Hydrophilic and Hydrophobic Effects on the Structure and Themodynamic Properties of Confined Water: Water in Solutions
Abstract
:1. Introduction
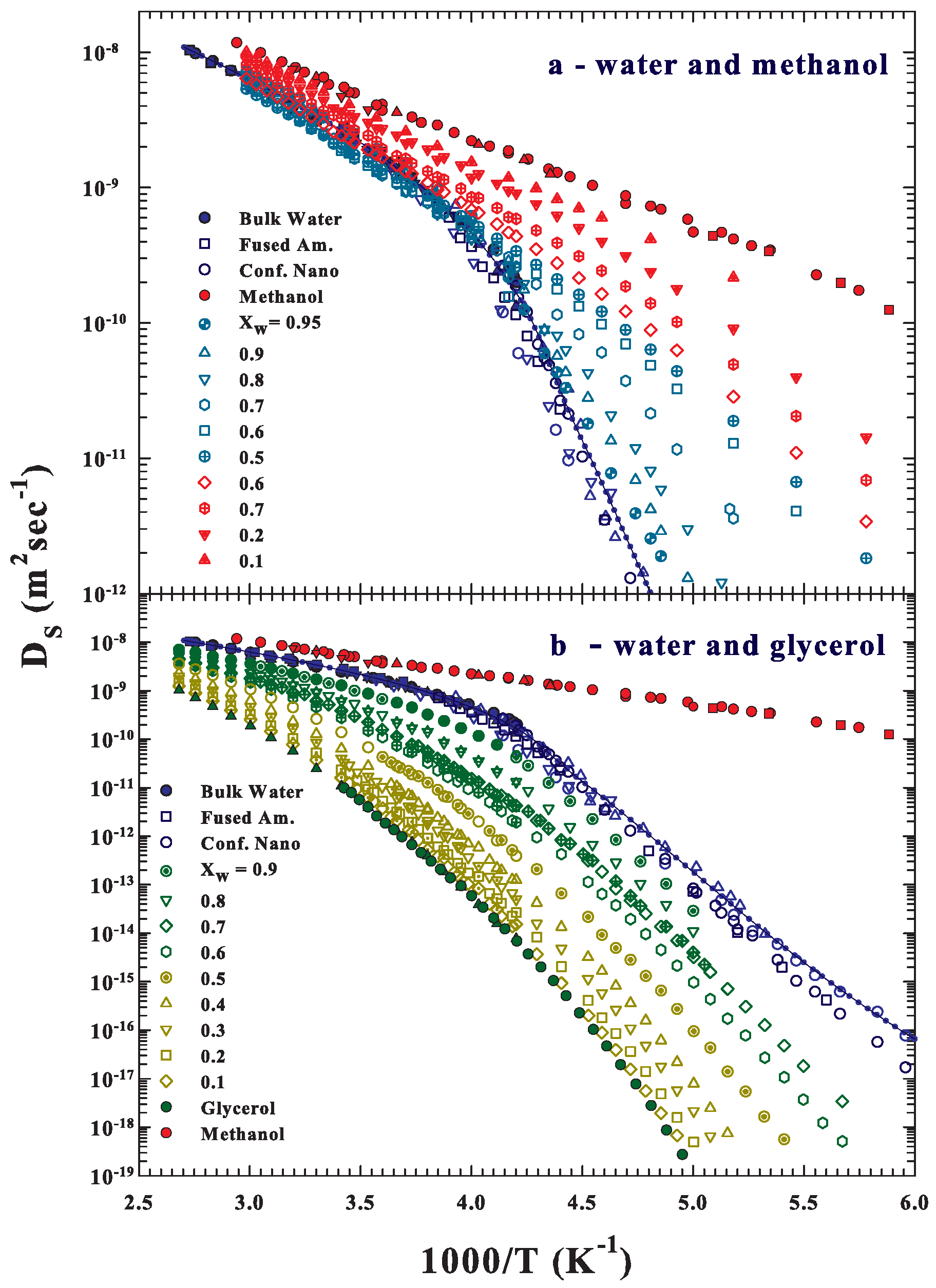
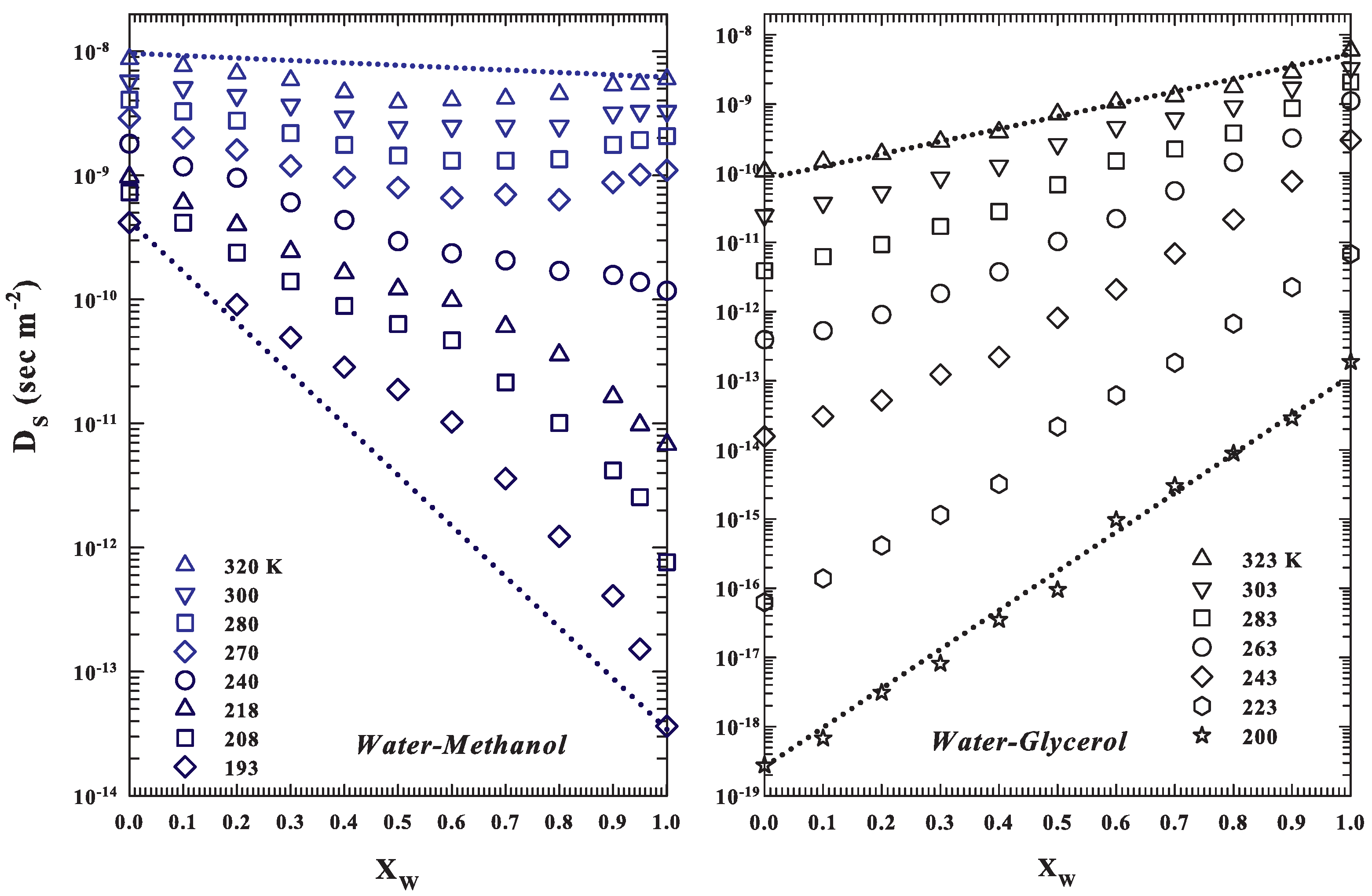
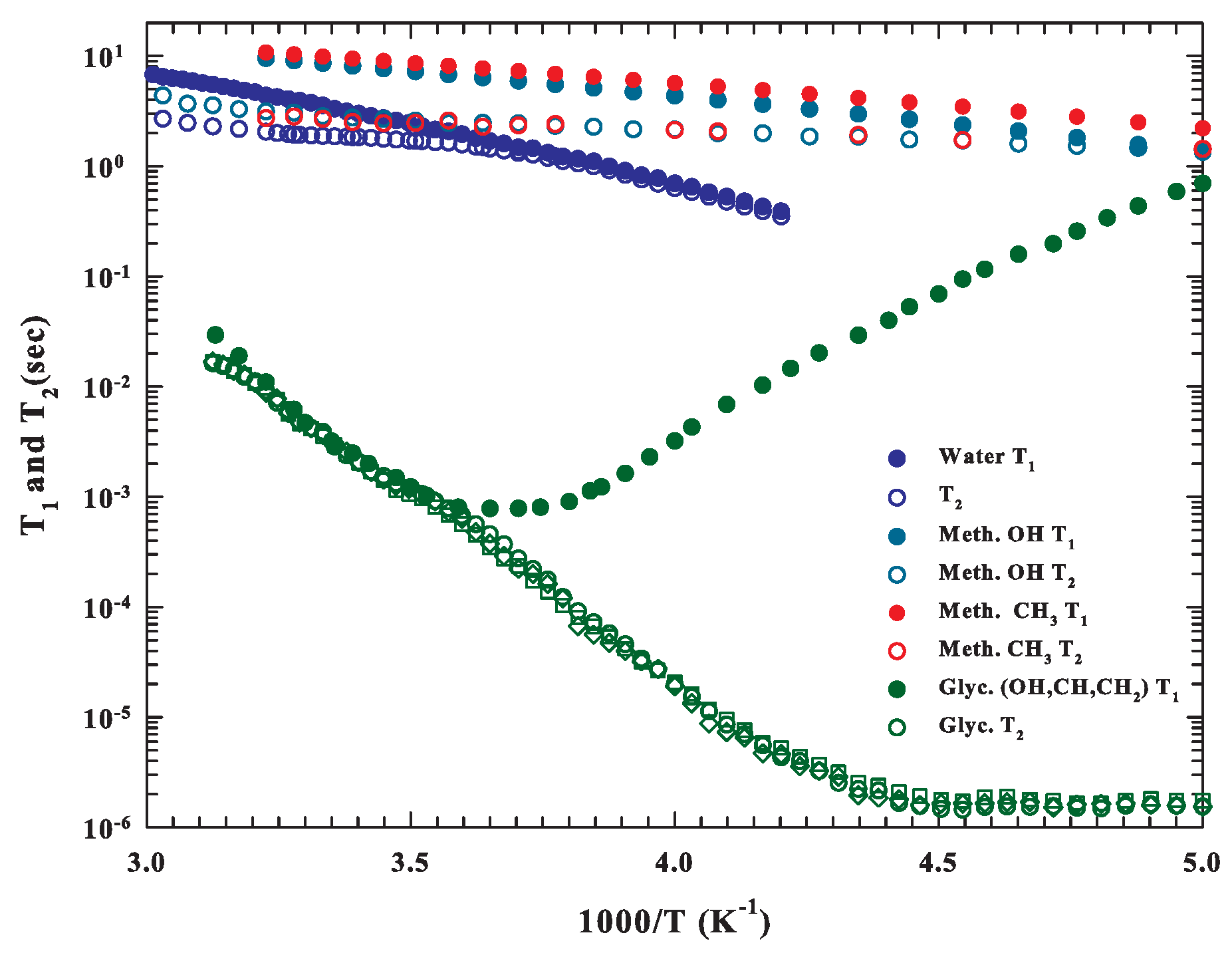
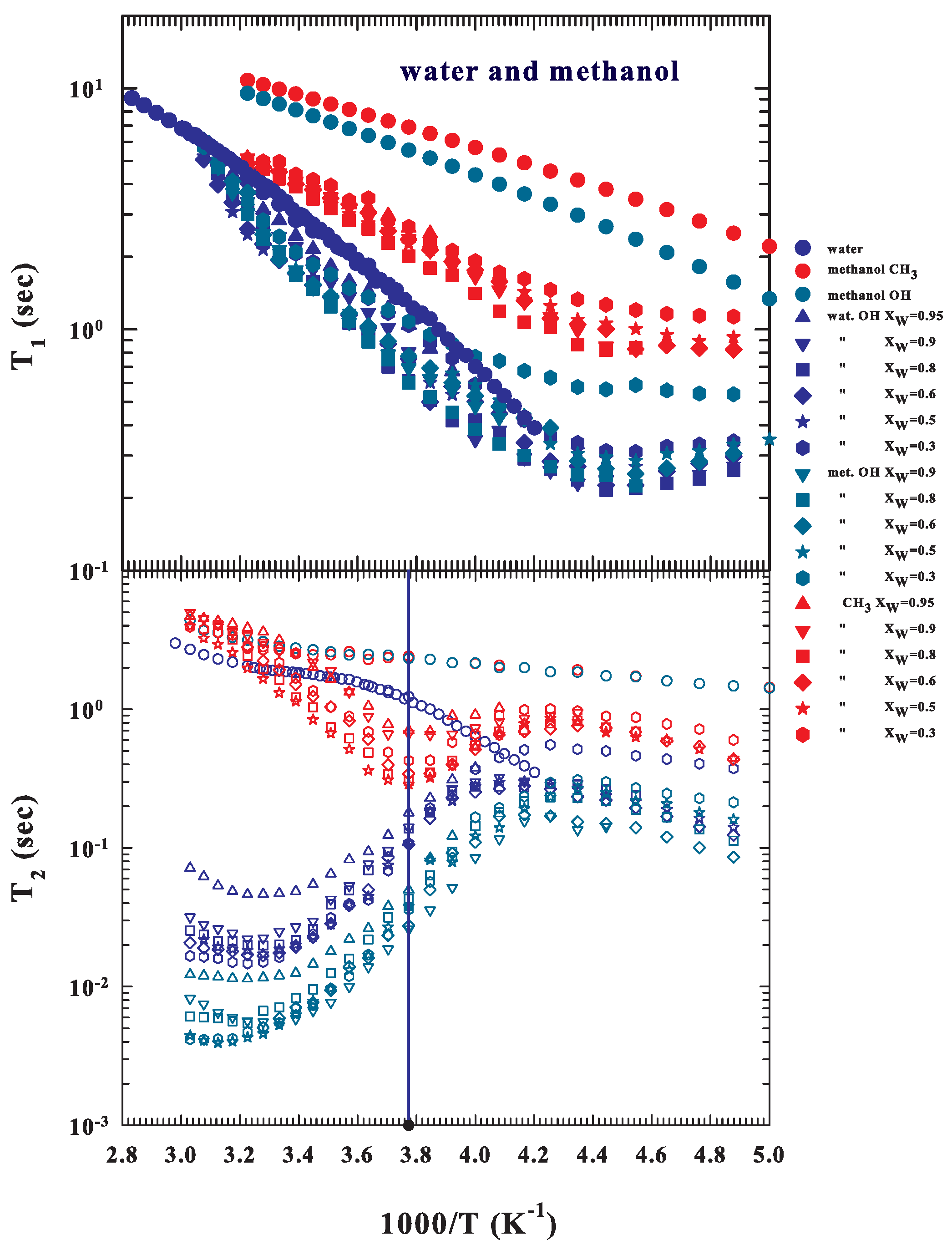
2. Data and Data Analysis
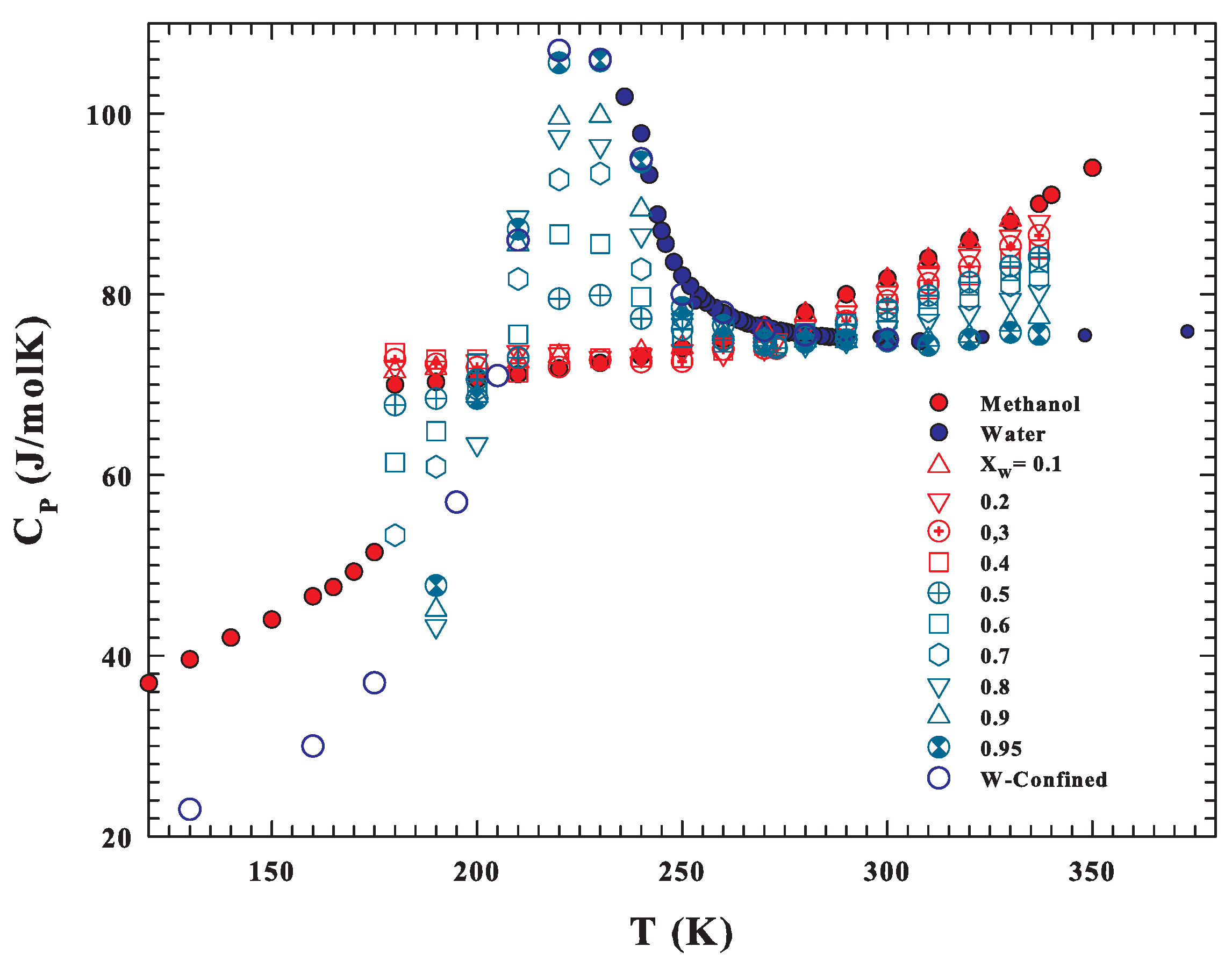
3. Results and Discussions
3.1. NMR Relaxation Time Data
3.2. Configurational Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ball, P. Life’s Matrix: A Biography of Water, 1st ed.; Farrar, Straus, and Giroux: New York, NY, USA, 2000; p. 417. [Google Scholar]
- Debenedetti, P.G.; Stanley, H.E. Supercooled and glassy water. Phys. Today 2003, 56, 40–46. [Google Scholar] [CrossRef]
- Speedy, R.J.; Angell, C.A. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45 ∘C. J. Chem. Phys. 1976, 65, 851–858. [Google Scholar] [CrossRef]
- Lobban, C.; Finney, J.L.; Kuhs, W.F. The structure of a new phase of ice. Nature 1998, 391, 268–270. [Google Scholar] [CrossRef]
- Mishima, O.; Calvert, L.D.; Whalley, E. Melting Ice-I at 77 K and 10 Kbar—A mew method of making amorphous solid. Nature 1984, 310, 393–397. [Google Scholar] [CrossRef]
- Mishima, O.; Calvert, L.D.; Whalley, E. An apparently first-order transition between two amorphous phases of ice induced by pressure. Nature 1985, 314, 76–78. [Google Scholar] [CrossRef]
- Mishima, O. Relationship between melting and amorphization of ice. Nature 1996, 384, 546–550. [Google Scholar] [CrossRef]
- Rapoport, E. Model for melting-curve at high pressure. J. Chem. Phys. 1967, 46, 2891. [Google Scholar] [CrossRef]
- Nemethy, G.; Scheraga, H. Structure of Water and Hydrophobic Bonding in Proteins. I. A Model for the Thermodynamic Properties of Liquid Water. J. Chem. Phys. 1962, 36, 3382. [Google Scholar] [CrossRef]
- Davis, C.M.; Litovitz, T.A. Two-State Theory of the Structure of Water. J. Chem. Phys. 1965, 42, 2563. [Google Scholar] [CrossRef]
- Jhon, M.S.; Grosh, J.; Ree, T.; Eyring, H. Significant-Structure Theory Applied to Water and Heavy Water. J. Chem. Phys. 1966, 44, 1465. [Google Scholar] [CrossRef]
- Kamb, B. Structure of high-pressure forms of ice. Science 1965, 150, 205. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.F.; Oliver, W.F. X-ray diffraction patterns of ice. Nature 1935, 135, 505–506. [Google Scholar] [CrossRef]
