The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization
Abstract
1. EPO and Erythropoiesis
2. EPOR/PI3K/AKT and Erythropoiesis
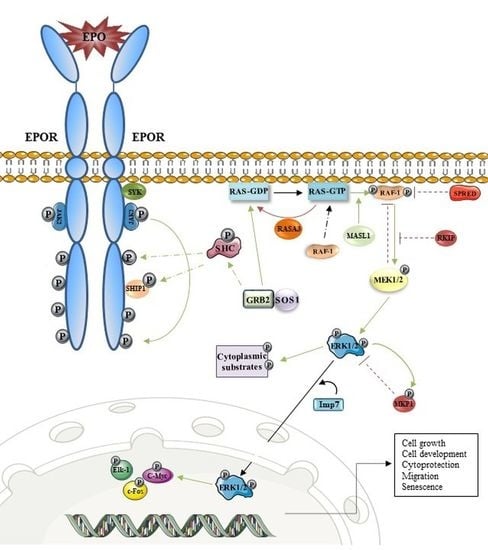
3. EPOR/MAPK and Erythropoiesis
4. The Interconnection between EPO Signaling Cascades
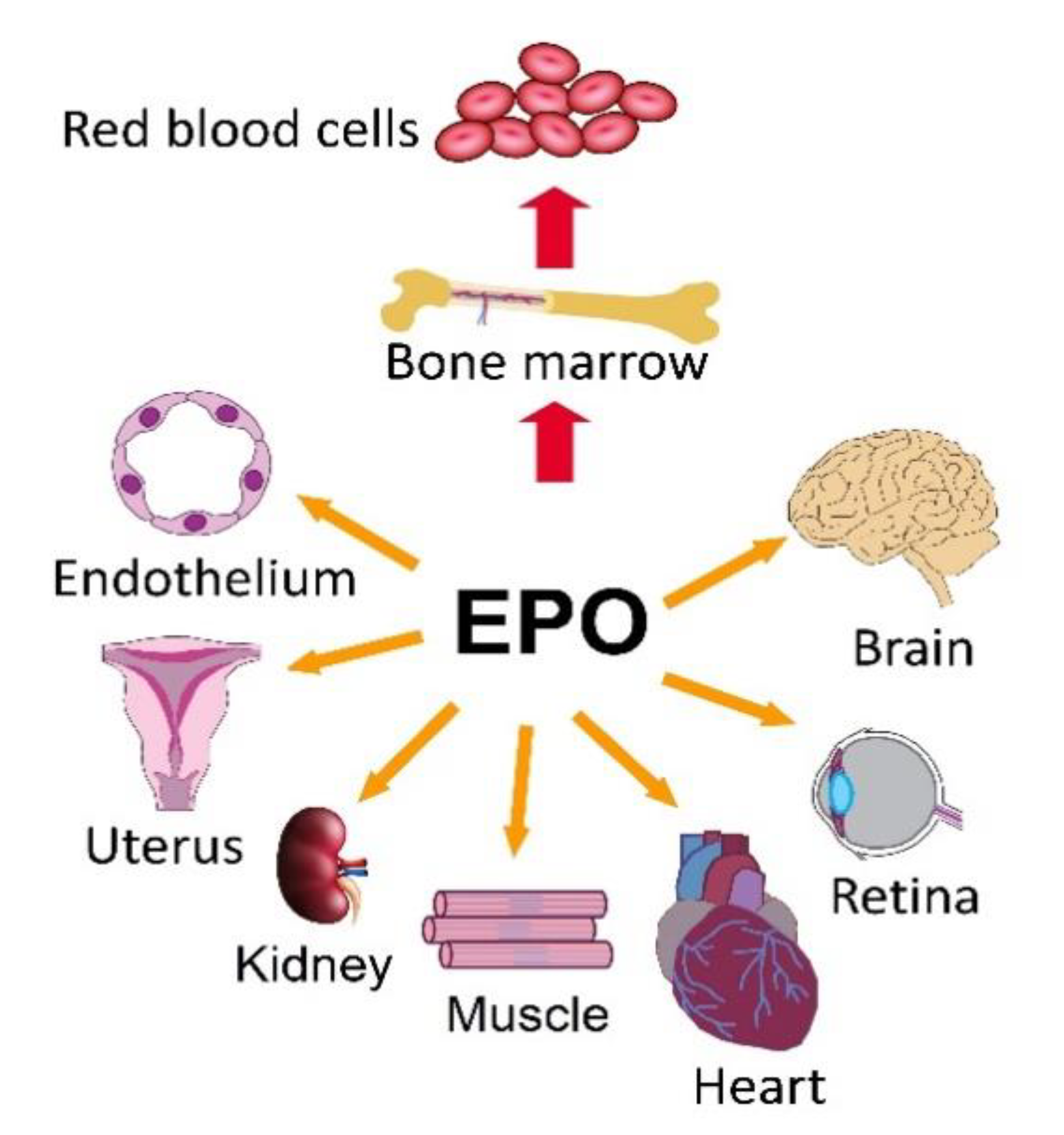
5. EPOR/PI3K/AKT and EPOR/MAPK in Non-Hematopoietic Tissues
5.1. Mitochondria
5.2. Nervous System
5.3. Bone and Bone Marrow
5.4. Heart
5.5. Kidney
5.6. Muscles
5.7. Retina
6. EPOR/PI3K/AKT and EPOR/MAPK in Cancer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef]
- Hirano, I.; Suzuki, N. The Neural Crest as the First Production Site of the Erythroid Growth Factor Erythropoietin. Front. Cell Dev. Biol. 2019, 7, 105. [Google Scholar] [CrossRef]
- Palis, J.; Koniski, A. Functional Analysis of Erythroid Progenitors by Colony-Forming Assays. Methods Mol. Biol 2018, 1698, 117–132. [Google Scholar]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Liu, Y.; Ji, P. Understanding terminal erythropoiesis: An update on chromatin condensation, enucleation, and reticulocyte maturation. Blood Rev. 2020, 46, 100740. [Google Scholar] [CrossRef]
- Bhoopalan, S.V.; Huang, L.J.; Weiss, M.J. Erythropoietin regulation of red blood cell production: From bench to bedside and back. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Gupta, R.; Musallam, K.M.; Taher, A.T.; Rivella, S. Ineffective Erythropoiesis: Anemia and Iron Overload. Hematol. Oncol. Clin. N. Am. 2018, 32, 213–221. [Google Scholar] [CrossRef]
- Sawada, K.; Krantz, S.B.; Dessypris, E.N.; Koury, S.T.; Sawyer, S.T. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J. Clin. Investig. 1989, 83, 1701–1709. [Google Scholar] [CrossRef]
- Millot, S.; Andrieu, V.; Letteron, P.; Lyoumi, S.; Hurtado-Nedelec, M.; Karim, Z.; Thibaudeau, O.; Bennada, S.; Charrier, J.L.; Lasocki, S.; et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation. Blood 2010, 116, 6072–6081. [Google Scholar] [CrossRef]
- Leimberg, M.J.; Prus, E.; Konijn, A.M.; Fibach, E. Macrophages function as a ferritin iron source for cultured human erythroid precursors. J. Cell Biochem. 2008, 103, 1211–1218. [Google Scholar] [CrossRef]
- Adlung, L.; Kar, S.; Wagner, M.C.; She, B.; Chakraborty, S.; Bao, J.; Lattermann, S.; Boerries, M.; Busch, H.; Wuchter, P.; et al. Protein abundance of AKT and ERK pathway components governs cell type-specific regulation of proliferation. Mol. Syst. Biol. 2017, 13, 904. [Google Scholar] [CrossRef]
- Tóthová, Z.; Tomc, J.; Debeljak, N.; Solár, P. STAT5 as a key protein of erythropoietin signalization. Int. J. Mol. Sci. 2021, 22, 7109. [Google Scholar] [CrossRef]
- Myklebust, J.H.; Blomhoff, H.K.; Rusten, L.S.; Stokke, T.; Smeland, E.B. Activation of phosphatidylinositol 3-kinase is important for erythropoietin-induced erythropoiesis from CD34(+) hematopoietic progenitor cells. Exp. Hematol. 2002, 30, 990–1000. [Google Scholar] [CrossRef]
- Held, M.A.; Greenfest-Allen, E.; Jachimowicz, E.; Stoeckert, C.J.; Stokes, M.P.; Wood, A.W.; Wojchowski, D.M. Phospho-proteomic discovery of novel signal transducers including thioredoxin-interacting protein as mediators of erythropoietin-dependent human erythropoiesis. Exp. Hematol. 2020, 84, 29–44. [Google Scholar] [CrossRef]
- Bulut, G.B.; Sulahian, R.; Yao, H.; Huang, L.J. Cbl ubiquitination of p85 is essential for Epo-induced EpoR endocytosis. Blood 2013, 122, 3964–3972. [Google Scholar] [CrossRef]
- Payrastre, B.; Cocco, L. Foreword: The PI3-kinase/Akt pathway: From signaling to diseases. Adv. Biol. Regul. 2015, 59, 1–3. [Google Scholar] [CrossRef]
- Missiroli, S.; Etro, D.; Buontempo, F.; Ye, K.; Capitani, S.; Neri, L.M. Nuclear translocation of active AKT is required for erythroid differentiation in erythropoietin treated K562 erythroleukemia cells. Int. J. Biochem. Cell Biol. 2009, 41, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kitidis, C.; Fleming, M.D.; Lodish, H.F.; Ghaffari, S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood 2006, 107, 907–915. [Google Scholar] [CrossRef]
