Anti-Inflammatory and Pro-Differentiating Properties of the Aryl Hydrocarbon Receptor Ligands NPD-0614-13 and NPD-0614-24: Potential Therapeutic Benefits in Psoriasis
Abstract
1. Introduction
2. Results
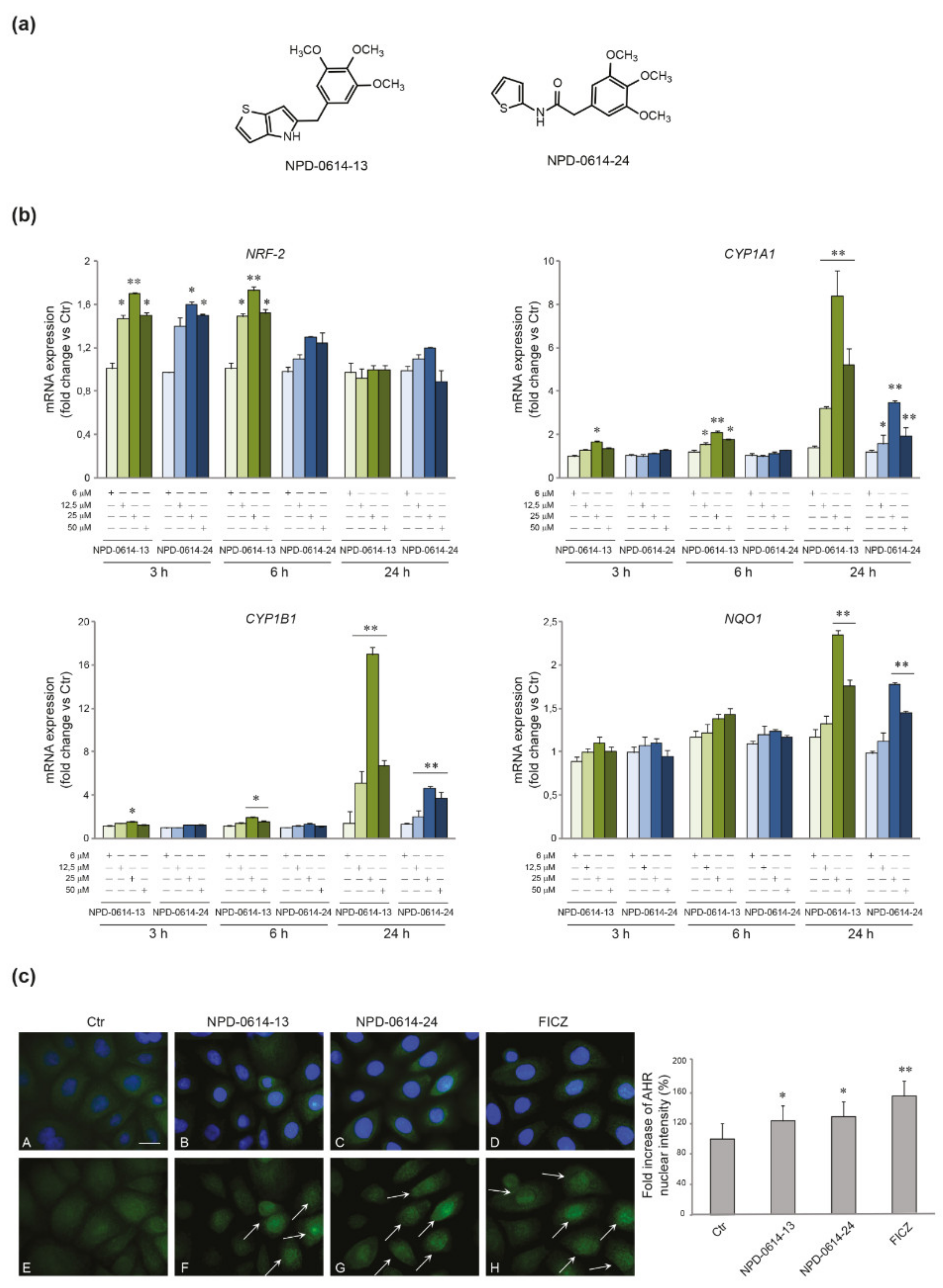
2.1. NPD-0614-13 and NPD-0614-24 Activated AhR Signaling in NHKs
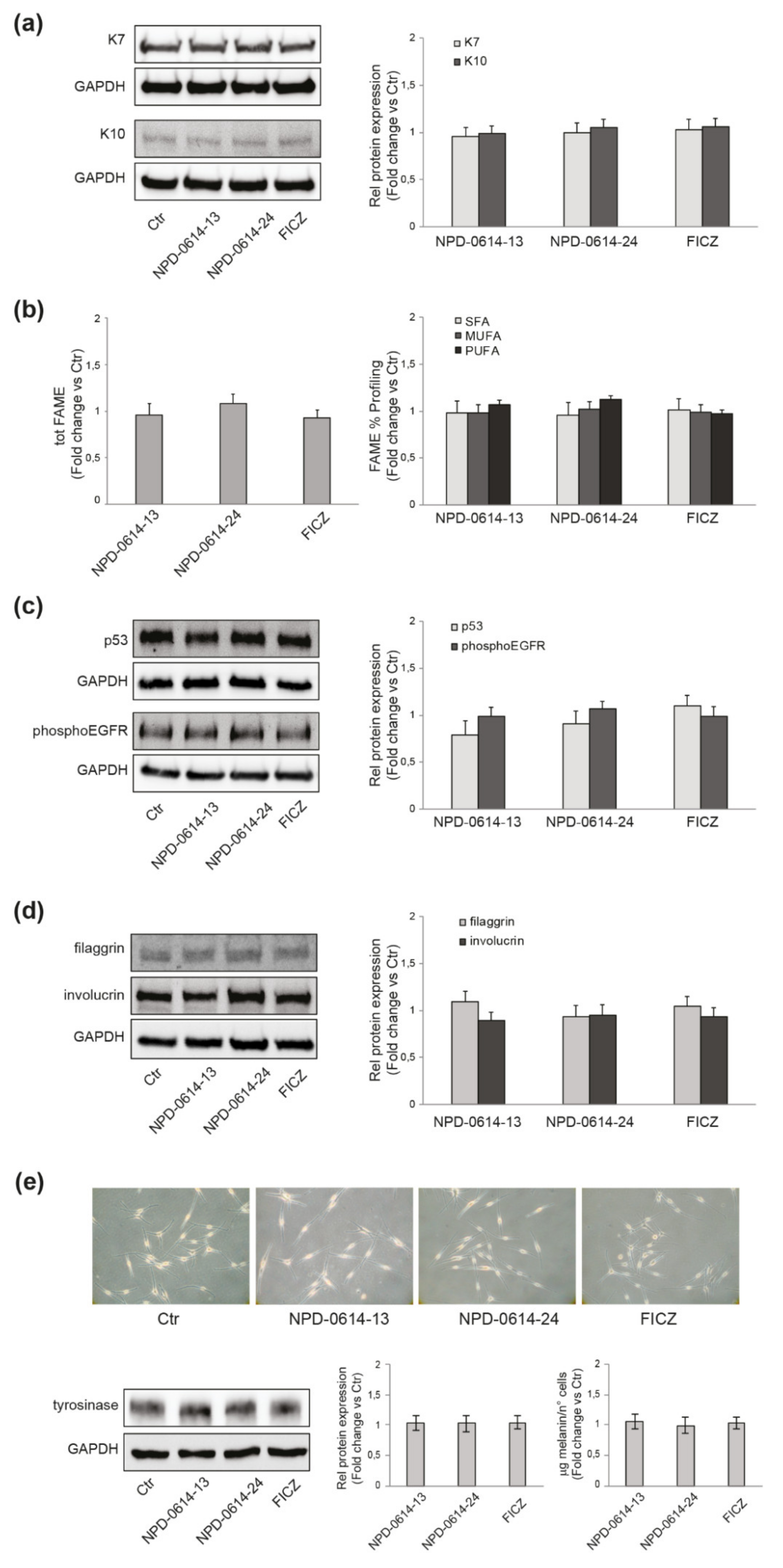
2.2. NPD-0614-13 and NPD-0614-24 Safety Profile in Different Skin Cell Populations
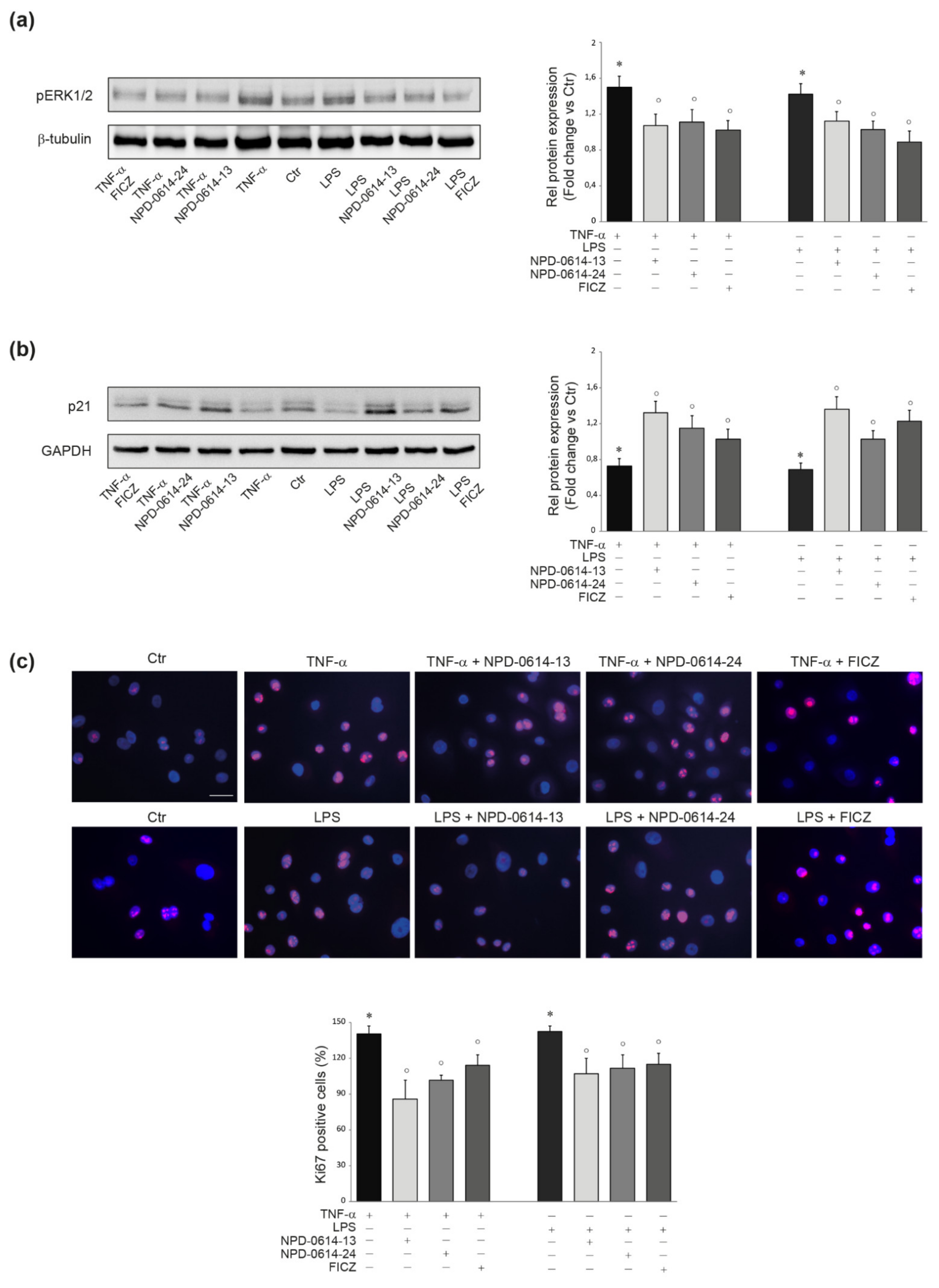
2.3. NPD-0614-13 and NPD-0614-24 Recovered the Alteration of Cell Proliferation Induced by LPS or TNF-α
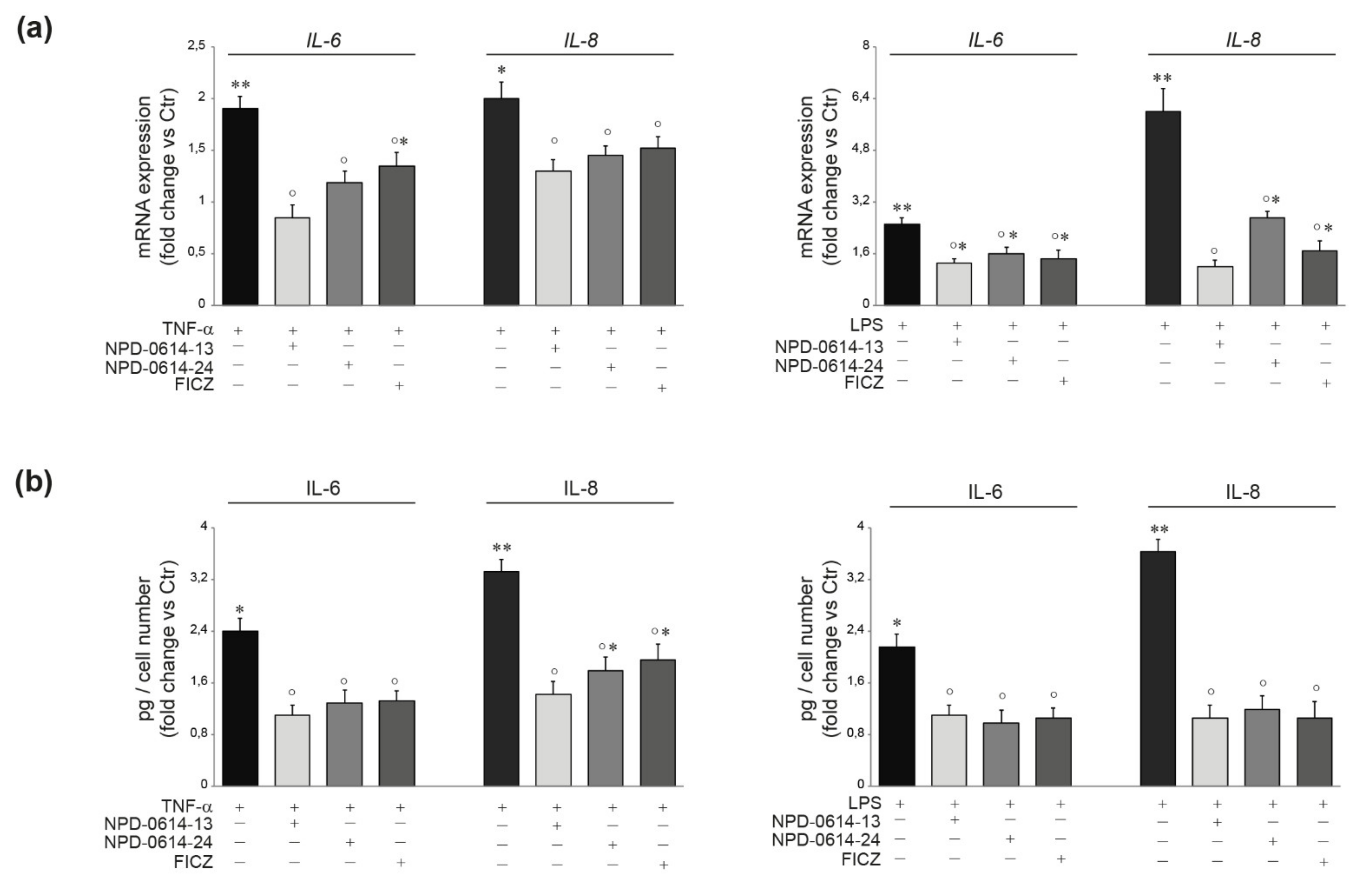
2.4. NPD-0614-13 and NPD-0614-24 Counteracted the Pro-Inflammatory Effects Induced by LPS or TNF-α
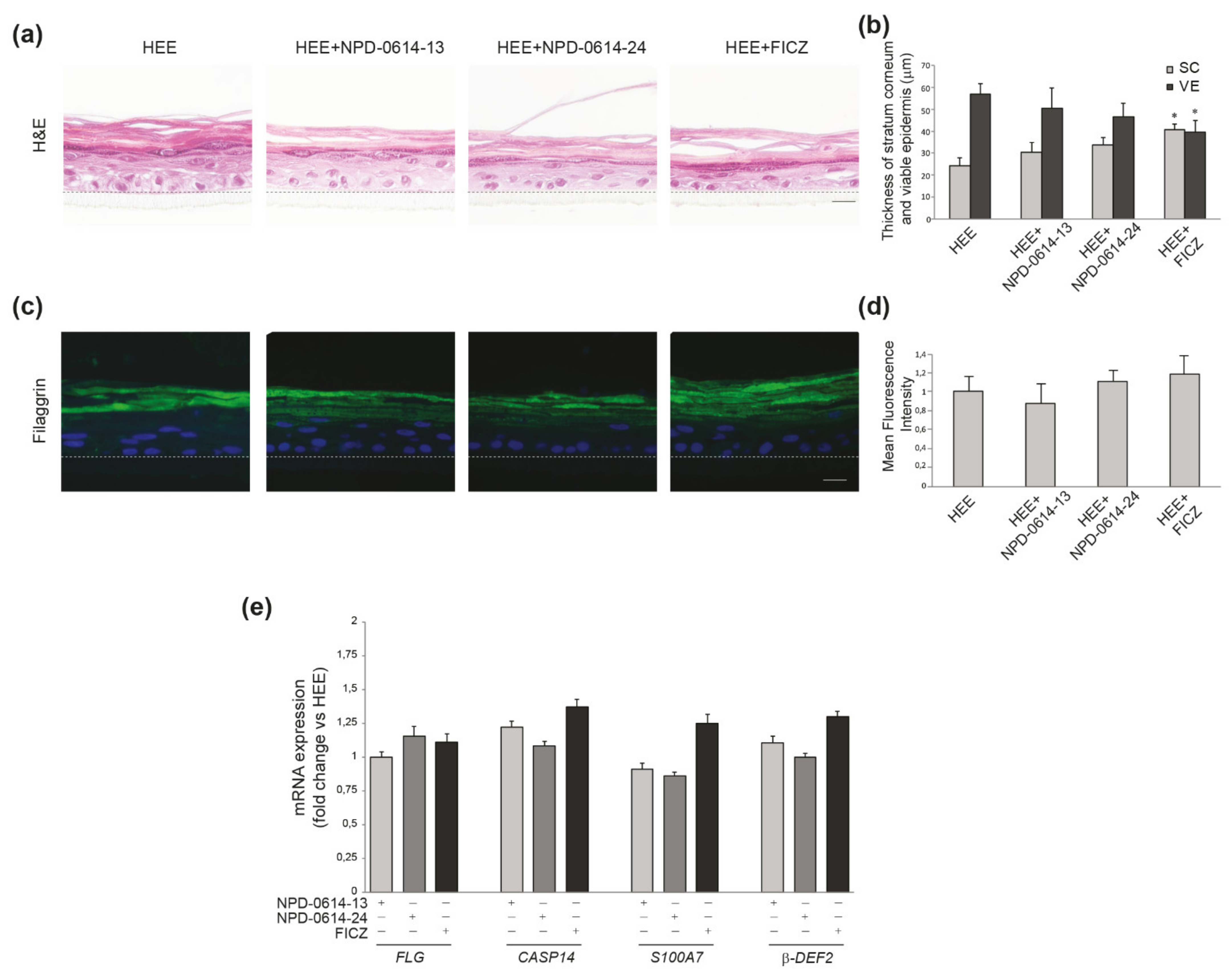
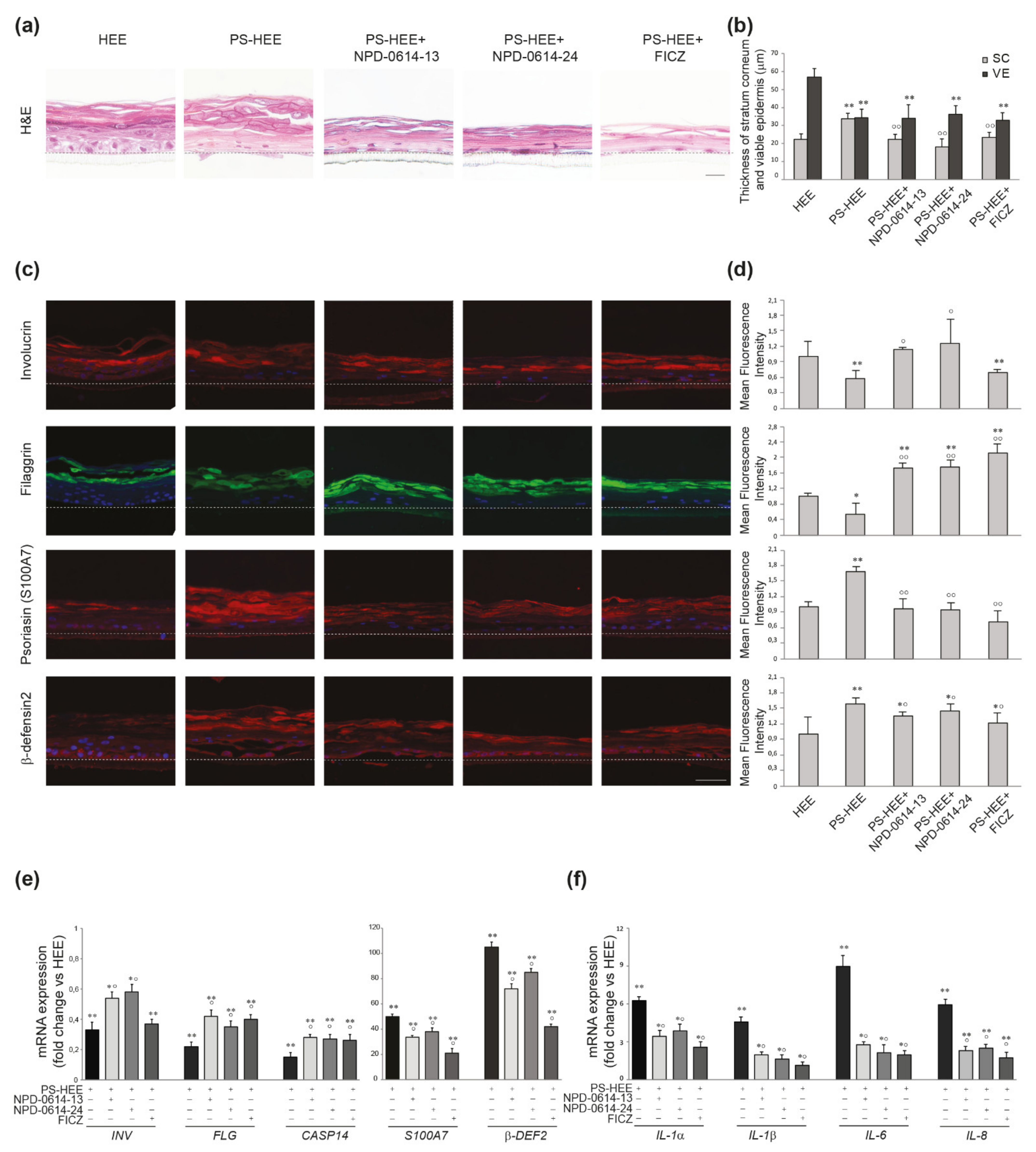
2.5. NPD-0614-13 and NPD-0614-24 Showed Pro-Differentiative and Anti-Inflammatory Effects in Psoriasis-Like Human Epidermal Equivalents (PS-HEE) and Reconstructed Human Psoriatic Skin Equivalents (PSE)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. New Chemical Ligands
4.3. Cell Culture
4.4. Morphologic Analysis by Inverted Phase Contrast Microscope
4.5. Human Epidermal Equivalents (HEEs) and Psoriasis-Like HEE (PS-HEEs) Preparation
4.6. D Full-Thickness Human Psoriasis Skin Equivalent (PSE)
4.7. RNA Extraction and Quantitative Real-Time RT-PCR
4.8. RNA Interference Experiments
4.9. Western Blot Analysis
4.10. Immunofluorescence Analysis
4.11. Fluorescence Measurement of ROS Levels
4.12. Protein Determination by Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Histology, Morphometry, and Immunofluorescence Analysis of HEEs and PS-HEEs
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ju, Q.; Yu, Q.; Song, N.; Tan, Y.; Xia, L.; Zouboulis, C.C. Expression and Significance of Aryl Hydrocarbon Receptor on Human Epidermis, Hair Follicle, and Sebaceous Gland. Chin. J. Dermatol. 2011, 44, 761–764. [Google Scholar]
- Denison, M.S.; Nagy, S.R. Activation of the Aryl Hydrocarbon Receptor by Structurally Diverse Exogenous and Endogenous Chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.S.; Soshilov, A.A.; He, G.; DeGroot, D.E.; Zhao, B. Exactly the Same but Different: Promiscuity and Diversity in the Molecular Mechanisms of Action of the Aryl Hydrocarbon (Dioxin) Receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the Aryl Hydrocarbon Receptor in the Skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A. The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT System in Skin Homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Haarmann-Stemmann, T.; Esser, C.; Krutmann, J. The Janus-Faced Role of Aryl Hydrocarbon Receptor Signaling in the Skin: Consequences for Prevention and Treatment of Skin Disorders. J. Investig. Dermatol. 2015, 135, 2572–2576. [Google Scholar] [CrossRef]