- Mishima, O. Reversible first-order transition between two H2O amorphs at ∼0.2 GPa and ∼135 K. J. Chem. Phys. 1994, 100, 5910–5912. [Google Scholar] [CrossRef]
- Loerting, T.; Salzmannm, C.; Kohl, I.; Mayer, E.; Hallbrucker, A. A second distinct structural “state” of high-density amorphous ice at 77 K and 1 bar. Phys. Chem. Chem. Phys. 2001, 3, 5355. [Google Scholar] [CrossRef]
- Poole, P.H.; Sciortino, F.; Essmann, U.; Stanley, H.E. Phase-behavior of metastable water. Nature 1992, 360, 324–328. [Google Scholar] [CrossRef]
- Palmer, J.C.; Poole, P.H.; Sciortino, F.; Debenedetti, P.G. Advances in Computational Studies of the Liquid-Liquid Transition in Water and Water-Like Models. Chem. Rev. 2018, 118, 9129–9151. [Google Scholar] [CrossRef]
- Mallamace, D.; Corsaro, E.; Mallamace, F.; Stanley, H.E. Experimental tests for a liquid-liquid critical point in water. Sci. China Phys. Mech. Astron. 2020, 63, 127001. [Google Scholar] [CrossRef]
- Prielmeier, F.X.; Lang, E.W.; Speedy, R.J.; Lüdemann, H.-D. The pressure-dependence of self-diffusion in supercooled light and heavy-water. Ber. Bunsenges Phys. Chem. 1988, 92, 1111–1117. [Google Scholar] [CrossRef]
- Cerveny, S.; Mallamace, F.; Swenson, J.; Vogel, M.; Xu, L.M. Confined water as model of supercooled water. Chem. Rev. 2016, 116, 7608–7625. [Google Scholar] [CrossRef]
- Mallamace, F.; Baglioni, P.; Corsaro, C.; Spooren, J.; Stanley, H.E.; Chen, S.-H. Transport properties of supercooled confined water. Riv. Nuovo Cimento 2011, 34, 253. [Google Scholar] [CrossRef]
- Xu, Y.; Petrik, N.G.; Scott Smith, R.; Kay, B.D.; Kimmel, G.A. Growth rate of crystalline ice and the diffusivity of supercooled water from 126 to 262 K. Proc. Natl. Acad. Sci. USA 2016, 113, 14921–14925. [Google Scholar] [CrossRef] [Green Version]
- Mallamace, F.; Branca, C.; Broccio, M.; Corsaro, C.; Mou, C.-Y.; Chen, S.-H. The anomalous behavior of the density of water in the range 30–373 K. Proc. Natl. Acad. Sci. USA 2007, 104, 18387–18391. [Google Scholar] [CrossRef] [Green Version]
- Erko, M.; Wallacher, D.; Hoell, A.; Hauß, T.; Zizak, I.; Paris, O. Density minimum of confined water at low temperatures: A combined study by small-angle scattering of X-rays and neutrons. Phys. Chem. Chem. Phys. 2012, 14, 3852–3858. [Google Scholar] [CrossRef]
