson, L.D.; Temkin, N.R.; Giacino, J.T.; Markowitz, A.J.; Maas, A.; Menon, D.K.; Teasdale, G.; Manley, G.T. A manual for the glasgow outcome scale-extended interview. J. Neurotrauma 2021. [Google Scholar] [CrossRef]
- Schall, T.J. Biology of the rantes/sis cytokine family. Cytokine 1991, 3, 165–183. [Google Scholar] [CrossRef]
- Tokami, H.; Ago, T.; Sugimori, H.; Kuroda, J.; Awano, H.; Suzuki, K.; Kiyohara, Y.; Kamouchi, M.; Kitazono, T.; Investigators, R. Rantes has a potential to play a neuroprotective role in an autocrine/paracrine manner after ischemic stroke. Brain Res. 2013, 1517, 122–132. [Google Scholar] [CrossRef]
- Buzas, E.I.; Toth, E.A.; Sodar, B.W.; Szabo-Taylor, K.E. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Aiello, A.; Giannessi, F.; Percario, Z.A.; Affabris, E. An emerging interplay between extracellular vesicles and cytokines. Cytokine Growth Factor Rev. 2020, 51, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, Z.Z.; Gong, J.; Xiang, M.; Zhang, P.; Zhao, G.N.; Li, M.; Zheng, A.; Zhu, X.; Lei, H.; et al. Oncostatin m confers neuroprotection against ischemic stroke. J. Neurosci. 2015, 35, 12047–12062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houben, E.; Hellings, N.; Broux, B. Oncostatin m, an underestimated player in the central nervous system. Front. Immunol. 2019, 10, 1165. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Kalani, M.Y.S.; Alsop, E.; Meechoovet, B.; Beecroft, T.; Agrawal, K.; Whitsett, T.G.; Huentelman, M.J.; Spetzler, R.F.; Nakaji, P.; Kim, S.; et al. Extracellular micrornas in blood differentiate between ischaemic and haemorrhagic stroke subtypes. J. Extracell Vesicles 2020, 9, 1713540. [Google Scholar] [CrossRef] [Green Version]
- Otero-Ortega, L.; Laso-Garcia, F.; Gomez-de Frutos, M.; Fuentes, B.; Diekhorst, L.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. Role of exosomes as a treatment and potential biomarker for stroke. Transl. Stroke Res. 2019, 10, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Price, C.I. A systematic review and meta-analysis of molecular biomarkers associated with early neurological deterioration following acute stroke. Cereb. Dis. 2018, 46, 230–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.; Zhu, Z.; Wang, A.; Xu, T.; Bu, X.; Peng, H.; Yang, J.; Han, L.; Chen, J.; Xu, T.; et al. Multiple biomarkers covering distinct pathways for predicting outcomes after ischemic stroke. Neurology 2019, 92, e295–e304. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Chen, X.; Huang, W.; Li, Q.; Zhang, H.; Chen, X.; Yang, S.; Jin, K.; Shao, B. The prognostic value of serum cytokines in patients with acute ischemic stroke. Aging Dis. 2019, 10, 544–556. [Google Scholar] [CrossRef] [Green Version]
- Watson, E.; Ding, D.; Khattar, N.K.; Everhart, D.E.; James, R.F. Neurocognitive outcomes after aneurysmal subarachnoid hemorrhage: Identifying inflammatory biomarkers. J. Neurol. Sci. 2018, 394, 84–93. [Google Scholar] [CrossRef]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Kooijman, R.; Sarre, S.; Michotte, Y.; De Keyser, J. Insulin-like growth factor i: A potential neuroprotective compound for the treatment of acute ischemic stroke? Stroke 2009, 40, e83–e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kawabori, M.; Yenari, M.A. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr. Med. Chem. 2014, 21, 2076–2097. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.C.; Barnes, T.Y.; Bullock, R.; Germanson, T.A.; Marmarou, A.; Young, H.F. Temporal profile of outcomes in severe head injury. J. Neurosurg. 1994, 81, 169–173. [Google Scholar] [CrossRef]
- Berthoud, T.K.; Dunachie, S.J.; Todryk, S.; Hill, A.V.; Fletcher, H.A. Mig (cxcl9) is a more sensitive measure than ifn-gamma of vaccine induced t-cell responses in volunteers receiving investigated malaria vaccines. J. Immunol. Methods 2009, 340, 33–41. [Google Scholar] [CrossRef]