- Di Meglio, P.; Duarte, J.H.; Ahlfors, H.; Owens, N.D.; Li, Y.; Villanova, F.; Tosi, I.; Hirota, K.; Nestle, F.O.; Mrowietz, U.; et al. Activation of the Aryl Hydrocarbon Receptor Dampens the Severity of Inflammatory Skin Conditions. Immunity 2014, 40, 989–1001. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef]
- Napolitano, M.; Patruno, C. Aryl Hydrocarbon Receptor (AhR) a Possible Target for the Treatment of Skin Disease. Med. Hypotheses 2018, 116, 96–100. [Google Scholar] [CrossRef]
- Hu, T.; Wang, D.; Yu, Q.; Li, L.; Mo, X.; Pan, Z.; Zouboulis, C.C.; Peng, L.; Xia, L.; Ju, Q. Aryl Hydrocarbon Receptor Negatively Regulates Lipid Synthesis and Involves in Cell Differentiation of SZ95 Sebocytes in Vitro. Chem. Biol. Interact. 2016, 258, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Mastrofrancesco, A.; Cardinali, G.; Rosato, E.; Salsano, F.; Su, D.S.; Serafino, A.; Picardo, M. Effects of Carbonaceous Nanoparticles from Low-Emission and Older Diesel Engines on Human Skin Cells. Carbon 2011, 49, 5038–5048. [Google Scholar] [CrossRef]
- Hao, N.; Whitelaw, M.L. The Emerging Roles of AhR in Physiology and Immunity. Biochem. Pharmacol. 2013, 86, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the Aryl Hydrocarbon Receptor in Tobacco Smoke Extract-Induced Matrix Metalloproteinase-1 Expression. Exp. Dermatol. 2013, 22, 349–353. [Google Scholar] [CrossRef]
- Szelest, M.; Walczak, K.; Plech, T. A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes. Int. J. Mol. Sci. 2021, 22, 1104. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Chen, G.; Chen, W.; Hou, X.; Hu, T.; Lu, L.; Wang, L.; Pan, Z.; Wu, Q.; Li, X.; et al. Formalin-Killed Propionibacterium Acnes Activates the Aryl Hydrocarbon Receptor and Modifies Differentiation of SZ95 Sebocytes in Vitro. Eur. J. Dermatol. 2021, 31, 32–40. [Google Scholar]
- Greenlee, W.F.; Dold, K.M.; Osborne, R. Actions of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) on Human Epidermal Keratinocytes in Culture. Vitr. Cell. Dev. Biol. 1985, 21, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Pan, Z.; Yu, Q.; Mo, X.; Song, N.; Yan, M.; Zouboulis, C.C.; Xia, L.; Ju, Q. Benzo(a)Pyrene Induces Interleukin (IL)-6 Production and Reduces Lipid Synthesis in Human SZ95 Sebocytes Via the Aryl Hydrocarbon Receptor Signaling Pathway. Environ. Toxicol. Pharmacol. 2016, 43, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Loertscher, J.A.; Sattler, C.A.; Allen-Hoffmann, B.L. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters the Differentiation Pattern of Human Keratinocytes in Organotypic Culture. Toxicol. Appl. Pharmacol. 2001, 175, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Poland, A.; Knutson, J.C. 2,3,7,8-Tetrachlorodibenzo-P-Dioxin and Related Halogenated Aromatic Hydrocarbons: Examination of the Mechanism of Toxicity. Annu. Rev. Pharmacol. Toxicol. 1982, 22, 517–554. [Google Scholar] [CrossRef]
