Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida
Abstract
:1. Introduction
2. Results
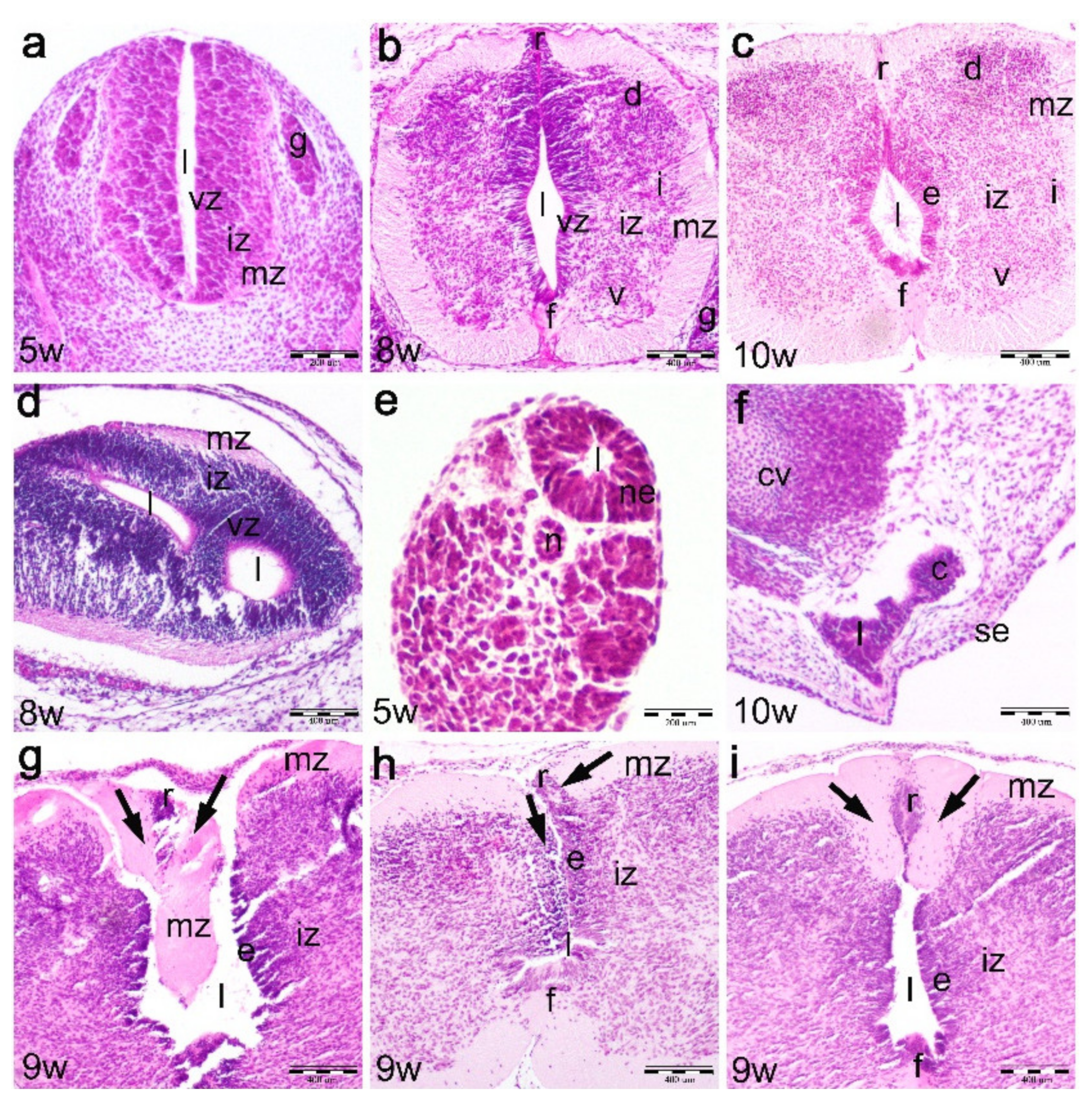
2.1. Normal Development of the Human Spinal Cord between the 5th and 10th Developmental Week (Hematoxylin and Eosin Staining)
2.2. Development of the Spinal Cord in the 9th Week Dysraphic Fetus (Hematoxylin and Eosin Staining)
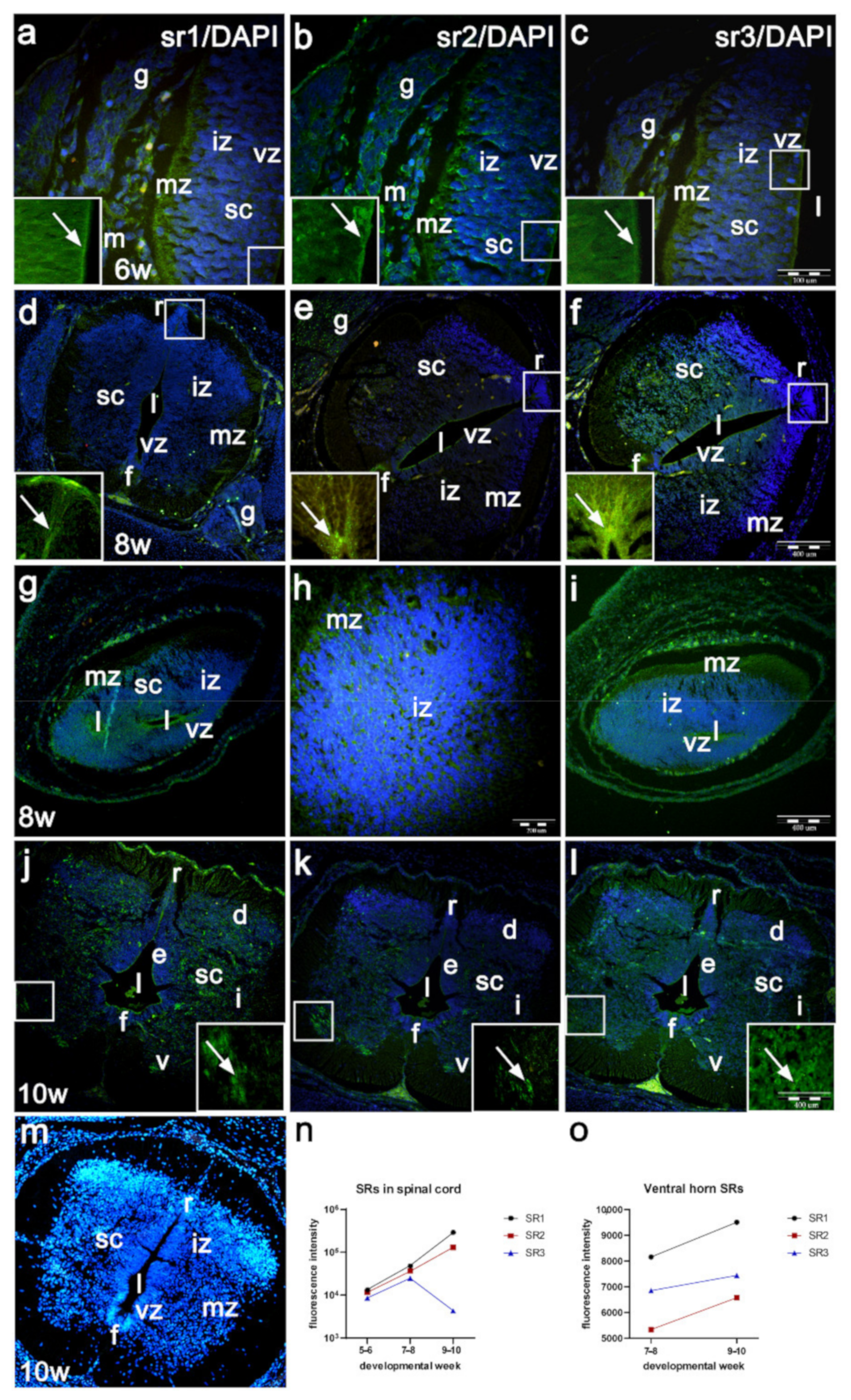
2.3. Expression of Serotonin Receptors (sr1, sr2 sr3) in the Developing Human Spinal Cord (Immunofluorescence Staining)
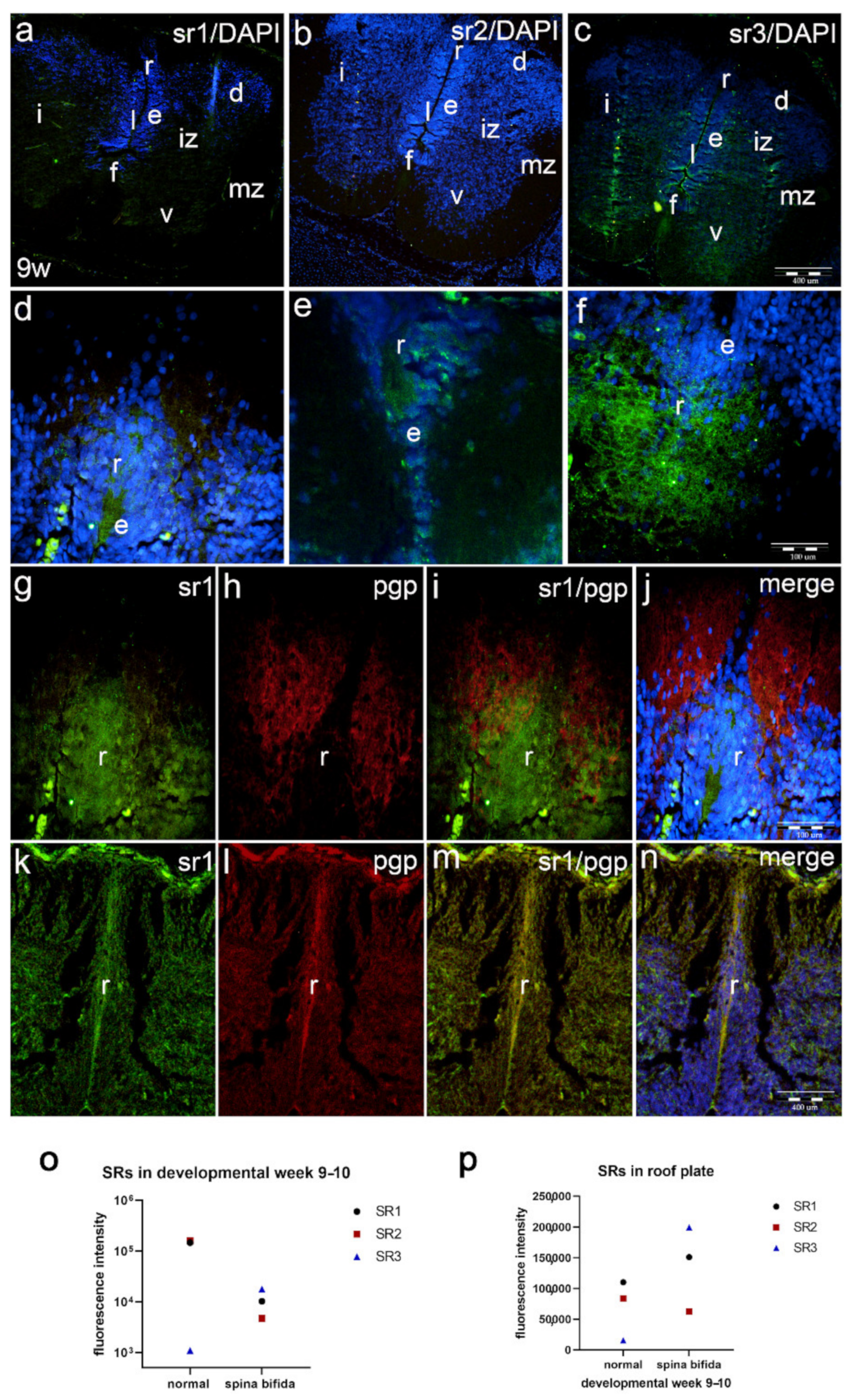
2.4. Co-Expression of Serotonin Receptors with Neuronal Marker (pgp) and Glial Cell Marker (GFAP) (Double Immunofluorescence Staining)
2.5. Expression of Serotonin Receptors in the Parts of the Spinal Cord Caudal to Dysraphic Cervical Region in Human Fetus with Cervical Spina Bifida
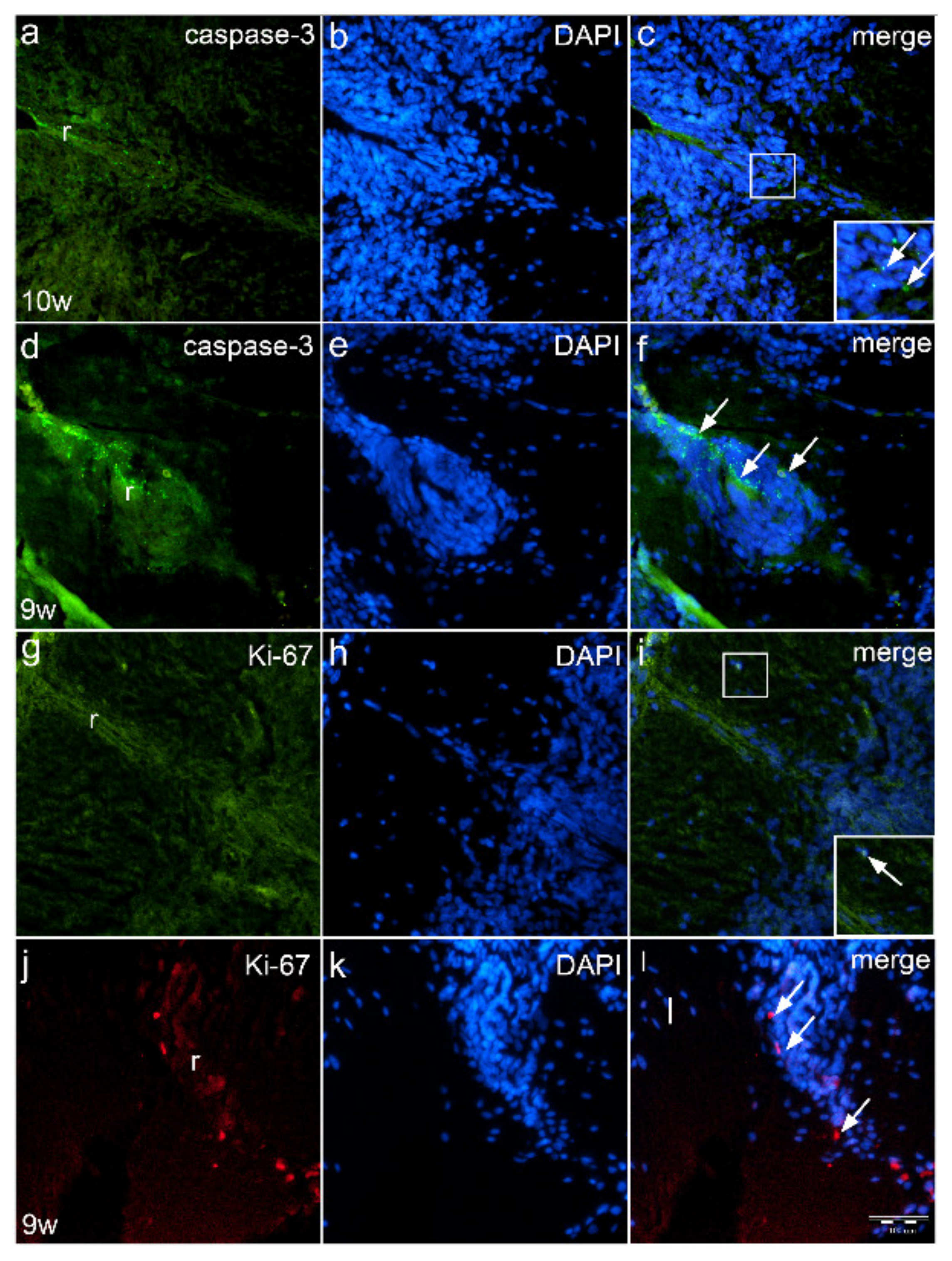