- Liang, R.; Menon, V.; Ghaffari, S. Following Transcriptome to Uncover FOXO Biological Functions. Methods Mol. Biol. 2019, 1890, 219–227. [Google Scholar]
- Wilkins, S.E.; Abboud, M.I.; Hancock, R.L.; Schofield, C.J. Targeting Protein-Protein Interactions in the HIF System. Chem. Med. Chem. 2016, 11, 773–786. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF1 signaling pathway in hypoxiaischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar]
- Zhao, S.; Fu, J.; Liu, F.; Rastogi, R.; Zhang, J.; Zhao, Y. Small interfering RNA directed against CTMP reduces acute traumatic brain injury in a mouse model by activating Akt. Neurol. Res. 2014, 36, 483–490. [Google Scholar] [CrossRef]
- Sivertsen, E.A.; Hystad, M.E.; Gutzkow, K.B.; Dosen, G.; Smeland, E.B.; Blomhoff, H.K.; Myklebust, J.H. PI3K/Akt-dependent Epo-induced signalling and target genes in human early erythroid progenitor cells. Br. J. Haematol. 2006, 135, 117–128. [Google Scholar] [CrossRef]
- Grover, A.; Mancini, E.; Moore, S.; Mead, A.J.; Atkinson, D.; Rasmussen, K.D.; O’Carroll, D.; Jacobsen, S.E.; Nerlov, C. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J. Exp. Med. 2014, 211, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Karayel, O.; Xu, P.; Bludau, I.; Velan Bhoopalan, S.; Yao, Y.; Ana Rita, F.C.; Santos, A.; Schulman, B.A.; Alpi, A.F.; Weiss, M.J.; et al. Integrative proteomics reveals principles of dynamic phosphosignaling networks in human erythropoiesis. Mol. Syst. Biol. 2020, 16, e9813. [Google Scholar] [CrossRef] [PubMed]
- Breig, O.; Theoleyre-Schaal, O.; Baklouti, F. Combined inhibition of PI3K and activation of MAPK p38 signaling pathways trigger erythroid alternative splicing switch of 4.1R pre-mRNA in DMSO-induced erythroleukemia cells. Cell Signal. 2013, 25, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Yue, J. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Beattie, B.K.; Liu, Q. The SH2 inositol 5-phosphatase Ship1 is recruited in an SH2-dependent manner to the erythropoietin receptor. J. Biol. Chem. 2000, 275, 4398–4406. [Google Scholar] [CrossRef]
- Baltanas, F.C.; Zarich, N.; Rojas-Cabaneros, J.M.; Santos, E. SOS GEFs in health and disease. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188445. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 2006, 24, 21–44. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, D.Y.; Wu, M.S.; Chu, C.L.; Tzeng, S.J.; Lin, W.W. Spleen tyrosine kinase mediates the actions of EPO and GM-CSF and coordinates with TGF-beta in erythropoiesis. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 687–696. [Google Scholar] [CrossRef]
- Fouquet, G.; Thongsa-Ad, U.; Lefevre, C.; Rousseau, A.; Tanhuad, N.; Khongkla, E.; Saengsawang, W.; Anurathapan, U.; Hongeng, S.; Maciel, T.T.; et al. Iron-loaded transferrin potentiates erythropoietin effects on erythroblast proliferation and survival: A novel role through transferrin receptors. Exp. Hematol. 2021. [Google Scholar] [CrossRef]
- Forejtnikova, H.; Vieillevoye, M.; Zermati, Y.; Lambert, M.; Pellegrino, R.M.; Guihard, S.; Gaudry, M.; Camaschella, C.; Lacombe, C.; Roetto, A.; et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood 2010, 116, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Kuhrt, D.; Wojchowski, D.M. Emerging EPO and EPO receptor regulators and signal transducers. Blood 2015, 125, 3536–3541. [Google Scholar] [CrossRef]
- Tamura, K.; Sudo, T.; Senftleben, U.; Dadak, A.M.; Johnson, R.; Karin, M. Requirement for p38alpha in erythropoietin expression: A role for stress kinases in erythropoiesis. Cell 2000, 102, 221–231. [Google Scholar] [CrossRef]