- Sutter, C.H.; Bodreddigari, S.; Campion, C.; Wible, R.S.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Increases the Expression of Genes in the Human Epidermal Differentiation Complex and Accelerates Epidermal Barrier Formation. Toxicol. Sci. 2011, 124, 128–137. [Google Scholar] [CrossRef]
- Tijet, N.; Boutros, P.C.; Moffat, I.D.; Okey, A.B.; Tuomisto, J.; Pohjanvirta, R. Aryl Hydrocarbon Receptor Regulates Distinct Dioxin-Dependent and Dioxin-Independent Gene Batteries. Mol. Pharmacol. 2006, 69, 140–153. [Google Scholar] [CrossRef]
- Wright, E.J.; De Castro, K.P.; Joshi, A.D.; Elferink, C.J. Canonical and Non-Canonical Aryl Hydrocarbon Receptor Signaling Pathways. Curr. Opin. Toxicol. 2017, 2, 87–92. [Google Scholar] [CrossRef]
- Ikuta, T.; Namiki, T.; Fujii-Kuriyama, Y.; Kawajiri, K. AhR Protein Trafficking and Function in the Skin. Biochem. Pharmacol. 2009, 77, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Kohle, C.; Bock, K.W. Coordinate Regulation of Phase I and II Xenobiotic Metabolisms by the Ah Receptor and Nrf2. Biochem. Pharmacol. 2007, 73, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Haarmann-Stemmann, T.; Bothe, H.; Abel, J. Growth Factors, Cytokines and their Receptors as Downstream Targets of Arylhydrocarbon Receptor (AhR) Signaling Pathways. Biochem. Pharmacol. 2009, 77, 508–520. [Google Scholar] [CrossRef]
- Fritsche, E.; Schafer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hubenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening Up the UV Response by Identification of the Arylhydrocarbon Receptor as a Cytoplasmatic Target for Ultraviolet B Radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.X.; Chen, G.; Hossini, A.M.; Hu, T.; Wang, L.; Pan, Z.; Lu, L.; Cao, K.; Ma, Y.; Zouboulis, C.C.; et al. Aryl Hydrocarbon Receptor Modulates the Expression of TNF-Alpha and IL-8 in Human Sebocytes Via the MyD88-p65NF-kappaB/p38MAPK Signaling Pathways. J. Innate Immun. 2019, 11, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Khan, E.M.; Leung, P.S.; Gershwin, M.E.; Chang, W.L.; Wu, D.; Haarmann-Stemmann, T.; Hoffmann, A.; Denison, M.S. Cross-Talk between Aryl Hydrocarbon Receptor and the Inflammatory Response: A Role for Nuclear Factor-kappaB. J. Biol. Chem. 2014, 289, 1866–1875. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl Hydrocarbon Receptor in Combination with Stat1 Regulates LPS-Induced Inflammatory Responses. J. Exp. Med. 2009, 206, 2027–2035. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl Hydrocarbon Receptor Regulates Stat1 Activation and Participates in the Development of Th17 Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Bureau, C.; Hanoun, N.; Torrisani, J.; Vinel, J.P.; Buscail, L.; Cordelier, P. Expression and Function of Kruppel Like-Factors (KLF) in Carcinogenesis. Curr. Genom. 2009, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Narla, G.; Heath, K.E.; Reeves, H.L.; Li, D.; Giono, L.E.; Kimmelman, A.C.; Glucksman, M.J.; Narla, J.; Eng, F.J.; Chan, A.M.; et al. KLF6, a Candidate Tumor Suppressor Gene Mutated in Prostate Cancer. Science 2001, 294, 2563–2566. [Google Scholar] [CrossRef]
- Vogel, C.F.; Sciullo, E.; Li, W.; Wong, P.; Lazennec, G.; Matsumura, F. RelB, a New Partner of Aryl Hydrocarbon Receptor-Mediated Transcription. Mol. Endocrinol. 2007, 21, 2941–2955. [Google Scholar] [CrossRef] [PubMed]
- Kiyomatsu-Oda, M.; Uchi, H.; Morino-Koga, S.; Furue, M. Protective Role of 6-Formylindolo [3,2-b]Carbazole (FICZ), an Endogenous Ligand for Arylhydrocarbon Receptor, in Chronic Mite-Induced Dermatitis. J. Dermatol. Sci. 2018, 90, 284–294. [Google Scholar] [CrossRef]
- Van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schroder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal Tar Induces AHR-Dependent Skin Barrier Repair in Atopic Dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef]
- Rannug, A.; Fritsche, E. The Aryl Hydrocarbon Receptor and Light. Biol. Chem. 2006, 387, 1149–1157. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl Hydrocarbon Receptor-Induced Signals Up-Regulate IL-22 Production and Inhibit Inflammation in the Gastrointestinal Tract. Gastroenterology 2011, 141, 237–248. [Google Scholar] [CrossRef]
- Marafini, I.; Di Fusco, D.; Dinallo, V.; Franze, E.; Stolfi, C.; Sica, G.; Monteleone, G.; Monteleone, I. NPD-0414-2 and NPD-0414-24, Two Chemical Entities Designed as Aryl Hydrocarbon Receptor (AhR) Ligands, Inhibit Gut Inflammatory Signals. Front. Pharmacol. 2019, 10, 380. [Google Scholar] [CrossRef]
- Ju, Q.; Fimmel, S.; Hinz, N.; Stahlmann, R.; Xia, L.; Zouboulis, C.C. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters Sebaceous Gland Cell Differentiation in Vitro. Exp. Dermatol. 2011, 20, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Forrester, A.R.; Elias, M.S.; Woodward, E.L.; Graham, M.; Williams, F.M.; Reynolds, N.J. Induction of a Chloracne Phenotype in an Epidermal Equivalent Model by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) is Dependent on Aryl Hydrocarbon Receptor Activation and is Not Reproduced by Aryl Hydrocarbon Receptor Knock Down. J. Dermatol. Sci. 2014, 73, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.; Yang, K.; Zouboulis, C.C.; Ring, J.; Chen, W. Chloracne: From Clinic to Research. Dermatol. Sin. 2018, 36, 2–6. [Google Scholar] [CrossRef]