2.6. Expression of Proliferation Marker Ki-67 and Apoptotic Marker Caspase-3 in the Roof Plate Area of Normal Fetus and Fetus with Spina Bifida (Immunofluorescence Staining)
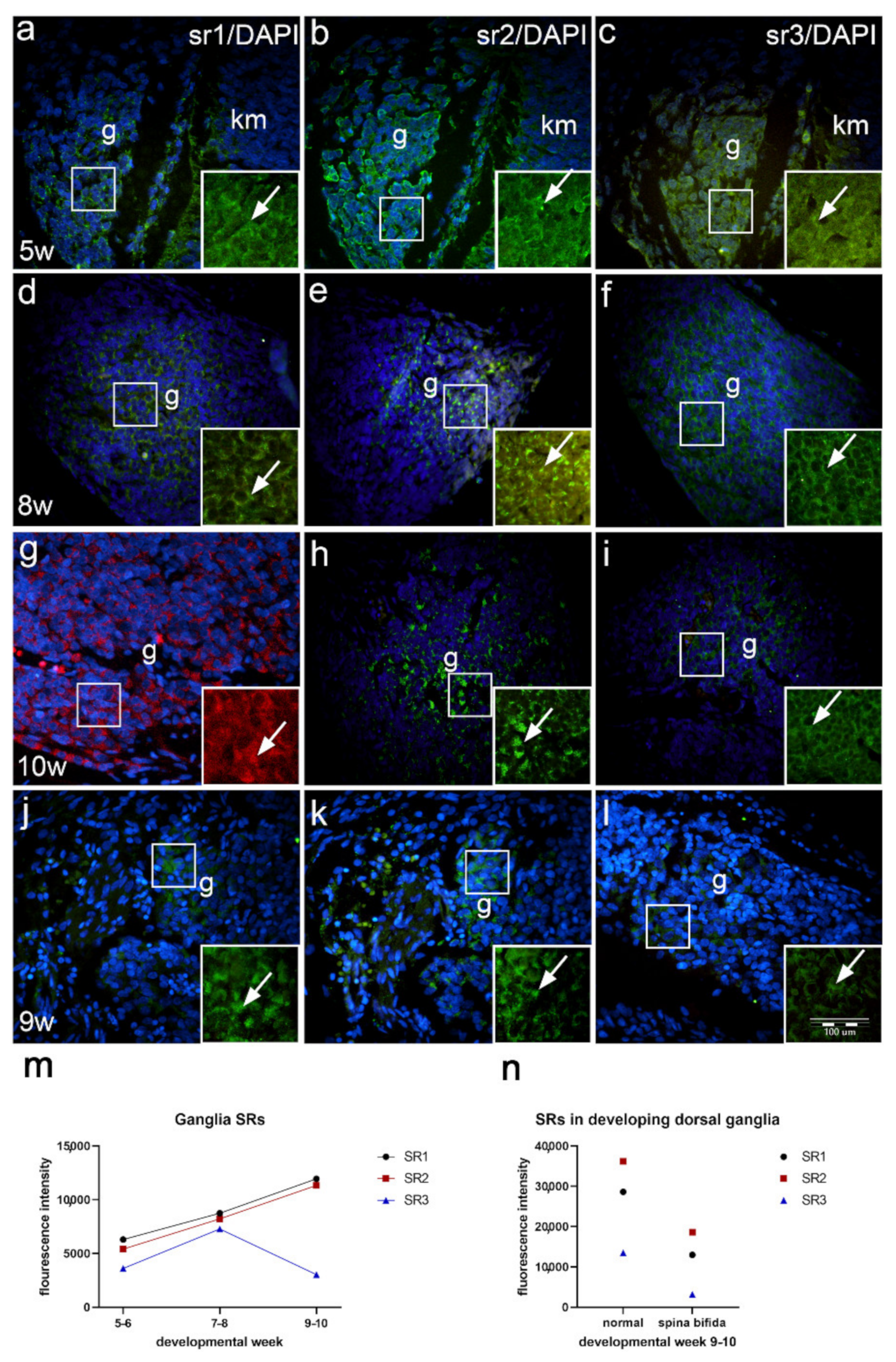
2.7. Expression of Serotonin Receptors in the Developing Dorsal Root Ganglia of Normal Fetuses and in Fetus with Cervical Spina Bifida (Immunofluorescence Staining)
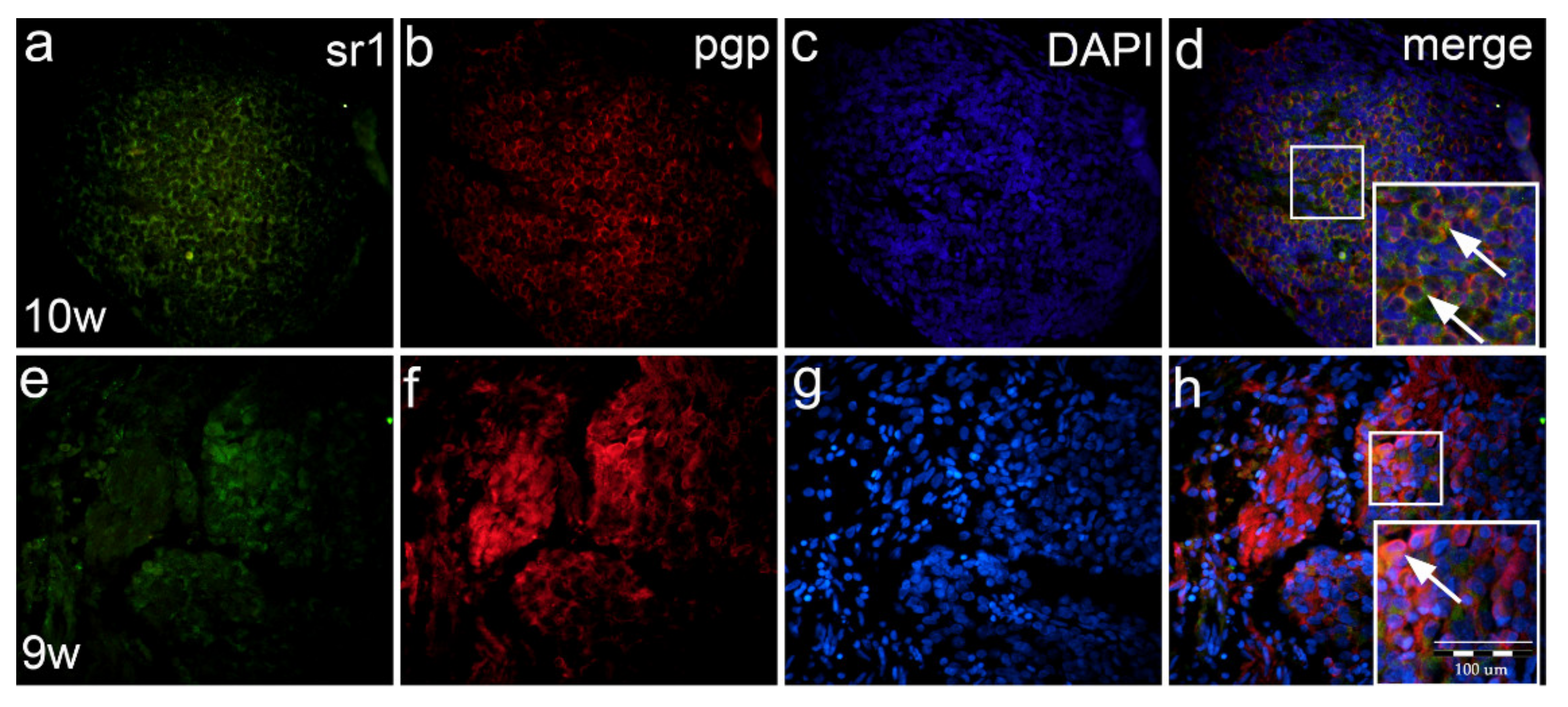
2.8. Co-Expression of Serotonin Markers with pgp in Normal and Malformed 9–10-Week Human Fetus (Double Immunofluorescence Staining)
3. Discussion
3.1. Development of Serotonergic Receptors during Early Development of the Human Spinal Cord
3.2. Expression of Serotonin Receptors in Malformed Human Fetus with Cervical Spina Bifida
3.3. Expression of Serotonin Receptors in Developing Spinal Ganglia and Ganglia of Fetus with Spina Bifida
4. Materials and Methods
4.1. Human Samples