- Bridgman, P.W. Water, in the liquid and five solid forms, under pressure. Proc. Am. Acad. Art. Sci. 1912, 47, 441–558. [Google Scholar] [CrossRef]
- Abascal, J.L.; Vega, C. Widom line and the liquid-liquid critical point for the TIP4P/2005 water model. J. Chem. Phys. 2010, 133, 234502. [Google Scholar] [CrossRef] [Green Version]
- Abascal, J.L.; Vega, C. Note: Equation of state and compressibility of supercooled water: Simulations and experiment. J. Chem. Phys. 2011, 134, 186101. [Google Scholar] [CrossRef]
- Ni, Y.; Skinner, J.L. Evidence for a liquid-liquid critical point in supercooled water within the E3B3 model and a possible interpretation of the kink in the homogeneous nucleation line. J. Chem. Phys. 2016, 144, 214501. [Google Scholar] [CrossRef] [PubMed]
- Sellberg, J.A.; Huang, C.; McQueen, T.A.; Loh, N.D.; Laksmono, H.; Schlesinger, D.; Sierra, R.G.; Nordlund, D.; Hampton, C.Y.; Starodub, D.; et al. Ultrafast X-ray probing of water structure below the homogeneous ice nucleation temperature. Nature 2014, 510, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Späh, A.; Pathak, H.; Perakis, F.; Mariedahl, D.; Amann-Winkel, K.; Sellberg, J.A.; Lee, J.H.; Kim, S.; Park, J.; et al. Maxima in the thermodynamic response and correlation functions of deeply supercooled water. Science 2017, 358, 1589–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Moynihan, C.T.; Angell, C.A. Thermodynamic determination of fragility in liquids and a fragile-to-strong liquid transition in water. Nature 1999, 398, 492. [Google Scholar] [CrossRef]
- Chen, S.H.; Mallamace, F.; Mou, C.Y.; Broccio, M.; Corsaro, C.; Faraone, A.; Liu, L. The violation of the Stokes-Einstein relation in supercooled water. Proc. Natl. Acad. Sci. USA 2006, 103, 12974–12978. [Google Scholar] [CrossRef] [Green Version]
- Stanley, H.E.; Buldyrev, S.V.; Franzese, G.; Kumar, P.; Mallamace, F.; Mazza, M.G.; Stokely, K.; Xu, L.L. Liquid polymorphism: Water in nanoconfined and biological environments. J. Phys. Condens. Matter 2010, 22, 284101. [Google Scholar] [CrossRef] [Green Version]
- Safran, S.A. Statistical thermodynamics of surfaces, interfaces and membranes. Phys. Today 1996, 49, 68. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals; Oxford Science Publication: Oxford, UK, 1974. [Google Scholar]