- Kennedy, L.H.; Sutter, C.H.; Leon Carrion, S.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Mediated Production of Reactive Oxygen Species is an Essential Step in the Mechanism of Action to Accelerate Human Keratinocyte Differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Panteleyev, A.A.; Bickers, D.R. Dioxin-Induced Chloracne--Reconstructing the Cellular and Molecular Mechanisms of a Classic Environmental Disease. Exp. Dermatol. 2006, 15, 705–730. [Google Scholar] [CrossRef]
- Furue, M.; Tsuji, G. Chloracne and Hyperpigmentation Caused by Exposure to Hazardous Aryl Hydrocarbon Receptor Ligands. Int. J. Environ. Res. Public Health 2019, 16, 4864. [Google Scholar] [CrossRef]
- Luecke, S.; Backlund, M.; Jux, B.; Esser, C.; Krutmann, J.; Rannug, A. The Aryl Hydrocarbon Receptor (AHR), a Novel Regulator of Human Melanogenesis. Pigment Cell Melanoma Res. 2010, 23, 828–833. [Google Scholar] [CrossRef]
- Puga, A.; Ma, C.; Marlowe, J.L. The Aryl Hydrocarbon Receptor Cross-Talks with Multiple Signal Transduction Pathways. Biochem. Pharmacol. 2009, 77, 713–722. [Google Scholar] [CrossRef]
- Kim, A.; Nam, Y.J.; Shin, Y.K.; Lee, M.S.; Sohn, D.S.; Lee, C.S. Rotundarpene Inhibits TNF-Alpha-Induced Activation of the Akt, mTOR, and NF-kappaB Pathways, and the JNK and p38 Associated with Production of Reactive Oxygen Species. Mol. Cell. Biochem. 2017, 434, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Intayoung, P.; Limtrakul, P.; Yodkeeree, S. Antiinflammatory Activities of Crebanine by Inhibition of NF-kappaB and AP-1 Activation through Suppressing MAPKs and Akt Signaling in LPS-Induced RAW264.7 Macrophages. Biol. Pharm. Bull. 2016, 39, 54–61. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wong, J.; Polyak, K.; Aronzon, D.; Enerback, C. Detection of psoriasin/S100A7 in the Sera of Patients with Psoriasis. Br. J. Dermatol. 2009, 160, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Broome, A.M.; Ryan, D.; Eckert, R.L. S100 Protein Subcellular Localization during Epidermal Differentiation and Psoriasis. J. Histochem. Cytochem. 2003, 51, 675–685. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, Anti-Inflammatory and Antiproliferative Effects of Delphinidin, a Dietary Anthocyanidin, in a Full-Thickness Three-Dimensional Reconstituted Human Skin Model of Psoriasis. Ski. Pharmacol. Physiol. 2015, 28, 177–188. [Google Scholar] [CrossRef]
