MicroRNAs, Multiple Sclerosis, and Depression
Abstract
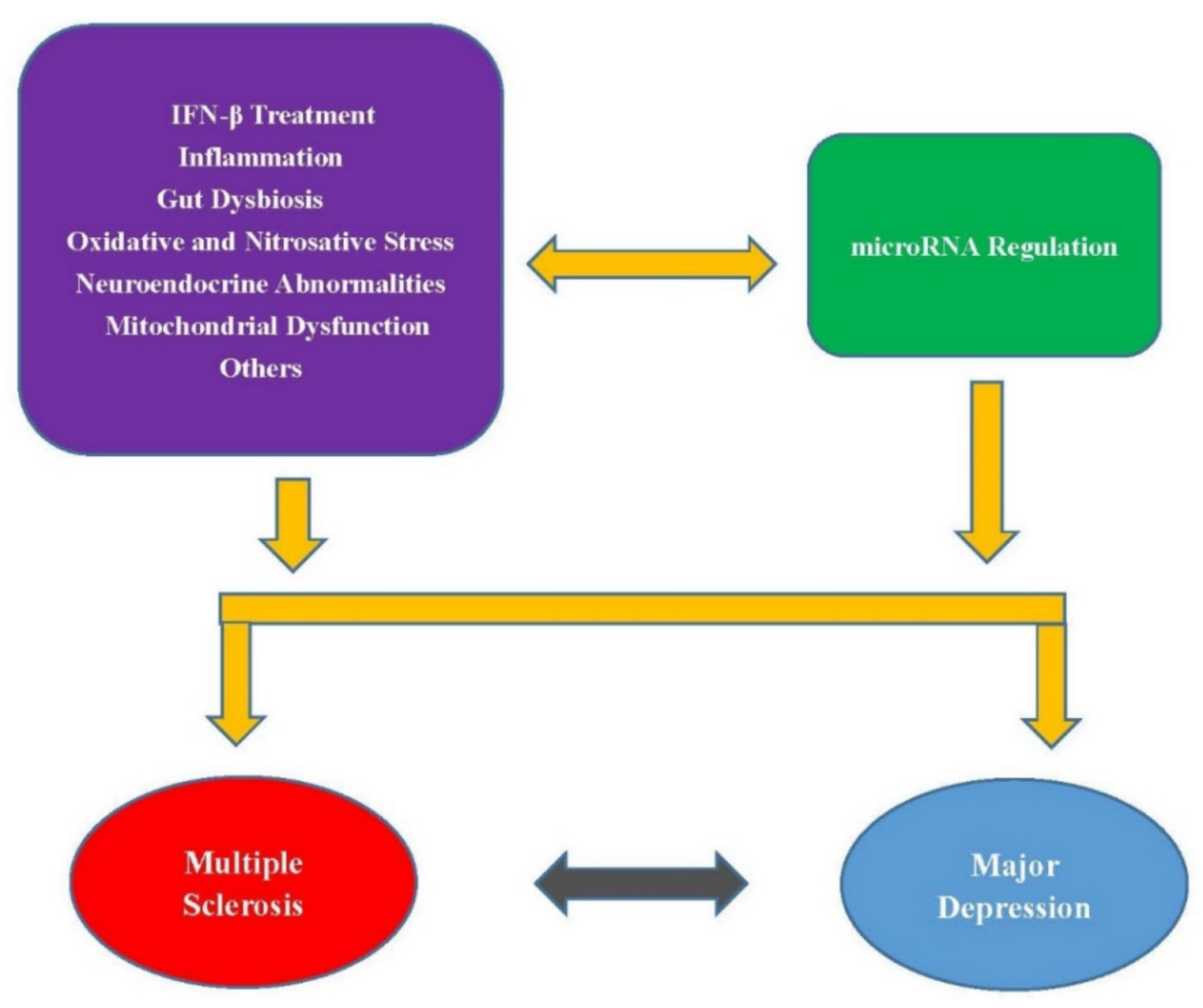
:1. Introduction
2. MicroRNA
3. MicroRNA Biomarkers
4. Other Mechanisms Connecting MS and MD
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of multiple sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16, s53–s59. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Tarlinton, R.E.; Khaibullin, T.; Granatov, E.; Martynova, E.; Rizvanov, A.; Khaiboullina, S. The interaction between viral and environmental risk factors in the pathogenesis of multiple sclerosis. Int. J. Mol. Sci. 2019, 20, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianfrancesco, M.A.; Stridh, P.; Rhead, B.; Shao, X.R.; Xu, E.; Graves, J.S.; Chitnis, T.; Waldman, A.; Lotze, T.; Schreiner, T.; et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology 2017, 88, 1623–1629. [Google Scholar] [CrossRef]
- Kepczynska, K.; Zajda, M.; Lewandowski, Z.; Przedlacki, J.; Zakrzewska-Pniewska, B. Bone metabolism and vitamin D status in patients with multiple sclerosis. Neurol. Neurochir. Pol. 2016, 50, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Handel, A.E.; Giovannoni, G.; Siegel, S.R.; Ebers, G.C.; Chaplin, G. Relationship of UV exposure to prevalence of multiple sclerosis in England. Neurology 2011, 76, 1410–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017, 19, 1. [Google Scholar]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ouakinin, S.R.; Barreira, D.P.; Gois, C.J. Depression and obesity: Integrating the role of stress, neuroendocrine dysfunction and inflammatory pathways. Front. Endocrinol. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.-J.; Kim, S.-Y.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Yoon, J.-S.; Kim, J.-M. Comorbidity of depression with physical disorders: Research and clinical implications. Chonnam Med. J. 2015, 51, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atlantis, E.; Fahey, P.; Foster, J. Collaborative care for comorbid depression and diabetes: A systematic review and meta-analysis. BMJ Open 2014, 4, e004706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.H.; Wang, H. The Association between depression and gastroesophageal reflux based on phylogenetic analysis of miRNA biomarkers. Curr. Med. Chem. 2020, 27, 6536–6547. [Google Scholar] [CrossRef]
- Cheung, S.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Patten, S.B.; Marrie, R.A.; Carta, M.G. Depression in multiple sclerosis. Int. Rev. Psychiatry 2017, 29, 463–472. [Google Scholar] [CrossRef]
- Feinstein, A.; Pavisian, B. Multiple sclerosis and suicide. Mult. Scler. 2017, 23, 923–927. [Google Scholar] [CrossRef]
- Pale, L.A.; Caballero, J.L.; Buxareu, B.S.; Serrano, P.S.; Sola, V.P. Systematic review of depression in patients with multiple sclerosis and its relationship to interferon beta treatment. Mult. Scler. Relat. Dis. 2017, 17, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodin, D.S.; Frohman, E.M.; Garmany, G.P.; Halper, J.; Likosky, W.H.; Lublin, F.D.; Silberberg, D.H.; Stuart, W.H.; van den Noort, S. Disease modifying therapies in multiple sclerosis—Report of the therapeutics and technology assessment subcommittee of the american academy of neurology and the ms council for clinical practice guidelines. Neurology 2002, 58, 169–178. [Google Scholar] [PubMed] [Green Version]
- Solaro, C.; Gamberini, G.; Masuccio, F.G. Depression in multiple sclerosis: Epidemiology, aetiology, diagnosis and treatment. CNS Drugs 2018, 32, 117–133. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal. Transduct Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef]
- Galka-Marciniak, P.; Urbanek-Trzeciak, M.O.; Nawrocka, P.M.; Dutkiewicz, A.; Giefing, M.; Lewandowska, M.A.; Kozlowski, P. Somatic mutations in miRNA genes in lung cancer-potential functional consequences of non-coding sequence variants. Cancers 2019, 11, 793. [Google Scholar] [CrossRef] [Green Version]
- Xian, Q.J.; Zhao, R.L.; Fu, J.J. MicroRNA-527 Induces proliferation and cell cycle in esophageal squamous cell carcinoma cells by repressing pH domain leucine-rich-repeats protein phosphatase 2. Dose Response 2020, 18, 1559325820928687. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Zhang, Z.X.; Ma, Y.; Su, H.X.; Xie, P.; Ran, J.T. LINC02381 promoted cell viability and migration via targeting miR-133b in cervical cancer cells. Cancer Manag. Res. 2020, 12, 3971–3979. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Predicting MicroRNA biomarkers for cancer using phylogenetic tree and microarray analysis. Int. J. Mol. Sci. 2016, 17, 773. [Google Scholar] [CrossRef] [Green Version]