- Guihard, S.; Clay, D.; Cocault, L.; Saulnier, N.; Opolon, P.; Souyri, M.; Pages, G.; Pouyssegur, J.; Porteu, F.; Gaudry, M. The MAPK ERK1 is a negative regulator of the adult steady-state splenic erythropoiesis. Blood 2010, 115, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.M.; Harandi, O.F.; Paulson, R.F. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood 2007, 109, 4494–4502. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.M.; Harandi, O.F.; Porayette, P.; Hegde, S.; Kannan, A.K.; Paulson, R.F. Maintenance of the BMP4-dependent stress erythropoiesis pathway in the murine spleen requires hedgehog signaling. Blood 2009, 113, 911–918. [Google Scholar] [CrossRef]
- Gong, J.; Yan, Z.; Liu, Q. Progress in experimental research on SPRED protein family. J. Int. Med. Res. 2020, 48, 300060520929170. [Google Scholar] [CrossRef]
- Gabriela-Freitas, M.; Pinheiro, J.; Raquel-Cunha, A.; Cardoso-Carneiro, D.; Martinho, O. RKIP as an Inflammatory and Immune System Modulator: Implications in Cancer. Biomolecules 2019, 9, 769. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef]
- Lake, D.; Correa, S.A.; Muller, J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol. Life Sci. 2016, 73, 4397–4413. [Google Scholar] [CrossRef]
- Kim, A.R.; Ulirsch, J.C.; Wilmes, S.; Unal, E.; Moraga, I.; Karakukcu, M.; Yuan, D.; Kazerounian, S.; Abdulhay, N.J.; King, D.S.; et al. Functional Selectivity in Cytokine Signaling Revealed Through a Pathogenic EPO Mutation. Cell 2017, 168, 1053–1064.e15. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ma, Y.; Hong, Z.; Zhao, L.; Monaghan, S.A.; Hu, M.C.; Huang, L.J. Activating JAK2 mutants reveal cytokine receptor coupling differences that impact outcomes in myeloproliferative neoplasm. Leukemia 2017, 31, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Ferrao, R.; Lupardus, P.J. The Janus Kinase (JAK) FERM and SH2 Domains: Bringing Specificity to JAK-Receptor Interactions. Front. Endocrinol. (Lausanne) 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.P.; Fu, P.; Jiang, X.; Salter, K.H.; Potti, A.; Arcasoy, M.O. Polycythemia vera erythroid precursors exhibit increased proliferation and apoptosis resistance associated with abnormal RAS and PI3K pathway activation. Exp. Hematol. 2009, 37, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Rommel, C.; Clarke, B.A.; Zimmermann, S.; Nunez, L.; Rossman, R.; Reid, K.; Moelling, K.; Yancopoulos, G.D.; Glass, D.J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 1999, 286, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, G.D.; Shi, Z.; Qi, L.L.; Zhou, L.Y.; Fu, Z.X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Pacold, M.E.; Suire, S.; Perisic, O.; Lara-Gonzalez, S.; Davis, C.T.; Walker, E.H.; Hawkins, P.T.; Stephens, L.; Eccleston, J.F.; Williams, R.L. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell 2000, 103, 931–943. [Google Scholar] [CrossRef]
- Fukumoto, T.; Kubota, Y.; Kitanaka, A.; Yamaoka, G.; Ohara-Waki, F.; Imataki, O.; Ohnishi, H.; Ishida, T.; Tanaka, T. Gab1 transduces PI3K-mediated erythropoietin signals to the Erk pathway and regulates erythropoietin-dependent proliferation and survival of erythroid cells. Cell Signal. 2009, 21, 1775–1783. [Google Scholar] [CrossRef]
- Kolch, W.; Heidecker, G.; Kochs, G.; Hummel, R.; Vahidi, H.; Mischak, H.; Finkenzeller, G.; Marme, D.; Rapp, U.R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature 1993, 364, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.M.; Bean, J.R.; Yang, W.; Shee, K.; Symonds, L.K.; Balko, J.M.; McDonald, W.H.; Liu, S.; Gonzalez-Angulo, A.M.; Mills, G.B.; et al. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene 2015, 34, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bonnas, C.; Wustefeld, L.; Winkler, D.; Kronstein-Wiedemann, R.; Dere, E.; Specht, K.; Boxberg, M.; Tonn, T.; Ehrenreich, H.; Stadler, H.; et al. EV-3, an endogenous human erythropoietin isoform with distinct functional relevance. Sci. Rep. 2017, 7, 3684. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, D.; Heinrich, R. Alternative Erythropoietin Receptors in the Nervous System. J. Clin. Med. 2018, 7, 24. [Google Scholar] [CrossRef]