- Flory, P. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Ashbaugh, H.S.; Pratt, L.R. Colloquium: Scaled particle theory and the length scales of hydrophobicity. Rev. Mod. Phys. 2006, 78, 160–178. [Google Scholar] [CrossRef]
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Altabet, Y.E.; Debenedetti, P.G. Communication: Relationship between local structure and the stability of water in hydrophobic confinement. J. Chem. Phys. 2017, 147, 241102. [Google Scholar] [CrossRef]
- Widom, B.; Ben-Amotz, D. Note on the energy density in the solvent induced by a solute. Proc. Natl. Acad. Sci. USA 2006, 103, 18887. [Google Scholar] [CrossRef] [Green Version]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Xi, K.K.; Kleinhammes, A.; Wu, Y. Temperature-induced hydrophobic-hydrophilic transition observed by water adsorption. Science 2008, 322, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Levy, Y.; Onuchic, J.N. Water mediation in protein folding and molecular recognition. Ann. Rev. Biophys. Biomol. Struct. 2006, 35, 389–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Baglioni, P.; Buldyrev, S.V.; Chen, S.-H.; Stanley, H.E. Energy landscape in protein folding and unfolding. Proc. Natl. Acad. Sci. USA 2016, 105, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallamace, D.; Chen, S.-H.; Corsaro, C.; Fazio, E.; Mallamace, F.; Stanley, H.E. Hydrophilic and hydrophobic competition in water-methanol solutions. Sci. China Phys. Mech. Astron. 2019, 62, 107003. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-Forming Liquids. J. Chem. Phys. 1965, 43, 139. [Google Scholar] [CrossRef] [Green Version]
- Corsaro, C.; Maisano, R.; Mallamace, D.; Dugo, G. H-1 NMR study of water/methanol solutions as a function of temperature and concentration. Physica A 2013, 392, 596–601. [Google Scholar] [CrossRef]
- Karger, N.; Vardag, T.; Ludemann, H.-D. Temperature dependence of self-diffusion in compressed monohydric alcohols. J. Chem. Phys. 1990, 93, 3437. [Google Scholar] [CrossRef]
- Denney, D.J.; Cole, R.H. Dielectric Properties of Methanol and Methanol-1-Propanol Solutions. J. Chem. Phys. 1955, 25, 1767. [Google Scholar] [CrossRef]
- Mandal, H.; Frood, D.G.; Saleh, M.A.; Morgan, B.K. Dielectric and viscosity studies of the principal relaxation process of liquid 1-alkanols and their solutions Walker, S. Chem. Phys. 1989, 134, 441–451. [Google Scholar] [CrossRef]