- Wang, H. Predicting cancer-related MiRNAs using expression profiles in tumor tissue. Curr. Pharm. Biotechnol. 2014, 15, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Si, W.G.; Shen, J.Y.; Zheng, H.L.; Fan, W.M. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics 2019, 11, 1–24. [Google Scholar] [CrossRef]
- Rizzuti, M.; Filosa, G.; Melzi, V.; Calandriello, L.; Dioni, L.; Bollati, V.; Bresolin, N.; Comi, G.P.; Barabino, S.; Nizzardo, M. MicroRNA expression analysis identifies a subset of downregulated miRNAs in ALS motor neuron progenitors. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.H.; Wang, H. Exploring microRNA biomarker for amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2018, 19, 1318. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.Y.; Chao, Y.X.; Dheen, S.T.; Tan, E.K.; Tay, S.S. Role of MicroRNAs in Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 5649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, M.; Piscopo, P.; Talarico, G.; Ricci, L.; Crestini, A.; Tosto, G.; Gasparini, M.; Bruno, G.; Denti, M.A.; Confaloni, A. Plasma microRNA profiling distinguishes patients with frontotemporal dementia from healthy subjects. Neurobiol. Aging 2019, 84, 240e1–240e12. [Google Scholar] [CrossRef] [PubMed]
- Magri, F.; Vanoli, F.; Corti, S. mi RNA in spinal muscular atrophy pathogenesis and therapy. J. Cell. Mol. Med. 2018, 22, 755–767. [Google Scholar] [PubMed] [Green Version]
- Wang, H. Phylogenetic analysis to explore the association between anti-NMDA receptor encephalitis and tumors based on microRNA biomarkers. Biomolecules 2019, 9, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Wang, H. The association between migraine and depression based on miRNA biomarkers and cohort studies. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Wang, H. Anti-NMDA receptor encephalitis and vaccination. Int. J. Mol. Sci. 2017, 18, 193. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The risks of miRNA therapeutics: In a drug target perspective. Drug Des. Devel. Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; de las Hazas, M.C.L.; Tome-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; Garcia-Ruiz, A.; Davalos, A. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.Z.; Zhang, X.M.; Yue, H.; Li, B.; Lin, L.; Fu, J. MicroRNA expression aberration in Chinese patients with relapsing remitting multiple sclerosis. J. Mol. Neurosci. 2014, 52, 131–137. [Google Scholar] [CrossRef]
- Lecca, D.; Marangon, D.; Coppolino, G.T.; Mendez, A.M.; Finardi, A.; Dalla Costa, G.; Martinelli, V.; Furlan, R.; Abbracchio, M.P. MiR-125a-3p timely inhibits oligodendroglial maturation and is pathologically up-regulated in human multiple sclerosis. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzziello, N.; Vilardo, L.; Pelucchi, P.; Consiglio, A.; Liuni, S.; Trojano, M.; Liguori, M. Investigating the role of microRNA and transcription factor co-regulatory networks in multiple sclerosis pathogenesis. Int. J. Mol. Sci. 2018, 19, 3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoruso, A.; Blonda, M.; Gironi, M.; Grasso, R.; Di Francescantonio, V.; Scaroni, F.; Furlan, R.; Verderio, C.; Avolio, C. Immune and central nervous system-related miRNAs expression profiling in monocytes of multiple sclerosis patients. Sci. Rep. 2020, 10, 6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, C.P.; McCoy, C.E. The role of MicroRNAs in repair processes in multiple sclerosis. Cells 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Y.; Wang, J.; Yan, Y.; Peng, L.; Qiu, W. Dysregulated MicroRNA involvement in multiple sclerosis by induction of T helper 17 cell differentiation. Front. Immunol. 2018, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Freiesleben, S.; Hecker, M.; Zettl, U.K.; Fuellen, G.; Taher, L. Analysis of microRNA and gene expression profiles in multiple sclerosis: Integrating interaction data to uncover regulatory mechanisms. Sci. Rep. 2016, 6, 34512. [Google Scholar] [CrossRef]