- He, L.; Cohen, E.B.; Edwards, A.P.B.; Xavier-Ferrucio, J.; Bugge, K.; Federman, R.S.; Absher, D.; Myers, R.M.; Kragelund, B.B.; Krause, D.S.; et al. Transmembrane Protein Aptamer Induces Cooperative Signaling by the EPO Receptor and the Cytokine Receptor beta-Common Subunit. iScience 2019, 17, 167–181. [Google Scholar] [CrossRef]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2020, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.G.; Kandel, E.S.; Cross, T.K.; Hay, N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell Biol. 1999, 19, 5800–5810. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, S.; Xiao, Y.; Chen, L. Erythropoietin enhances mitochondrial biogenesis in cardiomyocytes exposed to chronic hypoxia through Akt/eNOS signalling pathway. Cell Biol. Int. 2014, 38, 335–342. [Google Scholar] [CrossRef]
- Ong, S.B.; Hall, A.R.; Dongworth, R.K.; Kalkhoran, S.; Pyakurel, A.; Scorrano, L.; Hausenloy, D.J. Akt protects the heart against ischaemia-reperfusion injury by modulating mitochondrial morphology. Thromb. Haemost. 2015, 113, 513–521. [Google Scholar] [CrossRef]
- Kobayashi, H.; Miura, T.; Ishida, H.; Miki, T.; Tanno, M.; Yano, T.; Sato, T.; Hotta, H.; Shimamoto, K. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin. Exp. Pharmacol. Physiol. 2008, 35, 812–819. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Hou, J.; Shang, Y.C.; Wang, S.; Maiese, K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr. Neurovasc. Res. 2011, 8, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Ghilardi, G.; Bianciardi, P.; Latorre, E.; Rubino, F.; Bissi, M.; Di Giulio, A.M.; Samaja, M.; Gorio, A. Enhanced brain release of erythropoietin, cytokines and NO during carotid clamping. Neurol. Sci. 2016, 37, 243–252. [Google Scholar] [CrossRef]
- Juul, S.E.; Mayock, D.E.; Comstock, B.A.; Heagerty, P.J. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern. Health Neonatol. Perinatol. 2015, 1, 27. [Google Scholar] [CrossRef]
- Digicaylioglu, M.; Garden, G.; Timberlake, S.; Fletcher, L.; Lipton, S.A. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 2004, 101, 9855–9860. [Google Scholar] [CrossRef]
- Habib, P.; Stamm, A.S.; Zeyen, T.; Noristani, R.; Slowik, A.; Beyer, C.; Wilhelm, T.; Huber, M.; Komnig, D.; Schulz, J.B.; et al. EPO regulates neuroprotective Transmembrane BAX Inhibitor-1 Motif-containing (TMBIM) family members GRINA and FAIM2 after cerebral ischemia-reperfusion injury. Exp. Neurol. 2019, 320, 112978. [Google Scholar] [CrossRef] [PubMed]
- Komnig, D.; Gertz, K.; Habib, P.; Nolte, K.W.; Meyer, T.; Brockmann, M.A.; Endres, M.; Rathkolb, B.; Hrabe de Angelis, M.; Schulz, J.B.; et al. Faim2 contributes to neuroprotection by erythropoietin in transient brain ischemia. J. Neurochem. 2018, 145, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, J.; Chen, C.; Wei, N.; Xu, J.; Yang, G.; Wang, N.; Meng, Y.; Ren, J.; Xu, Z. Erythropoietin Rescues Memory Impairment in a Rat Model of Chronic Cerebral Hypoperfusion via the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3beta Pathway. Mol. Neurobiol. 2018, 55, 3290–3299. [Google Scholar] [CrossRef]
- Wang, M.; Yan, W.; Liu, Y.; Hu, H.; Sun, Q.; Chen, X.; Zang, W.; Chen, L. Erythropoietin ameliorates diabetes-associated cognitive dysfunction in vitro and in vivo. Sci. Rep. 2017, 7, 2801. [Google Scholar] [CrossRef]
- Lee, H.J.; Koh, S.H.; Song, K.M.; Seol, I.J.; Park, H.K. The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology 2016, 110, 93–100. [Google Scholar] [CrossRef]
- Jia, Y.; Mo, S.J.; Feng, Q.Q.; Zhan, M.L.; OuYang, L.S.; Chen, J.C.; Ma, Y.X.; Wu, J.J.; Lei, W.L. EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J. Mol. Neurosci. 2014, 53, 117–124. [Google Scholar] [CrossRef]