- Jordan, B.P.; Sheppard, R.J.; Szwarnowski, S. The dielectric properties of formamide, ethanediol and methanol. J. Phys. D Appl. Phys. 1978, 11, 695. [Google Scholar] [CrossRef]
- Bertolini, D.; Cassettari, M.; Salvetti, G.G. The dielectric properties of alcohols–water solutions. I. The alcohol rich region. J. Chem. Phys. 1983, 78, 365. [Google Scholar] [CrossRef]
- Noyel, G.A.; Jorat, L.J.; Derriche, O.; Huck, J.R. Dielectric properties of normal supercooled water obtained in alcohol/water mixtures. IEEE Trans. Electr. Ins. 1992, 27, 113. [Google Scholar] [CrossRef]
- Sun, M.; Wang, L.-M.; Tian, Y.; Liu, R.; Ngai, K.L.; Tan, C. Component Dynamics in Miscible Mixtures of Water and Methanol. J. Phys. Chem. B 2011, 115, 8242–8248. [Google Scholar] [CrossRef]
- Derlacki, Z.J.; Easteal, A.J.; Edge, A.V.J.; Woolf, L.A.; Roksandic, Z. Diffusion Coefficients of Methanol and Water and the Mutual Diffusion Coefficient in Methanol-Water Solutions at 278 and 298 K. J. Phys. Chem. 1985, 89, 5318–5322. [Google Scholar] [CrossRef]
- Puzenko, A.; Hayashi, Y.; Ryabov, Y.E.; Balin, I.; Feldman, Y.; Kaatze, U.; Behrends, R. Relaxation dynamics in glycerol-water mixtures: I. Glycerol-rich mixtures. J. Phys. Chem. B 2005, 109, 6031–6035. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sigmund, E.E.; Halperin, W.P. Stokes-Einstein relation in supercooled aqueous solutions of glycerol. Phys. Rev. Lett. 2006, 96, 145502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, I.; Greenbaum, A.; Sokolov, A.P.; Feldman, Y. The puzzling first-order phase transition in water-glycerol mixtures. Phys. Chem. Chem. Phys. 2015, 17, 18063. [Google Scholar] [CrossRef] [PubMed]
- Schröter, K.; Donth, E.J. Viscosity and shear response at the dynamic glass transition of glycerol. Chem. Phys. 2000, 113, 9101. [Google Scholar] [CrossRef]
- Trejo Gonzalez, J.; Longinotti, M.P.; Corti, H.R. The Viscosity of Glycerol-Water Mixtures Including the Supercooled Region. J. Chem. Eng. Data 2011, 56, 1397. [Google Scholar] [CrossRef]
- Simpson, J.H.; Carr, H.Y. Diffusion and nuclear spin relaxation in water. Phys. Rev. 1958, 111, 1201. [Google Scholar] [CrossRef]
- Price, W.S.; Ide, H.; Arata, Y. Self-diffusion of supercooled water to 238 K using PGSE NMR diffusion measurements. J. Phys. Chem. A 1999, 103, 448–450. [Google Scholar] [CrossRef]