- Becatti, M.; Barygina, V.; Mannucci, A.; Emmi, G.; Prisco, D.; Lotti, T.; Fiorillo, C.; Taddei, N. Sirt1 Protects Against Oxidative Stress-Induced Apoptosis in Fibroblasts from Psoriatic Patients: A New Insight into the Pathogenetic Mechanisms of Psoriasis. Int. J. Mol. Sci. 2018, 19, 1572. [Google Scholar] [CrossRef] [PubMed]
- Gegotek, A.; Domingues, P.; Wronski, A.; Skrzydlewska, E. Changes in Proteome of Fibroblasts Isolated from Psoriatic Skin Lesions. Int. J. Mol. Sci. 2020, 21, 5363. [Google Scholar] [CrossRef] [PubMed]
- Guban, B.; Vas, K.; Balog, Z.; Manczinger, M.; Bebes, A.; Groma, G.; Szell, M.; Kemeny, L.; Bata-Csorgo, Z. Abnormal Regulation of Fibronectin Production by Fibroblasts in Psoriasis. Br. J. Dermatol. 2016, 174, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Nicolaou, A. Bioactive Lipid Mediators in Skin Inflammation and Immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Luczaj, W.; Wronski, A.; Domingues, P.; Domingues, M.R.; Skrzydlewska, E. Lipidomic Analysis Reveals Specific Differences between Fibroblast and Keratinocyte Ceramide Profile of Patients with Psoriasis Vulgaris. Molecules 2020, 25, 630. [Google Scholar] [CrossRef]
- Miura, H.; Sano, S.; Higashiyama, M.; Yoshikawa, K.; Itami, S. Involvement of Insulin-Like Growth Factor-I in Psoriasis as a Paracrine Growth Factor: Dermal Fibroblasts Play a Regulatory Role in Developing Psoriatic Lesions. Arch. Dermatol. Res. 2000, 292, 590–597. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-kappaB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Nandal, S.; Dhir, A.; Kuhad, A.; Sharma, S.; Chopra, K. Curcumin Potentiates the Anti-Inflammatory Activity of Cyclooxygenase Inhibitors in the Cotton Pellet Granuloma Pouch Model. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 89–93. [Google Scholar]
- Rinaldi, A.L.; Morse, M.A.; Fields, H.W.; Rothas, D.A.; Pei, P.; Rodrigo, K.A.; Renner, R.J.; Mallery, S.R. Curcumin Activates the Aryl Hydrocarbon Receptor Yet significantly Inhibits (-)-Benzo(a)Pyrene-7R-Trans-7,8-Dihydrodiol Bioactivation in Oral Squamous Cell Carcinoma Cells and Oral Mucosa. Cancer Res. 2002, 62, 5451–5456. [Google Scholar] [PubMed]
- Vogel, C.F.; Matsumura, F. A New Cross-Talk between the Aryl Hydrocarbon Receptor and RelB, a Member of the NF-kappaB Family. Biochem. Pharmacol. 2009, 77, 734–745. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Zenz, R.; Wagner, E.F. Jun Signalling in the Epidermis: From Developmental Defects to Psoriasis and Skin Tumors. Int. J. Biochem. Cell Biol. 2006, 38, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.S.; Duculan, J.; Whynot, J.A.; Krueger, J.G. Increased JunB mRNA and Protein Expression in Psoriasis Vulgaris Lesions. J. Investig. Dermatol. 2006, 126, 912–914. [Google Scholar] [CrossRef][Green Version]