- Ding, J.; Li, Q.Y.; Yu, J.Z.; Wang, X.; Lu, C.Z.; Ma, C.G.; Xiao, B.G. The lack of CD131 and the inhibition of Neuro-2a growth by carbamylated erythropoietin. Cell Biol. Toxicol. 2015, 31, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, J.; Li, Q.Y.; Yu, J.Z.; Ma, C.G.; Wang, X.; Lu, C.Z.; Xiao, B.G. Neuroprotection and CD131/GDNF/AKT Pathway of Carbamylated Erythropoietin in Hypoxic Neurons. Mol. Neurobiol. 2017, 54, 5051–5060. [Google Scholar] [CrossRef] [PubMed]
- Byts, N.; Samoylenko, A.; Fasshauer, T.; Ivanisevic, M.; Hennighausen, L.; Ehrenreich, H.; Siren, A.L. Essential role for Stat5 in the neurotrophic but not in the neuroprotective effect of erythropoietin. Cell Death Differ. 2008, 15, 783–792. [Google Scholar] [CrossRef]
- Kilic, E.; Kilic, U.; Soliz, J.; Bassetti, C.L.; Gassmann, M.; Hermann, D.M. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J. 2005, 19, 2026–2028. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.A.; Satriotomo, I.; Mitchell, G.S. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J. Neurosci. 2012, 32, 5973–5983. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol.Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Miura, T.; Tanno, M.; Nishihara, M.; Naitoh, K.; Sato, T.; Takahashi, A.; Shimamoto, K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res. Cardiol. 2007, 102, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, M.; Yan, P.; Yao, Q.; Fan, J.; Gao, Z.; Wang, H. Erythropoietin suppresses D-galactose-induced aging of rats via the PI3K/Akt/Nrf2-ARE pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 2227–2240. [Google Scholar]
- Wu, H.; Zhao, J.; Chen, M.; Wang, H.; Yao, Q.; Fan, J.; Zhang, M. The Anti-Aging Effect of Erythropoietin via the ERK/Nrf2-ARE Pathway in Aging Rats. J. Mol. Neurosci. 2017, 61, 449–458. [Google Scholar] [CrossRef]
- Lee, S.M.; Nguyen, T.H.; Park, M.H.; Kim, K.S.; Cho, K.J.; Moon, D.C.; Kim, H.Y.; Yoon, D.Y.; Hong, J.T. EPO receptor-mediated ERK kinase and NF-kappaB activation in erythropoietin-promoted differentiation of astrocytes. Biochem. Biophys. Res. Commun. 2004, 320, 1087–1095. [Google Scholar] [CrossRef]
- Constanthin, P.E.; Contestabile, A.; Petrenko, V.; Quairiaux, C.; Salmon, P.; Huppi, P.S.; Kiss, J.Z. Endogenous erythropoietin signaling regulates migration and laminar positioning of upper-layer neurons in the developing neocortex. Development 2020, 147, dev190249. [Google Scholar] [CrossRef]
- Kaitsuka, T.; Kobayashi, K.; Otsuka, W.; Kubo, T.; Hakim, F.; Wei, F.Y.; Shiraki, N.; Kume, S.; Tomizawa, K. Erythropoietin facilitates definitive endodermal differentiation of mouse embryonic stem cells via activation of ERK signaling. Am. J. Physiol. Cell Physiol. 2017, 312, C573–C582. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Chen, S.H.; Lin, S.C.; Chen, W.T.; Lue, J.H.; Tsai, Y.J. Erythropoietin reduces nerve demyelination, neuropathic pain behavior and microglial MAPKs activation through erythropoietin receptors on Schwann cells in a rat model of peripheral neuropathy. Glia 2018, 66, 2299–2315. [Google Scholar] [CrossRef]
- Jones, N.M.; Bergeron, M. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J. Neurochem. 2004, 89, 157–167. [Google Scholar] [CrossRef]
- Lee, E.; Choi, S.Y.; Yang, J.H.; Lee, Y.J. Preventive effects of imperatorin on perfluorohexanesulfonate-induced neuronal apoptosis via inhibition of intracellular calcium-mediated ERK pathway. Korean J. Physiol. Pharmacol. 2016, 20, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.E.; Park, J.H.; Kim, C.S.; Lee, S.L.; Chung, H.L.; Kim, W.T.; Lee, E.J. Neuroprotective effects of erythropoietin against hypoxic injury via modulation of the mitogen-activated protein kinase pathway and apoptosis. Korean J. Pediatr. 2017, 60, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Hiram-Bab, S.; Neumann, D.; Gabet, Y. Erythropoietin in bone-Controversies and consensus. Cytokine 2017, 89, 155–159. [Google Scholar] [CrossRef]
- Liu, G.X.; Zhu, J.C.; Chen, X.Y.; Zhu, A.Z.; Liu, C.C.; Lai, Q.; Chen, S.T. Inhibition of adipogenic differentiation of bone marrow mesenchymal stem cells by erythropoietin via activating ERK and P38 MAPK. Genet. Mol. Res. 2015, 14, 6968–6977. [Google Scholar] [CrossRef]