- Sjöström, J.; Swenson, J.; Bergman, R.; Kittaka, S. Investigating hydration dependence of dynamics of confined water: Monolayer, hydration water and Maxwell-Wagner processes. J. Chem. Phys. 2008, 128, 154503. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, M.; Jonas, J. Reorientational motions in compressed viscous fluids: Selectively deuterated glycerol. J. Chem. Phys. 1979, 71, 3252. [Google Scholar] [CrossRef]
- Fujara, F.; Petry, W.; Diehl, R.M.; Schnauss, W.; Sillescu, H. Localized Motion in Supercooled Glycerol as Measured by2H-NMR Spin-Lattice Relaxation and Incoherent Neutron Scattering. Europhys. Lett. 1991, 14, 563. [Google Scholar] [CrossRef]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679. [Google Scholar] [CrossRef]
- Aroulmoji, V.; Rao, A.S. H-1 NMR relaxation studies on glycerine-water and dioxan-water with paramagnetic ions. Phys. Chem. Liquid 2000, 38, 723. [Google Scholar] [CrossRef]
- Egorov, A.V.; Lyubartsev, A.P.; Laaksonen, A. Molecular Dynamics Simulation Study of Glycerol-Water Liquid Mixtures. J. Phys. Chem. B 2011, 115, 14572–14581. [Google Scholar] [CrossRef]
- Mallamace, F.; Broccio, M.; Corsaro, C.; Faraone, A.; Majolino, D.; Venuti, V.; Liu, L.; Mou, C.Y.; Chen, S.-H. Evidence of the existence of the low-density liquid phase in supercooled, confined water. Proc. Natl. Acad. Sci. USA 2008, 104, 424–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallamace, F.; Corsaro, C.; Stanley, H.E. A singular thermodynamically consistent temperature at the origin of the anomalous behavior of liquid water. Sci. Rep. 2012, 2, 993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, C.; Stanley, H.E. The thermodynamical response functions and the origin of the anomalous behavior of liquid water. Farad. Disc. 2013, 167, 95. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; del Valle, J.C. Molecule 1-Methyl-5-nitroindoline Probes the Structural Change of Liquid Water with Temperature. ACS Omega 2018, 3, 18930–18934. [Google Scholar] [CrossRef]
- Gibson, G.E.; Giauque, W.F. The third law of thermodynamics evidence from the specific heats of glycerol that the entropy of a glass exceeds that of a crystal at the absolute zero. J. Am. Chem. Soc. 1923, 45, 93. [Google Scholar] [CrossRef]