- Swindell, W.R.; Johnston, A.; Carbajal, S.; Han, G.; Wohn, C.; Lu, J.; Xing, X.; Nair, R.P.; Voorhees, J.J.; Elder, J.T.; et al. Genome-Wide Expression Profiling of Five Mouse Models Identifies Similarities and Differences with Human Psoriasis. PLoS ONE 2011, 6, e18266. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of Ketoconazole as an AhR-Nrf2 Activator in Cultured Human Keratinocytes: The Basis of its Anti-Inflammatory Effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef]
- Kokot, A.; Metze, D.; Mouchet, N.; Galibert, M.D.; Schiller, M.; Luger, T.A.; Bohm, M. Alpha-Melanocyte-Stimulating Hormone Counteracts the Suppressive Effect of UVB on Nrf2 and Nrf-Dependent Gene Expression in Human Skin. Endocrinology 2009, 150, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Ding, J.; Johnston, A.; Tejasvi, T.; Guzman, A.M.; Nair, R.P.; Voorhees, J.J.; Abecasis, G.R.; Elder, J.T. Assessment of the Psoriatic Transcriptome in a Large Sample: Additional Regulated Genes and Comparisons with in Vitro Models. J. Investig. Dermatol. 2010, 130, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Krueger, J.G.; Li, K.; Jabbari, A.; Brodmerkel, C.; Lowes, M.A.; Suarez-Farinas, M. Meta-Analysis Derived (MAD) Transcriptome of Psoriasis Defines the “Core” Pathogenesis of Disease. PLoS ONE 2012, 7, e44274. [Google Scholar] [CrossRef] [PubMed]
- Bracke, S.; Desmet, E.; Guerrero-Aspizua, S.; Tjabringa, S.G.; Schalkwijk, J.; Van Gele, M.; Carretero, M.; Lambert, J. Identifying Targets for Topical RNAi Therapeutics in Psoriasis: Assessment of a New in Vitro Psoriasis Model. Arch. Dermatol. Res. 2013, 305, 501–512. [Google Scholar] [CrossRef]
- Desmet, E.; Ramadhas, A.; Lambert, J.; Van Gele, M. In Vitro Psoriasis Models with Focus on Reconstructed Skin Models as Promising Tools in Psoriasis Research. Exp. Biol. Med. 2017, 242, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Leroy, M.; Duque-Fernandez, A.; Bernard, G.; Soucy, J.; Pouliot, R. Characterization of a Psoriatic Skin Model Produced with Involved Or Uninvolved Cells. J. Tissue Eng. Regen. Med. 2015, 9, 789–798. [Google Scholar] [CrossRef]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.F.; Guillet, G.; Huguier, V.; Lecron, J.C.; et al. Inhibition of Keratinocyte Differentiation by the Synergistic Effect of IL-17A, IL-22, IL-1alpha, TNFalpha and Oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Schonthaler, H.B.; Guinea-Viniegra, J.; Tschachler, E. Psoriasis: What we have Learned from Mouse Models. Nat. Rev. Rheumatol. 