4.2. Hematoxylin and Eosin
4.3. Immunohistochemistry and Immunofluorescence Staining
4.4. Semi-Quantification
4.5. Statistics and Microphotograph Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, M.J.T.; Fitzgerald, M. Spinal Cord. In Human Embryology; Saunders, W.B., Ed.; Bailliere Tindall: London, UK, 1994; pp. 55–58. [Google Scholar]
- Griffith, C.M.; Wiley, M.J.; Sanders, E.J. The vertebrate tail bud: Three germ layers from one tissue. Anat. Embryol. 1992, 185, 101–113. [Google Scholar] [CrossRef]
- Nievelstein, R.A.; Hartwig, N.G.; Vermeij-Keers, C.; Valk, J. Embryonic development of the mammalian caudal neural tube. Teratology 1993, 48, 21–31. [Google Scholar] [CrossRef]
- Saraga-Babic, M.; Sapunar, D.; Wartiovaara, J. Variations in the formation of the human caudal spinal cord. J. Hirnforsch. 1995, 36, 341–347. [Google Scholar] [PubMed]
- Saraga-Babic, M.; Krolo, M.; Sapunar, D.; Terzic, J.; Biocic, M. Differences in origin and fate between the cranial and caudal spinal cord during normal and disturbed human development. Acta Neuropathol. 1996, 91, 194–199. [Google Scholar] [CrossRef]
- Sapunar, D.; Vilovic, K.; England, M.; Saraga-Babic, M. Morphological diversity of dying cells during regression of the human tail. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2001, 183, 217–222. [Google Scholar] [CrossRef]
- Fallon, J.F.; Simandl, B.K. Evidence of a role for cell death in the disappearance of the embryonic human tail. Am. J. Anat. 1978, 152, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Vukojevic, K.; Skobic, H.; Saraga-Babic, M. Proliferation and differentiation of glial and neuronal progenitors in the development of human spinal ganglia. Differ. Res. Biol. Divers. 2009, 78, 91–98. [Google Scholar] [CrossRef]