- Enatescu, V.R.; Papava, I.; Enatescu, I.; Antonescu, M.; Anghel, A.; Seclaman, E.; Sirbu, I.O.; Marian, C. Circulating plasma micro RNAs in patients with major depressive disorder treated with antidepressants: A pilot study. Psychiatry Investig. 2016, 13, 549. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, A.; Vincenti, S.; de Vito, F.; Bozzoni, I.; Oliverio, A.; Presutti, C.; Fragapane, P.; Mele, A. Stress induces region specific alterations in microRNAs expression in mice. Behav. Brain Res. 2010, 208, 265–269. [Google Scholar] [CrossRef]
- Dwivedi, Y. Emerging role of microRNAs in major depressive disorder: Diagnosis and therapeutic implications. Dialogues Clin. Neurosci. 2014, 16, 43–61. [Google Scholar] [PubMed]
- Ferrua, C.P.; Giorgi, R.; da Rosa, L.C.; do Amaral, C.C.; Ghisleni, G.C.; Pinheiro, R.T.; Nedel, F. MicroRNAs expressed in depression and their associated pathways: A systematic review and a bioinformatics analysis. J. Chem. Neuroanat. 2019, 100, 101650. [Google Scholar] [CrossRef]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.P.; Fiori, L.M.; Cruceanu, C.; Lin, R.; Labonte, B.; Cates, H.M.; Heller, E.A.; Vialou, V.; Ku, S.M.; Gerald, C. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, Y.; Roy, B.; Lugli, G.; Rizavi, H.; Zhang, H.; Smalheiser, N. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: Relevance to depression pathophysiology. Transl. Psychiatry 2015, 5, e682. [Google Scholar] [CrossRef]
- Quintana, E.; Ortega, F.J.; Robles-Cedeno, R.; Villar, M.L.; Buxo, M.; Mercader, J.M.; Alvarez-Cermeno, J.C.; Pueyo, N.; Perkal, H.; Fernandez-Real, J.M.; et al. miRNAs in cerebrospinal fluid identify patients with MS and specifically those with lipid-specific oligoclonal IgM bands. Mult. Scler. J. 2017, 23, 1716–1726. [Google Scholar] [CrossRef]
- Li, J.; Meng, H.; Cao, W.; Qiu, T. MiR-335 is involved in major depression disorder and antidepressant treatment through targeting GRM4. Neurosci. Lett. 2015, 606, 167–172. [Google Scholar] [CrossRef]
- Ghadiri, N.; Emamnia, N.; Ganjalikhani-Hakemi, M.; Ghaedi, K.; Etemadifar, M.; Salehi, M.; Shirzad, H.; Nasr-Esfahani, M.H. Analysis of the expression of mir-34a, mir-199a, mir-30c and mir-19a in peripheral blood CD4+T lymphocytes of relapsing-remitting multiple sclerosis patients. Gene 2018, 659, 109–117. [Google Scholar] [CrossRef]
- Regev, K.; Healy, B.C.; Paul, A.; Diaz-Cruz, C.; Mazzola, M.A.; Raheja, R.; Glanz, B.I.; Kivisakk, P.; Chitnis, T.; Jagodic, M.; et al. Identification of MS-specific serum miRNAs in an international multicenter study. Neurol. Neuroimmunol. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffioletti, E.; Cattaneo, A.; Rosso, G.; Maina, G.; Maj, C.; Gennarelli, M.; Tardito, D.; Bocchio-Chiavetto, L. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J. Affect. Disord. 2016, 200, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.M.; Wang, N.L.; Burmeister, M.; McInnis, M.G. MicroRNA expression changes in lymphoblastoid cell lines in response to lithium treatment. Int. J. Neuropsychoph. 2009, 12, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Teuber-Hanselmann, S.; Meinl, E.; Junker, A. MicroRNAs in gray and white matter multiple sclerosis lesions: Impact on pathophysiology. J. Pathol. 2020, 250, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Liu, C.; Kang, J.; Zhao, G.; Ye, Z.; Huang, S.; Li, Z.; Wu, Z.; Pei, G. MicroRNA miR-326 regulates T H-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 2009, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Menge, T.; Wingerath, B.; Kaya, D.; Graf, J.; Prozorovski, T.; Keller, A.; Backes, C.; Beier, M.; Scheffler, M.; et al. Natalizumab restores aberrant miRNA expression profile in multiple sclerosis and reveals a critical role for miR-20b. Ann. Clin. Transl. Neurol. 2015, 2, 43–55. [Google Scholar] [CrossRef]
- Honardoost, M.A.; Kiani-Esfahani, A.; Ghaedi, K.; Etemadifar, M.; Salehi, M. miR-326 and miR-26a, two potential markers for diagnosis of relapse and remission phases in patient with relapsing-remitting multiple sclerosis. Gene 2014, 544, 128–133. [Google Scholar] [CrossRef]