- Boulakirba, S.; Pfeifer, A.; Mhaidly, R.; Obba, S.; Goulard, M.; Schmitt, T.; Chaintreuil, P.; Calleja, A.; Furstoss, N.; Orange, F.; et al. Il-34 and csf-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci. Rep. 2018, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. On the mechanism determining the th1/th2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand. J. Immunol. 2014, 79, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Gray, P.A.; Van Damme, J.; Sozzani, S. Macrophage-derived chemokine (mdc). J. Leukoc. Biol. 2000, 68, 400–404. [Google Scholar] [PubMed]
- Hendrix, S.; Nitsch, R. The role of t helper cells in neuroprotection and regeneration. J. Neuroimmunol. 2007, 184, 100–112. [Google Scholar] [CrossRef]
- Rayasam, A.; Hsu, M.; Hernandez, G.; Kijak, J.; Lindstedt, A.; Gerhart, C.; Sandor, M.; Fabry, Z. Contrasting roles of immune cells in tissue injury and repair in stroke: The dark and bright side of immunity in the brain. Neurochem. Int. 2017, 107, 104–116. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, R.; Zhu, X.; Smerin, D.; Zhong, Y.; Gu, L.; Fang, W.; Xiong, X. The involvement and therapy target of immune cells after ischemic stroke. Front. Immunol. 2019, 10, 2167. [Google Scholar] [CrossRef] [Green Version]
- Palomino, D.C.; Marti, L.C. Chemokines and immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Canestro, C.; Reiner, M.F.; Bonetti, N.R.; Liberale, L.; Merlini, M.; Wust, P.; Amstalden, H.; Briand-Schumacher, S.; Semerano, A.; Giacalone, G.; et al. Ap-1 (activated protein-1) transcription factor jund regulates ischemia/reperfusion brain damage via il-1beta (interleukin-1beta). Stroke 2019, 50, 469–477. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.M.; Fish, E.N. Chemokines: Attractive mediators of the immune response. Semin. Immunol. 2003, 15, 5–14. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.; Zhou, Y.; Gan, Y.; Zhu, W.; Xia, Y.; Jiang, X.; Watkins, S.; Vazquez, A.; Thomson, A.W.; et al. C-c chemokine receptor type 5 (ccr5)-mediated docking of transferred tregs protects against early blood-brain barrier disruption after stroke. J. Am. Heart Assoc. 2017, 6, e006387. [Google Scholar] [CrossRef]
- Victoria, E.C.G.; de Brito Toscano, E.C.; de Sousa Cardoso, A.C.; da Silva, D.G.; de Miranda, A.S.; da Silva Barcelos, L.; Sugimoto, M.A.; Sousa, L.P.; de Assis Lima, I.V.; de Oliveira, A.C.P.; et al. Knockdown of c-c chemokine receptor 5 (ccr5) is protective against cerebral ischemia and reperfusion injury. Curr. Neurovasc. Res. 2017, 14, 125–131. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (mcp-1): An overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Perera, P.Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, H.A.; Collier, L.A.; Chapman, C.B.; Benkovic, S.A.; Willing, A.E.; Pennypacker, K.R. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J. Neuroimmune Pharm. 2014, 9, 679–689. [Google Scholar] [CrossRef]
- Saenger, A.K.; Christenson, R.H. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010, 56, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, A.G.; Anthony, D.C.; Ruitenberg, M.J.; Couch, Y. Systemic immune response to traumatic cns injuries-are extracellular vesicles the missing link? Front. Immunol. 2019, 10, 2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvjetkovic, A.; Jang, S.C.; Konecna, B.; Hoog, J.L.; Sihlbom, C.; Lasser, C.; Lotvall, J. Detailed analysis of protein topology of extracellular vesicles-evidence of unconventional membrane protein orientation. Sci. Rep. 2016, 6, 36338. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [Green Version]
- Epple, L.M.; Griffiths, S.G.; Dechkovskaia, A.M.; Dusto, N.L.; White, J.; Ouellette, R.J.; Anchordoquy, T.J.; Bemis, L.T.; Graner, M.W. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS ONE 2012, 7, e42064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009, 23, 1541–1557. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Chen, C. The crown and the scepter: Roles of the protein corona in nanomedicine. Adv. Mater. 2019, 31, e1805740. [Google Scholar] [CrossRef] [PubMed]