- Suresh, S.; de Castro, L.F.; Dey, S.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7, 21. [Google Scholar] [CrossRef]
- Lin, H.; Luo, X.; Jin, B.; Shi, H.; Gong, H. The Effect of EPO Gene Overexpression on Proliferation and Migration of Mouse Bone Marrow-Derived Mesenchymal Stem Cells. Cell Biochem. Biophys. 2015, 71, 1365–1372. [Google Scholar] [CrossRef]
- Li, J.; Huang, Z.; Li, B.; Zhang, Z.; Liu, L. Mobilization of Transplanted Bone Marrow Mesenchymal Stem Cells by Erythropoietin Facilitates the Reconstruction of Segmental Bone Defect. Stem. Cells Int. 2019, 2019, 5750967. [Google Scholar] [CrossRef]
- Cokic, B.B.; Cokic, V.P.; Suresh, S.; Wirt, S.; Noguchi, C.T. Nitric oxide and hypoxia stimulate erythropoietin receptor via MAPK kinase in endothelial cells. Microvasc. Res. 2014, 92, 34–40. [Google Scholar] [CrossRef]
- Jun, J.H.; Jun, N.H.; Shim, J.K.; Shin, E.J.; Kwak, Y.L. Erythropoietin protects myocardium against ischemia-reperfusion injury under moderate hyperglycemia. Eur. J. Pharmacol. 2015, 745, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Yang, X.H.; Wang, X.J.; Li, S.M.; Sun, N.; Zhang, T. Erythropoietin Decreases the Occurrence of Myocardial Fibrosis by Inhibiting the NADPH-ERK-NF-x03BA;B Pathway. Cardiology 2016, 133, 97–108. [Google Scholar] [CrossRef]
- Klopsch, C.; Skorska, A.; Ludwig, M.; Lemcke, H.; Maass, G.; Gaebel, R.; Beyer, M.; Lux, C.; Toelk, A.; Muller, K.; et al. Intramyocardial angiogenetic stem cells and epicardial erythropoietin save the acute ischemic heart. Dis. Model. Mech. 2018, 11, dmm033282. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wen, Y.; Kang, J.; Wei, C.; Wang, M.; Zheng, Z.; Peng, J. Regulation of TLR4 expression mediates the attenuating effect of erythropoietin on inflammation and myocardial fibrosis in rat heart. Int. J. Mol. Med. 2018, 42, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Wu, Y.; Yang, B. Renoprotection and Mechanisms of Erythropoietin and Its Derivatives Helix B Surface Peptide in Kidney Injuries. Curr. Protein Pept. Sci. 2017, 18, 1183–1190. [Google Scholar] [CrossRef]
- Shiou, S.R.; Yu, Y.; Chen, S.; Ciancio, M.J.; Petrof, E.O.; Sun, J.; Claud, E.C. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J. Biol. Chem. 2011, 286, 12123–12132. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, D.; Na, N.; Li, H.; Miao, B.; Hong, L.; Huang, Z. Renoprotective effect of erythropoietin via modulation of the STAT6/MAPK/NF-kappaB pathway in ischemia/reperfusion injury after renal transplantation. Int. J. Mol. Med. 2018, 41, 25–32. [Google Scholar]
- Ranjbaran, M.; Kadkhodaee, M.; Seifi, B.; Adelipour, M.; Azarian, B. Erythropoietin attenuates experimental haemorrhagic shock-induced renal damage through an iNOS- dependent mechanism in male Wistar rats. Injury 2017, 48, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Seifi, B.; Kadkhodaee, M.; Ranjbaran, M.; Bakhshi, E. Nephroprotection through the Akt/eNOS pathway by centrally administered erythropoietin in a rat model of fixed-volume hemorrhage. Life Sci. 2018, 193, 180–185. [Google Scholar] [CrossRef]
- Park, S.L.; Won, S.Y.; Song, J.H.; Kambe, T.; Nagao, M.; Kim, W.J.; Moon, S.K. EPO gene expression promotes proliferation, migration and invasion via the p38MAPK/AP-1/MMP-9 pathway by p21WAF1 expression in vascular smooth muscle cells. Cell Signal. 2015, 27, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, C.; Xiao, F.; Wang, H.; Wu, Z. JAK2/STAT2/STAT3 are required for myogenic differentiation. J. Biol. Chem. 2008, 283, 34029–34036. [Google Scholar] [CrossRef]
- Lamon, S.; Zacharewicz, E.; Arentson-Lantz, E.; Gatta, P.A.; Ghobrial, L.; Gerlinger-Romero, F.; Garnham, A.; Paddon-Jones, D.; Russell, A.P. Erythropoietin Does Not Enhance Skeletal Muscle Protein Synthesis Following Exercise in Young and Older Adults. Front. Physiol. 2016, 7, 292. [Google Scholar] [CrossRef]