2010, 6, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Pouliot-Berube, C.; Zaniolo, K.; Guerin, S.L.; Pouliot, R. Tissue-Engineered Human Psoriatic Skin Supplemented with Cytokines as an in Vitro Model to Study Plaque Psoriasis. Regen. Med. 2016, 11, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Flori, E.; Maresca, V.; Ottaviani, M.; Aspite, N.; Dell’Anna, M.L.; Panzella, L.; Napolitano, A.; Picardo, M.; d’Ischia, M. The Eumelanin Intermediate 5,6-Dihydroxyindole-2-Carboxylic Acid is a Messenger in the Cross-Talk among Epidermal Cells. J. Investig. Dermatol. 2012, 132, 1196–1205. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Seltmann, H.; Neitzel, H.; Orfanos, C.E. Establishment and Characterization of an Immortalized Human Sebaceous Gland Cell Line (SZ95). J. Investig. Dermatol. 1999, 113, 1011–1020. [Google Scholar] [CrossRef]
- Smits, J.P.H.; Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.M.J.J.; van de Zande, G.W.H.J.F.; Zeeuwen, P.L.J.M.; Schalkwijk, J.; van den Bogaard, E.H. Immortalized N/TERT Keratinocytes as an Alternative Cell Source in 3D Human Epidermal Models. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinali, G.; Flori, E.; Mastrofrancesco, A.; Mosca, S.; Ottaviani, M.; Dell’Anna, M.L.; Truglio, M.; Vento, A.; Zaccarini, M.; Zouboulis, C.C.; et al. Anti-Inflammatory and Pro-Differentiating Properties of the Aryl Hydrocarbon Receptor Ligands NPD-0614-13 and NPD-0614-24: Potential Therapeutic Benefits in Psoriasis. Int. J. Mol. Sci. 2021, 22, 7501. https://doi.org/10.3390/ijms22147501
Cardinali G, Flori E, Mastrofrancesco A, Mosca S, Ottaviani M, Dell’Anna ML, Truglio M, Vento A, Zaccarini M, Zouboulis CC, et al. Anti-Inflammatory and Pro-Differentiating Properties of the Aryl Hydrocarbon Receptor Ligands NPD-0614-13 and NPD-0614-24: Potential Therapeutic Benefits in Psoriasis. International Journal of Molecular Sciences. 2021; 22(14):7501. https://doi.org/10.3390/ijms22147501
Chicago/Turabian StyleCardinali, Giorgia, Enrica Flori, Arianna Mastrofrancesco, Sarah Mosca, Monica Ottaviani, Maria Lucia Dell’Anna, Mauro Truglio, Antonella Vento, Marco Zaccarini, Christos C. Zouboulis, and et al. 2021. "Anti-Inflammatory and Pro-Differentiating Properties of the Aryl Hydrocarbon Receptor Ligands NPD-0614-13 and NPD-0614-24: Potential Therapeutic Benefits in Psoriasis" International Journal of Molecular Sciences 22, no. 14: 7501. https://doi.org/10.3390/ijms22147501
APA StyleCardinali, G., Flori, E., Mastrofrancesco, A., Mosca, S., Ottaviani, M., Dell’Anna, M. L., Truglio, M., Vento, A., Zaccarini, M., Zouboulis, C. C., & Picardo, M. (2021). Anti-Inflammatory and Pro-Differentiating Properties of the Aryl Hydrocarbon Receptor Ligands NPD-0614-13 and NPD-0614-24: Potential Therapeutic Benefits in Psoriasis. International Journal of Molecular Sciences, 22(14), 7501. https://doi.org/10.3390/ijms22147501