- Palviainen, M.; Saraswat, M.; Varga, Z.; Kitka, D.; Neuvonen, M.; Puhka, M.; Joenvaara, S.; Renkonen, R.; Nieuwland, R.; Takatalo, M.; et al. Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo-implications for biomarker discovery. PLoS ONE 2020, 15, e0236439. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef] [Green Version]
- Ziai, W.C. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke 2013, 44, S74–S78. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubramanian, C.; Mlynash, M.; Finley-Caulfield, A.; Eyngorn, I.; Kalimuthu, R.; Snider, R.W.; Wijman, C.A. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 2011, 42, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Ironside, N.; Chen, C.J.; Ding, D.; Mayer, S.A.; Connolly, E.S., Jr. Perihematomal edema after spontaneous intracerebral hemorrhage. Stroke 2019, 50, 1626–1633. [Google Scholar] [CrossRef]
- Ironside, N.; Chen, C.J.; Mutasa, S.; Sim, J.L.; Ding, D.; Marfatiah, S.; Roh, D.; Mukherjee, S.; Johnston, K.C.; Southerland, A.M.; et al. Fully automated segmentation algorithm for perihematomal edema volumetry after spontaneous intracerebral hemorrhage. Stroke 2020, 51, 815–823. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3d active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.K.; Song, K.S.; Chung, J.H.; Lee, K.R.; Lee, S.N. Platelet microparticles induce angiogenesis in vitro. Br. J. Haematol. 2004, 124, 376–384. [Google Scholar] [CrossRef]
- Mause, S.F.; von Hundelshausen, P.; Zernecke, A.; Koenen, R.R.; Weber, C. Platelet microparticles: A transcellular delivery system for rantes promoting monocyte recruitment on endothelium. Arter. Thromb. Vasc. Biol. 2005, 25, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta 2014, 1838, 2954–2965. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.C.; Connors, A.F., Jr.; Wagner, D.P.; Haley, E.C., Jr. Predicting outcome in ischemic stroke: External validation of predictive risk models. Stroke 2003, 34, 200–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, W.E.; Hess, R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ich score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.T.; Hareendran, A.; Grant, M.; Baird, T.; Schulz, U.G.; Muir, K.W.; Bone, I. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified rankin scale. Stroke 2002, 33, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Snoek, J.; Bond, M.R.; Brooks, N. Disability after severe head injury: Observations on the use of the Glasgow outcome scale. J. Neurol. Neurosurg. Psychiatry 1981, 44, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fringuello, A.; Tatman, P.D.; Wroblewski, T.; Thompson, J.A.; Yu, X.; Lillehei, K.O.; Kowalski, R.G.; Graner, M.W. Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident. Int. J. Mol. Sci. 2021, 22, 7847. https://doi.org/10.3390/ijms22157847
Fringuello A, Tatman PD, Wroblewski T, Thompson JA, Yu X, Lillehei KO, Kowalski RG, Graner MW. Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident. International Journal of Molecular Sciences. 2021; 22(15):7847. https://doi.org/10.3390/ijms22157847
Chicago/Turabian StyleFringuello, Anthony, Philip D. Tatman, Tadeusz Wroblewski, John A. Thompson, Xiaoli Yu, Kevin O. Lillehei, Robert G. Kowalski, and Michael W. Graner. 2021. "Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident" International Journal of Molecular Sciences 22, no. 15: 7847. https://doi.org/10.3390/ijms22157847
APA StyleFringuello, A., Tatman, P. D., Wroblewski, T., Thompson, J. A., Yu, X., Lillehei, K. O., Kowalski, R. G., & Graner, M. W. (2021). Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident. International Journal of Molecular Sciences, 22(15), 7847. https://doi.org/10.3390/ijms22157847