- Aschrafi, A.; Verheijen, J.M.; Gordebeke, P.M.; Loohuis, N.F.O.; Menting, K.; Jager, A.; Palkovits, M.; Geenen, B.; Kos, A.; Martens, G.J.M.; et al. MicroRNA-326 acts as a molecular switch in the regulation of midbrain urocortin 1 expression. J. Psychiatry Neurosci. 2016, 41, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, I.; Iaccino, E.; Dattilo, V.; Barone, S.; Vecchio, E.; Mimmi, S.; Filippelli, E.; Demonte, G.; Polidoro, S.; Granata, A.; et al. Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. FASEB J. 2018, 32, 4241–4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalheiser, N.R.; Lugli, G.; Rizavi, H.S.; Torvik, V.I.; Turecki, G.; Dwivedi, Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS ONE 2012, 7, e33201. [Google Scholar]
- Lee, S.Y.; Lu, R.B.; Wang, L.J.; Chang, C.H.; Lu, T.; Wang, T.Y.; Tsai, K.W. Serum miRNA as a possible biomarker in the diagnosis of bipolar II disorder. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ruhrmann, S.; Ewing, E.; Piket, E.; Kular, L.; Cetrulo Lorenzi, J.C.; Fernandes, S.J.; Morikawa, H.; Aeinehband, S.; Sayols-Baixeras, S.; Aslibekyan, S. Hypermethylation of MIR21 in CD4+ T cells from patients with relapsing-remitting multiple sclerosis associates with lower miRNA-21 levels and concomitant up-regulation of its target genes. Mult. Scler. J. 2018, 24, 1288–1300. [Google Scholar] [CrossRef] [Green Version]
- Miguel-Hidalgo, J.J.; Hall, K.O.; Bonner, H.; Roller, A.M.; Syed, M.; Park, C.J.; Ball, J.P.; Rothenberg, M.E.; Stockmeier, C.A.; Romero, D.G.; et al. MicroRNA-21: Expression in oligodendrocytes and correlation with low myelin mRNAs in depression and alcoholism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 503–514. [Google Scholar] [CrossRef]
- Ahmadian-Elmi, M.; Bidmeshki Pour, A.; Naghavian, R.; Ghaedi, K.; Tanhaei, S.; Izadi, T.; Nasr-Esfahani, M.H. miR-27a and miR-214 exert opposite regulatory roles in Th17 differentiation via mediating different signaling pathways in peripheral blood CD4+ T lymphocytes of patients with relapsing-remitting multiple sclerosis. Immunogenetics 2016, 68, 43–54. [Google Scholar] [CrossRef]
- Qi, B.; Fiori, L.M.; Turecki, G.; Trakadis, Y.J. Machine learning analysis of blood microRNA data in major depression: A case-control study for biomarker discovery. Int. J. Neuropsychopharmacol. 2020, 23, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, J.; Zhong, Y.; Jiang, L.; Mu, P.; Li, Y.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Expression, regulation and function of microRNAs in multiple sclerosis. Int. J. Med. Sci. 2014, 11, 810–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbett, K.A.; Vereczkei, A.; Kálmán, S.; Brown, J.A.; Taylor, W.D.; Faludi, G.; Korade, Ž.; Shelton, R.C.; Mirnics, K. Coordinated messenger RNA/microRNA changes in fibroblasts of patients with major depression. Biol. Psychiatry 2015, 77, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, J.C.; Brum, D.G.; Zanette, D.L.; de Paula Alves Souza, A.; Barbuzano, F.G.; Dos Santos, A.C.; Barreira, A.A.; da Silva, W.A. miR-15a and 16-1 are downregulated in CD4+ T cells of multiple sclerosis relapsing patients. Int. J. Neurosci. 2012, 122, 466–471. [Google Scholar] [CrossRef]
- Volk, N.; Pape, J.C.; Engel, M.; Zannas, A.S.; Cattane, N.; Cattaneo, A.; Binder, E.B.; Chen, A. Amygdalar MicroRNA-15a is essential for coping with chronic stress. Cell Rep. 2016, 17, 1882–1891. [Google Scholar] [CrossRef] [Green Version]
- Bocchio-Chiavetto, L.; Maffioletti, E.; Bettinsoli, P.; Giovannini, C.; Bignotti, S.; Tardito, D.; Corrada, D.; Milanesi, L.; Gennarelli, M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur. Neuropsychopharmacol. 2013, 23, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.R.; Tahmasebivand, M.; Khorrami, M.; Ayromlou, H.; Khalili, S.K.; Khorvash, F.; Rikhtegar, R.; Khademi, B.; Bahmanpour, Z.; Emamalizadeh, B. Connection of miR-185 and miR-320a expression levels with response to interferon-beta in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020, 44, 102264. [Google Scholar] [CrossRef] [PubMed]
- Camkurt, M.A.; Acar, Ş.; Coşkun, S.; Güneş, M.; Güneş, S.; Yılmaz, M.F.; Görür, A.; Tamer, L. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J. Psychiatr. Res. 2015, 69, 67–71. [Google Scholar] [CrossRef]
- Deng, Z.-F.; Zheng, H.-L.; Chen, J.-G.; Luo, Y.; Xu, J.-F.; Zhao, G.; Lu, J.-J.; Li, H.-H.; Gao, S.-Q.; Zhang, D.-Z. miR-214-3p targets β-catenin to regulate depressive-like behaviors induced by chronic social defeat stress in mice. Cereb. Cortex 2019, 29, 1509–1519. [Google Scholar] [CrossRef]