- Archer, D.G.; Carter, R.W. Thermodynamic properties of the NaCl+H2O system. 4. Heat capacities of H2O and NaCl(aq) in cold-stable and supercooled states. J. Phys. Chem. B 2000, 104, 8563. [Google Scholar] [CrossRef]
- Tombari, E.; Ferrari, C.; Salvetti, G. Heat capacity anomaly in a large sample of supercooled water. Chem. Phys. Lett. 1999, 300, 749. [Google Scholar] [CrossRef]
- Angell, C.A.; Sichina, W.J.; Oguni, M. Heat capacity of water at extremes of super cooling and superheating. J. Phys. Chem. 1982, 86, 998–1002. [Google Scholar] [CrossRef]
- Handa, Y.P.; Mishima, O.; Whalley, E. High-density amorphous ice. III. Thermal properties. J. Chem. Phys. 1986, 84, 2766. [Google Scholar] [CrossRef]
- Kelley, K.K. The heat capacity of methyl alcohol from 16 degrees K to 298 degrees K and the corresponding entropy and free energy. J. Am. Chem. Soc. 1929, 51, 180. [Google Scholar] [CrossRef]
- Carlson, H.G.; Westrum, E.F. Methanol: Heat Capacity, Enthalpies of Transition and Melting, and Thermodynamic Properties from 5–300 K. J. Chem. Phys. 1971, 54, 1464. [Google Scholar] [CrossRef]
- Pathak, H.; Späh, A.; Esmaeildoost, N.; Sellberg, J.A.; Kim, H.K.; Perakis, F.; Amann-Winkel, K.; Ladd-Parada, M.; Koliyadu, J.; Lane, T.J.; et al. Enhancement and maximum in the isobaric specific-heat capacity measurements of deeply supercooled water using ultrafast calorimetry. Proc. Natl. Acad. Sci. USA 2021, 118, e2018379118. [Google Scholar] [CrossRef]
- Starr, F.; Angell, C.A.; Stanley, H.E. Prediction of entropy and dynamic properties of water below the homogeneous nucleation temperature. Physica A 2003, 323, 51. [Google Scholar] [CrossRef] [Green Version]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Fazio, E.; Chen, S.-H.; Cupane, A. Specific Heat and Transport Functions of Water. Int. J. Mol. Sci. 2020, 21, 622. [Google Scholar] [CrossRef] [Green Version]
- Oguni, M.; Maruyama, S.; Wakabayashi, K.; Nagoe, A. Glass transitions of ordinary and heavy water within silica-gel nanopores. Chem. Asian J. 2007, 2, 514. [Google Scholar] [CrossRef]
- Oguni, M.; Kanke, Y.; Namba, S. Thermal properties of the water confined within nanopores of silica MCM-41. AIP Conf. Proc. 2008, 982, 34. [Google Scholar]
- Kauzmann, W. Some Factors in the Interpretation of Protein Denaturation. Adv. Prot. Chem. 1959, 14, 1. [Google Scholar]