- Lee, J.I.; Hur, J.M.; You, J. Functional recovery with histomorphometric analysis of nerves and muscles after combination treatment with erythropoietin and dexamethasone in acute peripheral nerve injury. PLoS ONE 2020, 15, e0238208. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Lu, I.C.; Tai, M.H.; Chai, C.Y.; Kwan, A.L.; Huang, S.H. Erythropoietin Alleviates Burn-induced Muscle Wasting. Int. J. Med. Sci. 2020, 17, 33–44. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, C.; Liu, D.; Yang, Q.; Tang, L.; Wang, T.; Tian, H.; Lu, L.; Xu, J.Y.; Gao, F.; et al. Erythropoietin protects the inner blood-retinal barrier by inhibiting microglia phagocytosis via Src/Akt/cofilin signalling in experimental diabetic retinopathy. Diabetologia 2020, 64, 211–225. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Yang, Q.; Yang, Y.; Li, W.; Tian, H.; Lu, L.; Wang, F.; Xu, J.Y.; Gao, F.; et al. Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin. Clin. Exp. Ophthalmol. 2019, 47, 1182–1197. [Google Scholar] [CrossRef]
- Xu, G.; Kang, D.; Zhang, C.; Lou, H.; Sun, C.; Yang, Q.; Lu, L.; Xu, G.T.; Zhang, J.; Wang, F. Erythropoietin Protects Retinal Cells in Diabetic Rats Through Upregulating ZnT8 via Activating ERK Pathway and Inhibiting HIF-1alpha Expression. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8166–8178. [Google Scholar] [CrossRef]
- Broudy, V.C.; Lin, N.; Brice, M.; Nakamoto, B.; Papayannopoulou, T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood 1991, 77, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Solar, P.; Hrckova, G.; Varinska, L.; Solarova, Z.; Kriska, J.; Uhrinova, I.; Kello, M.; Mojzis, J.; Fedorocko, P.; Sytkowski, A.J. Location and the functionality of erythropoietin receptor(s) in A2780 cells. Oncol. Rep. 2012, 28, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.; Huang, J.; Mora, E.M.; Nick, A.M.; Cho, M.S.; Wu, S.Y.; Noh, K.; Pecot, C.V.; Rupaimoole, R.; Stein, M.A.; et al. Erythropoietin Stimulates Tumor Growth via EphB4. Cancer Cell 2015, 28, 610–622. [Google Scholar] [CrossRef]
- Paragh, G.; Kumar, S.M.; Rakosy, Z.; Choi, S.C.; Xu, X.; Acs, G. RNA interference-mediated inhibition of erythropoietin receptor expression suppresses tumor growth and invasiveness in A2780 human ovarian carcinoma cells. Am. J. Pathol. 2009, 174, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, N.; Wang, X.; Zhang, C.; Li, T.; Ning, X.; Gong, K. The erythropoietin/erythropoietin receptor signaling pathway promotes growth and invasion abilities in human renal carcinoma cells. PLoS ONE 2012, 7, e45122. [Google Scholar] [CrossRef] [PubMed]
- Frille, A.; Leithner, K.; Olschewski, A.; Olschewski, H.; Wohlkonig, C.; Hrzenjak, A. No erythropoietin-induced growth is observed in non-small cell lung cancer cells. Int. J. Oncol. 2018, 52, 518–526. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kim, H.P.; Han, S.W.; Oh, D.Y.; Im, S.A.; Bang, Y.J.; Kim, T.Y. KRAS mutant lung cancer cells are differentially responsive to MEK inhibitor due to AKT or STAT3 activation: Implication for combinatorial approach. Mol. Carcinog. 2010, 49, 353–362. [Google Scholar] [CrossRef]
- Kumar, S.M.; Yu, H.; Fong, D.; Acs, G.; Xu, X. Erythropoietin activates the phosphoinositide 3-kinase/Akt pathway in human melanoma cells. Melanoma Res. 2006, 16, 275–283. [Google Scholar] [CrossRef]
- Vazquez-Mellado, M.J.; Aguilar, C.; Rocha-Zavaleta, L. Erythropoietin protects neuroblastoma cells against etoposide and vincristine by activating ERK and AKT pathways but has no effect in kidney cells. Life Sci. 2015, 137, 142–149. [Google Scholar] [CrossRef]