- Afrang, N.; Tavakoli, R.; Tasharrofi, N.; Alian, A.; Naderi Sohi, A.; Kabiri, M.; Fathi-Roudsari, M.; Soufizomorrod, M.; Rajaei, F.; Soleimani, M.; et al. A critical role for miR-184 in the fate determination of oligodendrocytes. Stem Cell Res. Ther. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Silva, A.P.; Fujimura, P.T.; Silva, J.R.D.; Teixeira, A.L.; Vieira, E.M.; Guedes, P.H.G.; Barroso, L.S.S.; Nicolau, M.D.; Ferreira, J.D.R.; Bertola, L.; et al. Brain-enriched MicroRNA-184 is downregulated in older adults with major depressive disorder: A translational study. J. Psychiatr. Res. 2019, 111, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Smalheiser, N.R.; Lugli, G.; Rizavi, H.S.; Zhang, H.; Torvik, V.I.; Pandey, G.N.; Davis, J.M.; Dwivedi, Y. MicroRNA expression in rat brain exposed to repeated inescapable shock: Differential alterations in learned helplessness vs. non-learned helplessness. Int. J. Neuropsychoph. 2011, 14, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.X.; Xie, G.J.; Mao, X.; Zou, X.P.; Liao, Y.J.; Liu, Q.S.; Wang, H.; Cheng, Y. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology 2020, 45, 1050–1058. [Google Scholar] [CrossRef]
- Liang, J.Q.; Liao, H.R.; Xu, C.X.; Li, X.L.; Wei, Z.X.; Xie, G.J.; Cheng, Y. Serum exosome-derived miR-139-5p as a potential biomarker for major depressive disorder. Neuropsychiatr. Dis. Treat. 2020, 16, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Xia, Q.H.; Xia, Q.R.; Zhang, X.L.; Liang, J. MicroRNA-based biomarkers in the diagnosis and monitoring of therapeutic response in patients with depression. Neuropsychiatr. Dis. Treat. 2019, 15, 3583–3597. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Han, J.J.; Liang, X.Y.; Zhao, L.; Zhang, F.; Rasouli, J.; Wang, Z.Z.; Zhang, G.X.; Li, X. miR-23b suppresses leukocyte migration and pathogenesis of experimental autoimmune encephalomyelitis by targeting CCL7. Mol. Ther. 2018, 26, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Kramer, S.; Haghikia, A.; Bang, C.; Scherf, K.; Pfanne, A.; Duscha, A.; Kaisler, J.; Gisevius, B.; Gold, R.; Thum, T.; et al. Elevated levels of miR-181c and miR-633 in the CSF of patients with MS: A validation study. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghikia, A.; Hellwig, K.; Baraniskin, A.; Holzmann, A.; Decard, B.F.; Thum, T.; Gold, R. Regulated microRNAs in the CSF of patients with multiple sclerosis: A case-control study. Neurology 2012, 79, 2166–2170. [Google Scholar] [CrossRef]
- Gao, Y.; Han, D.; Feng, J. MicroRNA in multiple sclerosis. Clin. Chim. Acta 2021, 516, 92–99. [Google Scholar] [CrossRef]
- Guerau-de-Arellano, M.; Smith, K.M.; Godlewski, J.; Liu, Y.; Winger, R.; Lawler, S.E.; Whitacre, C.C.; Racke, M.K.; Lovett-Racke, A.E. Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain 2011, 134, 3578–3589. [Google Scholar] [CrossRef]
- Issler, O.; Haramati, S.; Paul, E.D.; Maeno, H.; Navon, I.; Zwang, R.; Gil, S.; Mayberg, H.S.; Dunlop, B.W.; Menke, A.; et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 2014, 83, 344–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolati, S.; Marofi, F.; Babaloo, Z.; Aghebati-Maleki, L.; Roshangar, L.; Ahmadi, M.; Rikhtegar, R.; Yousefi, M. Dysregulated network of miRNAs involved in the pathogenesis of multiple sclerosis. Biomed. Pharm. 2018, 104, 280–290. [Google Scholar] [CrossRef]
- Belzeaux, R.; Bergon, A.; Jeanjean, V.; Loriod, B.; Formisano-Tréziny, C.; Verrier, L.; Loundou, A.; Baumstarck-Barrau, K.; Boyer, L.; Gall, V. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl. Psychiatry 2012, 2, e185. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.; Teuber-Hanselmann, S.; Soub, D.; Harnisch, K.; Mairinger, F.; Junker, A. MicroRNA profiles of MS gray matter lesions identify modulators of the synaptic protein synaptotagmin-7. Brain Pathol. 2019, 30, 524–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.Y.; Huang, Y.L.; Chang, C.; Kang, H.Y. Deficiency in androgen receptor aggravates the depressive-like behaviors in chronic mild stress model of depression. Cells 2019, 8, 1021. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.; Suderman, M.; Cattane, N.; Mazzelli, M.; Begni, V.; Maj, C.; D’Aprile, I.; Pariante, C.M.; Luoni, A.; Berry, A.; et al. Long-term effects of stress early in life on microRNA-30a and its network: Preventive effects of lurasidone and potential implications for depression vulnerability. Neurobiol. Stress 2020, 13, 100271. [Google Scholar] [CrossRef] [PubMed]