- Frank, H.S.; Evans, M.J. Free Volume and Entropy in Condensed Systems III. Entropy in Binary Liquid Mixtures; Partial Molal Entropy in Dilute Solutions; Structure and Thermodynamics in Aqueous Electrolytes. J. Chem. Phys. 1945, 13, 507. [Google Scholar] [CrossRef]
- Skipper, N.T. Computer simulation of methane—Water solutions. Evidence for a temperature-dependent hydrophobic attraction. Chem. Phys. Lett. 1993, 207, 424. [Google Scholar] [CrossRef]
- Soper, A.K.; Finney, J.L. Hydration of methanol in aqueous solution. Phys. Rev. Lett. 1993, 71, 4346. [Google Scholar] [CrossRef] [PubMed]
- Micali, N.; Trusso, S.; Vasi, C.; Blaudez, D.; Mallamace, F. Dynamical properties of water-methanol solutions studied by depolarized Rayleigh scattering. Phys. Rev. E 1996, 54, 1720. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, C.; Vasi, S.; Stanley, H.E. Dynamical properties of water-methanol solutions. J. Chem. Phys. 2016, 144, 064506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
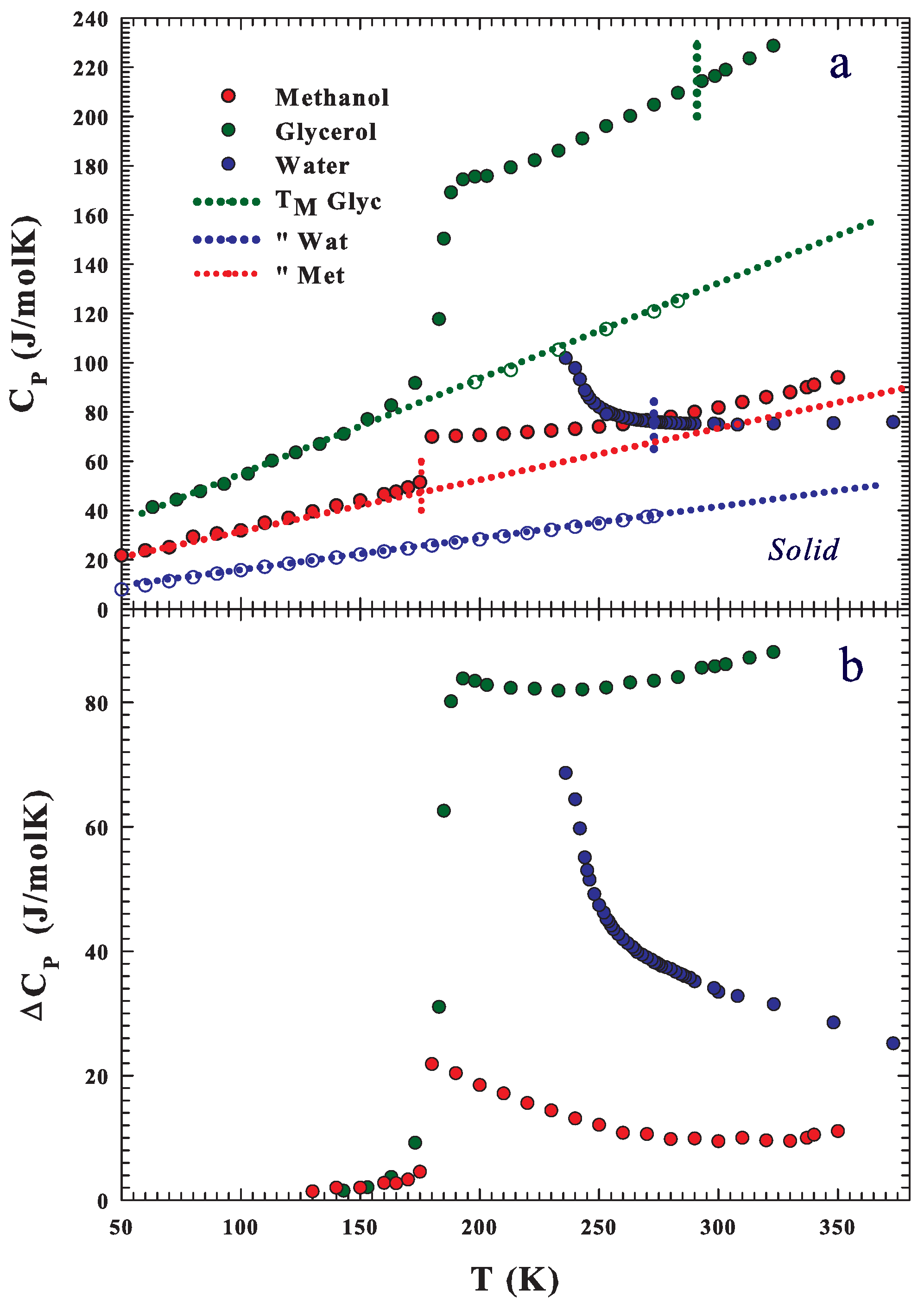
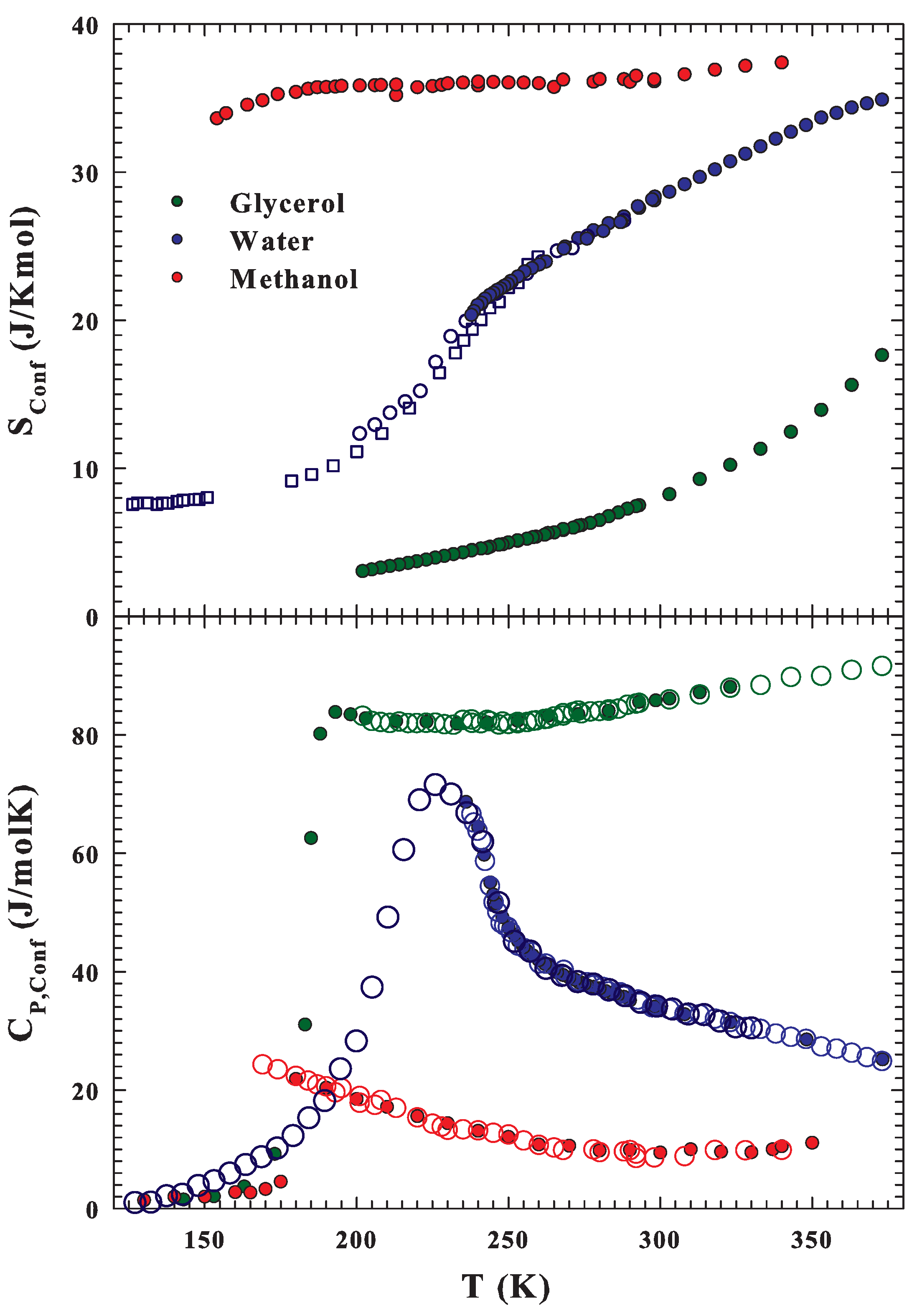
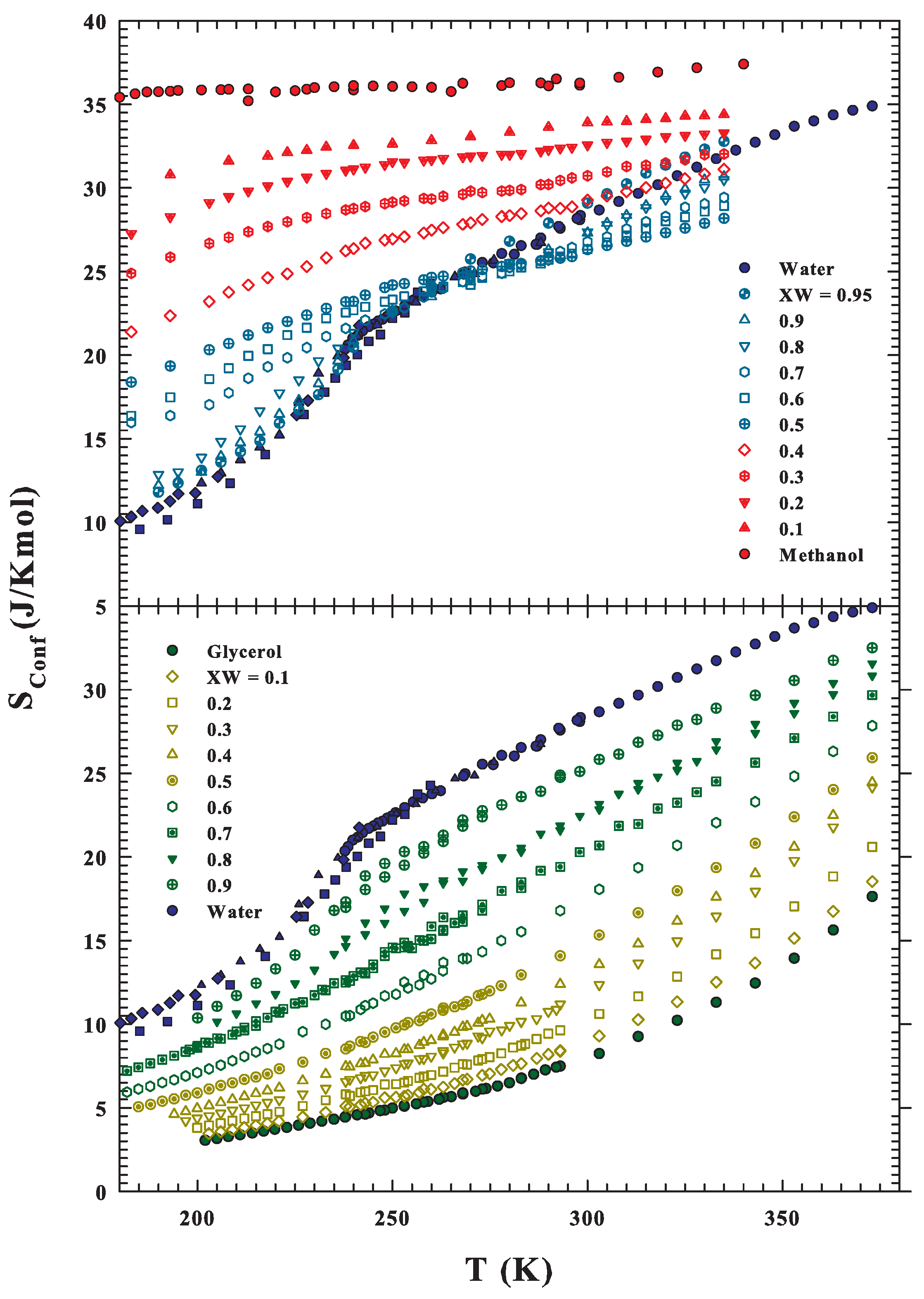
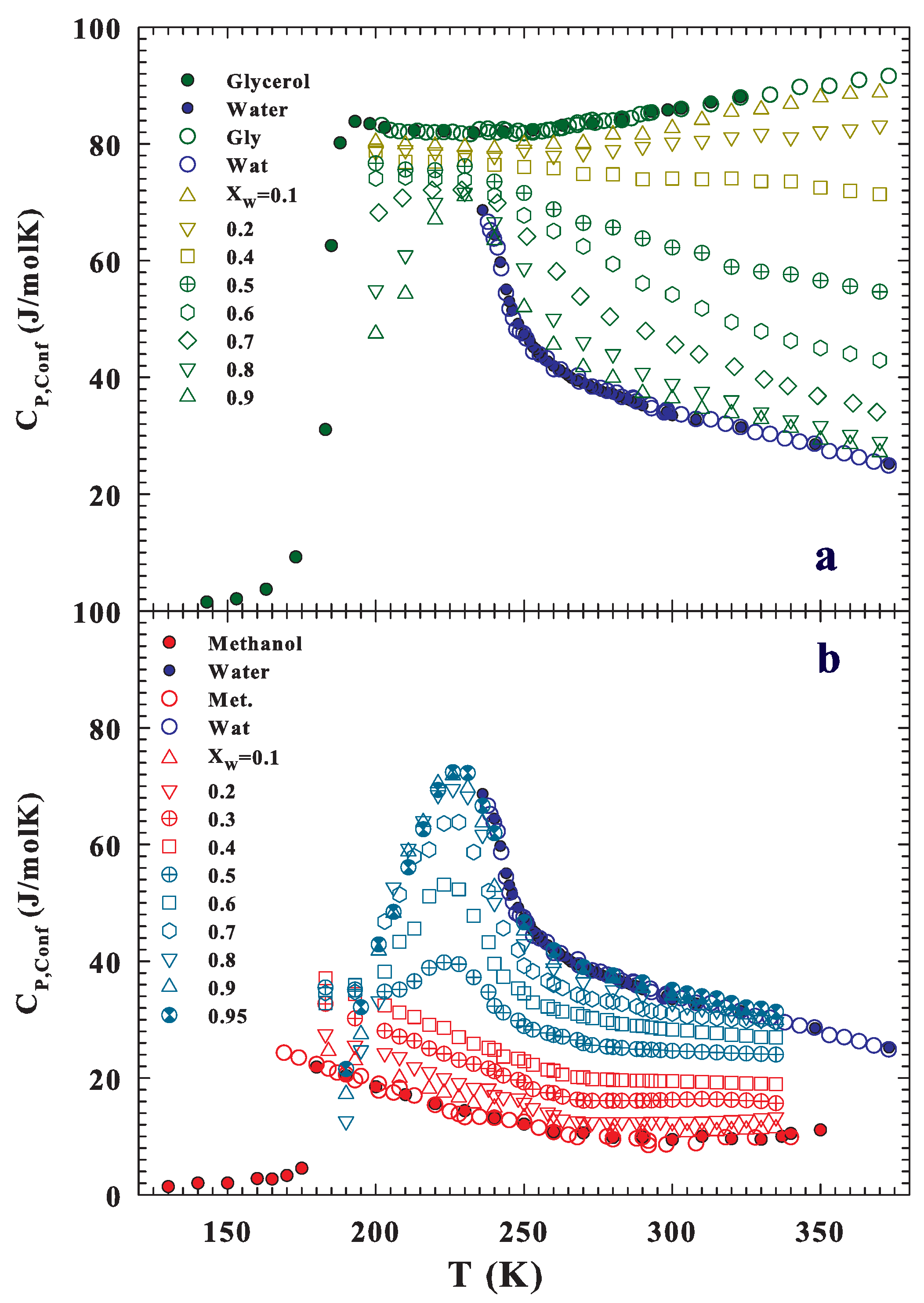
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamace, F.; Mallamace, D.; Chen, S.-H.; Lanzafame, P.; Papanikolaou, G. Hydrophilic and Hydrophobic Effects on the Structure and Themodynamic Properties of Confined Water: Water in Solutions. Int. J. Mol. Sci. 2021, 22, 7547. https://doi.org/10.3390/ijms22147547
Mallamace F, Mallamace D, Chen S-H, Lanzafame P, Papanikolaou G. Hydrophilic and Hydrophobic Effects on the Structure and Themodynamic Properties of Confined Water: Water in Solutions. International Journal of Molecular Sciences. 2021; 22(14):7547. https://doi.org/10.3390/ijms22147547
Chicago/Turabian StyleMallamace, Francesco, Domenico Mallamace, Sow-Hsin Chen, Paola Lanzafame, and Georgia Papanikolaou. 2021. "Hydrophilic and Hydrophobic Effects on the Structure and Themodynamic Properties of Confined Water: Water in Solutions" International Journal of Molecular Sciences 22, no. 14: 7547. https://doi.org/10.3390/ijms22147547
APA StyleMallamace, F., Mallamace, D., Chen, S.-H., Lanzafame, P., & Papanikolaou, G. (2021). Hydrophilic and Hydrophobic Effects on the Structure and Themodynamic Properties of Confined Water: Water in Solutions. International Journal of Molecular Sciences, 22(14), 7547. https://doi.org/10.3390/ijms22147547