- Tankiewicz-Kwedlo, A.; Hermanowicz, J.; Surazynski, A.; Rozkiewicz, D.; Pryczynicz, A.; Domaniewski, T.; Pawlak, K.; Kemona, A.; Pawlak, D. Erythropoietin accelerates tumor growth through increase of erythropoietin receptor (EpoR) as well as by the stimulation of angiogenesis in DLD-1 and Ht-29 xenografts. Mol. Cell Biochem. 2016, 421, 1–18. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, G.; Wang, X.; Chen, F.; Liao, Z.; Zhang, Z.; Liu, Z.; Zeng, W.; Fang, M.; Wang, W.; et al. AKT/GSK-3beta/beta-catenin signaling pathway participates in erythropoietin-promoted glioma proliferation. J. Neurooncol. 2020, 149, 231–242. [Google Scholar] [CrossRef]
- Uddin, S.; Kottegoda, S.; Stigger, D. Activation of the AKT/FKHRL1 pathway mediates the antiapoptotic effects of erythropoietin in primary human erythroid progenitors. Biochem. Biophys. Res. Commun. 2000, 275, 16–19. [Google Scholar] [CrossRef]
- Um, M.; Lodish, H.F. Antiapoptotic effects of erythropoietin in differentiated neuroblastoma SH-SY5Y cells require activation of both the STAT5 and AKT signaling pathways. J. Biol. Chem. 2006, 281, 5648–5656. [Google Scholar] [CrossRef]
- Chan, K.K.; Matchett, K.B.; Coulter, J.A.; Yuen, H.F.; McCrudden, C.M.; Zhang, S.D.; Irwin, G.W.; Davidson, M.A.; Rulicke, T.; Schober, S.; et al. Erythropoietin drives breast cancer progression by activation of its receptor EPOR. Oncotarget 2017, 8, 38251–38263. [Google Scholar] [CrossRef]
- Fu, P.; Jiang, X.; Arcasoy, M.O. Constitutively active erythropoietin receptor expression in breast cancer cells promotes cellular proliferation and migration through a MAP-kinase dependent pathway. Biochem. Biophys. Res. Commun. 2009, 379, 696–701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamadmad, S.N.; Hohl, R.J. Erythropoietin stimulates cancer cell migration and activates RhoA protein through a mitogen-activated protein kinase/extracellular signal-regulated kinase-dependent mechanism. J. Pharmacol. Exp. Ther. 2008, 324, 1227–1233. [Google Scholar] [CrossRef]
- Aguilar, C.; Aguilar, C.; Lopez-Marure, R.; Jimenez-Sanchez, A.; Rocha-Zavaleta, L. Co-stimulation with stem cell factor and erythropoietin enhances migration of c-Kit expressing cervical cancer cells through the sustained activation of ERK1/2. Mol. Med. Rep. 2014, 9, 1895–1902. [Google Scholar] [CrossRef]
- Breig, O.; Theoleyre, O.; Douablin, A.; Baklouti, F. Subtle distinct regulations of late erythroid molecular events by PI3K/AKT-mediated activation of Spi-1/PU.1 oncogene autoregulation loop. Oncogene 2010, 29, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Roversi, F.M.; Pericole, F.V.; Machado-Neto, J.A.; da Silva Santos Duarte, A.; Longhini, A.L.; Corrocher, F.A.; Palodetto, B.; Ferro, K.P.; Rosa, R.G.; Baratti, M.O.; et al. Hematopoietic cell kinase (HCK) is a potential therapeutic target for dysplastic and leukemic cells due to integration of erythropoietin/PI3K pathway and regulation of erythropoiesis: HCK in erythropoietin/PI3K pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, H. Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 319–331. [Google Scholar]
- Najafi, M.; Ahmadi, A.; Mortezaee, K. Extracellular-signal-regulated kinase/mitogen-activated protein kinase signaling as a target for cancer therapy: An updated review. Cell Biol. Int. 2019, 43, 1206–1222. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7682. https://doi.org/10.3390/ijms22147682
Tóthová Z, Šemeláková M, Solárová Z, Tomc J, Debeljak N, Solár P. The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. International Journal of Molecular Sciences. 2021; 22(14):7682. https://doi.org/10.3390/ijms22147682
Chicago/Turabian StyleTóthová, Zuzana, Martina Šemeláková, Zuzana Solárová, Jana Tomc, Nataša Debeljak, and Peter Solár. 2021. "The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization" International Journal of Molecular Sciences 22, no. 14: 7682. https://doi.org/10.3390/ijms22147682
APA StyleTóthová, Z., Šemeláková, M., Solárová, Z., Tomc, J., Debeljak, N., & Solár, P. (2021). The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization. International Journal of Molecular Sciences, 22(14), 7682. https://doi.org/10.3390/ijms22147682