- Selmaj, I.; Cichalewska, M.; Namiecinska, M.; Galazka, G.; Horzelski, W.; Selmaj, K.W.; Mycko, M.P. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann. Neurol. 2017, 81, 703–717. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, H.; Wei, G.; Dong, Z.; Zhao, H.; Han, X.; Song, X.; Zhang, H.; Zong, X.; Baloch, Z.; et al. Identification of key genes, pathways, and miRNA/mRNA regulatory networks of CUMS-induced depression in nucleus accumbens by integrated bioinformatics analysis. Neuropsychiatr. Dis. Treat. 2019, 15, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhu, J.; Wang, Z.; Yao, X.; Wu, X.; Liu, F.; Zheng, W.; Li, Z.; Lin, A. MicroRNAs correlate with multiple sclerosis and neuromyelitis optica spectrum disorder in a Chinese population. Med. Sci. Monit. 2017, 23, 2565–2583. [Google Scholar] [CrossRef] [Green Version]
- Gururajan, A.; Naughton, M.; Scott, K.A.; O’connor, R.; Moloney, G.; Clarke, G.; Dowling, J.; Walsh, A.; Ismail, F.; Shorten, G. MicroRNAs as biomarkers for major depression: A role for let-7b and let-7c. Transl. Psychiatry 2016, 6, e862. [Google Scholar] [CrossRef] [Green Version]
- Dutta, R.; Chang, A.; Doud, M.K.; Kidd, G.J.; Ribaudo, M.V.; Young, E.A.; Fox, R.J.; Staugaitis, S.M.; Trapp, B.D. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 2011, 69, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.P.; Silva, M.; de Souza, E.; Nicolau, S.; Pereira, K.S.; do Nascimento, K.K.F.; Silva, C.M.; Ferreira, M.; Tolentino, G.T.; Araujo, T.; et al. Biological pathways found in common between Alzheimer’s disease and major depression: A study on microrna expression in a systematic review. Alzheimer’s Dement. 2016, 7, P886–P887. [Google Scholar] [CrossRef]
- Majd, M.; Hosseini, A.; Ghaedi, K.; Kiani-Esfahani, A.; Tanhaei, S.; Shiralian-Esfahani, H.; Rahnamaee, S.Y.; Mowla, S.J.; Nasr-Esfahani, M.H. MiR-9-5p and miR-106a-5p dysregulated in CD4(+) T-cells of multiple sclerosis patients and targeted essential factors of T helper17/regulatory T-cells differentiation. Iran. J. Basic Med. Sci. 2018, 21, 277–283. [Google Scholar]
- Sun, X.; Song, Z.; Si, Y.; Wang, J.H. microRNA and mRNA profiles in ventral tegmental area relevant to stress-induced depression and resilience. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 150–165. [Google Scholar] [CrossRef]
- Mohammed, E.M. Environmental influencers, MicroRNA, and multiple sclerosis. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573519894955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Liu, Y. Effects of duloxetine on microRNA expression profile in frontal lobe and hippocampus in a mouse model of depression. Int. J. Clin. Exp. Pathol. 2015, 8, 15454. [Google Scholar]
- Ma, K.; Guo, L.; Xu, A.; Cui, S.; Wang, J.H. Molecular mechanism for stress-induced depression assessed by sequencing miRNA and mRNA in medial prefrontal cortex. PLoS ONE 2016, 11, e0159093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-San Martin, M.; Reverter, G.; Robles-Cedeno, R.; Buxo, M.; Ortega, F.J.; Gomez, I.; Tomas-Roig, J.; Celarain, N.; Villar, L.M.; Perkal, H.; et al. Analysis of miRNA signatures in CSF identifies upregulation of miR-21 and miR-146a/b in patients with multiple sclerosis and active lesions. J. Neuroinflamm. 2019, 16, 220. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimkhani, S.; Vafaee, F.; Young, P.E.; Hur, S.S.J.; Hawke, S.; Devenney, E.; Beadnall, H.; Barnett, M.H.; Suter, C.M.; Buckland, M.E. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci. Rep. 2017, 7, 14293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelwal, N.; Dey, S.K.; Chakravarty, S.; Kumar, A. miR-30 family miRNAs Mediate the effect of chronic social defeat stress on hippocampal neurogenesis in mouse depression model. Front. Mol. Neurosci. 2019, 12, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, R.; Liu, Y.; Liu, D.; Jiang, H.; Pan, F. FKBP5 and specific microRNAs via glucocorticoid receptor in the basolateral amygdala involved in the susceptibility to depressive disorder in early adolescent stressed rats. J. Psychiatr. Res. 2017, 95, 102–113. [Google Scholar] [CrossRef]
- Roy, B.; Dunbar, M.; Shelton, R.C.; Dwivedi, Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology 2017, 42, 864–875. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Liu, X.; Jiang, K.; Peng, D.; Hong, W.; Fang, Y.; Qian, Y.; Yu, S.; Li, H. Alterations of microRNA-124 expression in peripheral blood mononuclear cells in pre-and post-treatment patients with major depressive disorder. J. Psychiatr. Res. 2016, 78, 65–71. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Y.; Wang, X.; Wu, J.; Liu, K.; Zhou, J.; Liu, L.; Zhang, C. Identification of Differential MicroRNAs in Cerebrospinal Fluid and Serum of Patients with Major Depressive Disorder. PLoS ONE 2015, 10, e0121975. [Google Scholar] [CrossRef]
- Dwivedi, Y. MicroRNAs in depression and suicide: Recent insights and future perspectives. J. Affect. Disord. 2018, 240, 146–154. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, R.; Zhang, Z.; Han, Z.; Yao, C.; Hou, G.; Dai, D.; Jin, W.; Tang, Y.; Yu, X.; et al. The MicroRNA miR-22 Represses Th17 cell pathogenicity by targeting PTEN-regulated pathways. Immunohorizons 2020, 4, 308–318. [Google Scholar] [CrossRef]
- Mandolesi, G.; Rizzo, F.R.; Balletta, S.; Bassi, M.S.; Gilio, L.; Guadalupi, L.; Nencini, M.; Moscatelli, A.; Ryan, C.P.; Licursi, V.; et al. The microRNA let-7b-5p is negatively associated with inflammation and disease severity in multiple sclerosis. Cells 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Piket, E.; Zheleznyakova, G.Y.; Kular, L.; Jagodic, M. Small non-coding RNAs as important players, biomarkers and therapeutic targets in multiple sclerosis: A comprehensive overview. J. Autoimmun. 2019, 101, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Roy, B.; Dwivedi, Y. Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects. Neuropsychopharmacology 2021, 46, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Bai, M.; Zhang, L.; Xue, L.; Yi, J. Maternal deprivation enhances behavioral vulnerability to stress associated with miR-504 expression in nucleus accumbens of rats. PLoS ONE 2013, 8, e69934. [Google Scholar] [CrossRef] [Green Version]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozicz, T.; Tilburg-Ouwens, D.; Faludi, G.; Palkovits, M.; Roubos, E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience 2008, 152, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Niu, Q.H.; Lei, Y.; Li, X.; Li, Y.H.; Song, X.Q. MiR-221 is involved in depression by regulating Wnt2/CREB/BDNF axis in hippocampal neurons. Cell Cycle 2018, 17, 2745–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, T.A. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028472. [Google Scholar] [CrossRef]
- Friedman, R.M. Clinical uses of interferons. Br. J. Clin. Pharm. 2008, 65, 158–162. [Google Scholar] [CrossRef]
- Pinto, E.F.; Andrade, C. Interferon-related depression: A primer on mechanisms, treatment, and prevention of a common clinical problem. Curr. Neuropharmacol. 2016, 14, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reder, A.T.; Oger, J.F.; Kappos, L.; O’Connor, P.; Rametta, M. Short-term and long-term safety and tolerability of interferon beta-1b in multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol. Neurobiol. 2018, 55, 6282–6306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Lee, C.H.; Wang, H.Y. Exploring the association of autism spectrum disorders and constipation through analysis of the gut microbiome. Int. J. Environ. Res. Pub. Health 2021, 18, 667. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in multiple sclerosis: Where are we, what we know and do not know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Van den Hoogen, W.J.; Laman, J.D.; t Hart, B.A. Modulation of multiple sclerosis and its animal model experimental autoimmune encephalomyelitis by food and gut microbiota. Front. Immunol. 2017, 8, 1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A gut (microbiome) feeling about the brain. Curr. Opin. Gastroen. 2016, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial dysfunction and multiple sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Bansal, Y.; Kuhad, A. Mitochondrial dysfunction in depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, B.; Peplow, P.V. MicroRNAs in blood and cerebrospinal fluid as diagnostic biomarkers of multiple sclerosis and to monitor disease progression. Neural. Regen. Res. 2020, 15, 606. [Google Scholar] [PubMed]
- Gheysarzadeh, A.; Sadeghifard, N.; Afraidooni, L.; Pooyan, F.; Mofid, M.R.; Valadbeigi, H.; Bakhtiari, H.; Keikhavani, S. Serum-based microRNA biomarkers for major depression: MiR-16, miR-135a, and miR-1202. J. Res. Med. Sci. 2018, 23, 69. [Google Scholar] [CrossRef]
- Mycko, M.P.; Baranzini, S.E. microRNA and exosome profiling in multiple sclerosis. Mult. Scler. J. 2020, 26, 599–604. [Google Scholar] [CrossRef]
| miRNA | MS miRNA Expression | MS References | MD miRNA Expression | MD References |
|---|---|---|---|---|
| miR-125a | ↑, ↓(Natalizumab) | [41,42,43,44,45,46,47] | ↓,↑, ↓(Escitalopram) | [48,49,50,51] |
| miR-146b | ↑ | [41,52] | ↓(Escitalopram), ↑(Duloxetine) | [48] [53] |
| miR-200c | ↑ | [41,52] | ↓ | [54] |
| miR-328 | ↓,↑ | [41,47,52,55] | ↑ | [51,56] |
| miR-199a | ↑,↓ | [41,52,55,57,58] | ↑ | [51,59] |
| miR-152 | ↓,↑ | [41] | ↑(Lithium) | [60] |
| miR-650 | ↑ | [52,61] | ↓ | [51,54] |
| miR-326 | ↑, ↓(Natalizumab) | [42,52,62,63,64] | ↓ | [65] |
| miR-142 | ↑,↓ | [52,58,66] | ↓,↑ | [67,68] |
| miR-21 | ↑,↓ | [52,69] | ↓ | [70] |
| miR-27a | ↑ | [45,52,71] | ↓ | [48,67,72] |
| miR-193a | ↑ | [52,73] | ↓ | [74] |
| miR-15a | ↑ | [52,75] | ↑ | [76] |
| miR-130a | ↑ | [52] | ↓ | [67] |
| miR-22 | ↑ | [52,66] | ↓,↑(Escitalopram) | [72,74,77] |
| miR-320 | ↑ | [43,52,78] | ↓ | [51,79] |
| miR-214 | ↑,↓ | [52,71] | ↑ | [80] |
| miR-184 | ↓ | [45,52,81] | ↓,↑ | [50,82,83] |
| miR-139 | ↓ | [52,61] | ↑ | [51,84,85,86] |
| miR-23b | ↓ | [52,87] | ↑ | [68] |
| miR-487b | ↓ | [52,61] | ↑ | [49,54] |
| miR-181c | ↓ | [52,88,89] | ↓,↑ | [50,54,83] |
| miR-340 | ↓,↑ | [52,90,91] | ↓ | [92] |
| miR-629 | ↑ | [52,93] | ↑,↑(Escitalopram) | [72,74,77] |
| miR-148a | ↑ | [52,90] | ↑ | [72,94] |
| miR-28 | ↑ | [52] | [72] | |
| miR-195 | ↑ | [52] | ↑ | [86] |
| miR-497 | ↑,↓ | [52,73,93] | ↓ | [67] |
| miR-135a | ↑ | [52,61,95] | ↓ | [54,92] |
| miR-204 | ↑ | [52,61,95] | ↑ | [96] |
| miR-660 | ↑,↓ | [52,61,66,95] | ↓ | [67] |
| miR-30a | ↑,↓ | [45,47,52,55,61,93,95] | ↑ | [51,97] |
| miR-365 | ↑,↓ | [52,55] | ↑ | [54] |
| miR-532 | ↑,↓ | [52,98] | ↓,↑(Escitalopram) | [48,51,99] |
| miR-126 | ↑ | [52,73,100] | ↓,↓(Escitalopram) | [48,49,72] |
| Let-7c | ↑, ↓(natalizumab) | [46,52,61,95] | ↓ | [101] |
| miR-20b | ↑,↓ | [47,52,63] | ↓ | [67] |
| miR-30d | ↑ | [52,102] | ↑,↑(Escitalopram) | [77,103] |
| miR-9 | ↑ | [45,52,104] | ↓ | [49] |
| miR-219 | ↓ | [45,52] | ↓ | [105] |
| miR-338 | ↓ | [45,52] | ↑ | [99] |
| miR-642 | ↓, ↑(natalizumab) | [46,52] | ↑ | [67] |
| miR-181b | ↓ | [52,106] | ↓(Escitalopram) | [48] |
| miR-18a | ↓ | [52,63] | ↑,↑(Duloxetine) | [50,83,107] |
| miR-190 | ↓ | [52,61] | ↓ | [67] |
| miR-213 | ↓ | [52,61] | ↑ | [108] |
| miR-330 | ↓ | [52,58] | ↑ | [51,59] |
| miR-151 | ↓ | [52,61] | ↓(Escitalopram) | [48] |
| miR-140 | ↓ | [52,61] | ↑(Escitalopram) | [77,83] |
| miR-146a | ↑,↓ | [44,45,47,52,55,66,109] | ↓, ↑(Duloxetine), ↓(Escitalopram) | [48,53,54,67] |
| miR-223 | ↑,↓ | [44,45,52,110] | ↑,↓(Escitalopram) | [48,51] |
| miR-30c | ↑ | [44,57] | ↓ | [49,111] |
| miR-155 | ↑,↓ | [44,45,52] | ↑,↓,↑(Lithium) | [51,54,60,67,72] |
| miR-124 | ↓ | [44,45] | ↑,↓,↓(Duloxetine) | [54,99,107,112,113,114] |
| miR-34a | ↑,↓ | [45,52,57,73] | ↑,↑(Lithium) | [51,60,115] |
| miR-19a | ↑,↓ | [57,66] | ↑ | [116] |
| miR-21 | ↑,↓ | [55,109] | ↑,↓ | [70,74] |
| miR-22 | ↑ | [66,117] | ↓,↑(Escitalopram) | [74,77] |
| miR-486 | ↓ | [66,73] | ↓ | [99] |
| miR-451a | ↓,↑ | [66,110] | ↑ | [51,79] |
| let-7b | ↓,↑ | [47,66,118] | ↓ | [101] |
| miR-320b | ↓ | [66,119] | ↓ | [74] |
| miR-122 | ↓,↑ | [61,66,95] | ↓ | [74] |
| miR-215 | ↓ | [66,106] | ↑ | [120] |
| miR-26a | ↓,↑ | [64,66] | ↑(Escitalopram), ↓(Escitalopram) | [48,77] |
| miR-15b | ↓ | [66,110] | ↑,↓ | [51,121] |
| miR-221 | ↑ | [47,106] | ↑ | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H. MicroRNAs, Multiple Sclerosis, and Depression. Int. J. Mol. Sci. 2021, 22, 7802. https://doi.org/10.3390/ijms22157802
Wang H. MicroRNAs, Multiple Sclerosis, and Depression. International Journal of Molecular Sciences. 2021; 22(15):7802. https://doi.org/10.3390/ijms22157802
Chicago/Turabian StyleWang, Hsiuying. 2021. "MicroRNAs, Multiple Sclerosis, and Depression" International Journal of Molecular Sciences 22, no. 15: 7802. https://doi.org/10.3390/ijms22157802
APA StyleWang, H. (2021). MicroRNAs, Multiple Sclerosis, and Depression. International Journal of Molecular Sciences, 22(15), 7802. https://doi.org/10.3390/ijms22157802