- Vukojevic, K.; Filipovic, N.; Tica Sedlar, I.; Restovic, I.; Bocina, I.; Pintaric, I.; Saraga-Babic, M. Neuronal differentiation in the developing human spinal ganglia. Anat. Rec. 2016, 299, 1060–1072. [Google Scholar] [CrossRef]
- Masliukov, P.M.; Porseva, V.V.; Korzina, M.V.; Nozdrachev, A.D. Neurochemical properties of sensory neurons in the development. Ross. Fiziol. Zhurnal Im. I.M. Sechenova 2013, 99, 777–792. [Google Scholar]
- Yew, D.T.; Chan, W.Y.; Luo, C.B.; Zheng, D.R.; Yu, M.C. Neurotransmitters and neuropeptides in the developing human central nervous system. A review. Biol. Signals Recept. 1999, 8, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.S.; Tsui, T.Y.; Lam, J.C.; Wai, M.S.; Chan, W.M.; Yew, D.T. Serotonin and its receptors in the human CNS with new findings—A mini review. Curr. Med. Chem. 2011, 18, 5281–5288. [Google Scholar] [CrossRef]
- Whitaker-Azmitia, P.M.; Clarke, C.; Azmitia, E.C. Localization of 5-HT1A receptors to astroglial cells in adult rats: Implications for neuronal-glial interactions and psychoactive drug mechanism of action. Synapse 1993, 14, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Medullary serotonergic system: Organization, effects, and clinical correlations. Neurology 2014, 83, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, T.; Schwartz, J.P. Somatostatin enhances neurofilament expression and neurite outgrowth in cultured rat cerebellar granule cells. Brain Res. Dev. Brain Res. 1995, 88, 109–116. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Q.Y.; Jung, D.; Manos, P.; Pancrazio, J.J.; Schaffner, A.E.; Barker, J.L.; Stenger, D.A. Central neuronal synapse formation on micropatterned surfaces. Brain Res. Dev. Brain Res. 1998, 111, 231–243. [Google Scholar] [CrossRef]
- Festoff, B.W.; Nelson, P.G.; Brenneman, D.E. Prevention of activity-dependent neuronal death: Vasoactive intestinal polypeptide stimulates astrocytes to secrete the thrombin-inhibiting neurotrophic serpin, protease nexin I. J. Neurobiol. 1996, 30, 255–266. [Google Scholar] [CrossRef]
- Gimenez y Ribotta, M.; Privat, A. Biological interventions for spinal cord injury. Curr. Opin. Neurol. 1998, 11, 647–654. [Google Scholar] [CrossRef]
- Gimenez y Ribotta, M.; Sandillon, F.; Privat, A. Influence of hypergravity on the development of monoaminergic systems in the rat spinal cord. Brain Res. Dev. Brain Res. 1998, 111, 147–157. [Google Scholar] [CrossRef]
- Hains, B.C.; Fullwood, S.D.; Eaton, M.J.; Hulsebosch, C.E. Subdural engraftment of serotonergic neurons following spinal hemisection restores spinal serotonin, downregulates serotonin transporter, and increases BDNF tissue content in rat. Brain Res. 2001, 913, 35–46. [Google Scholar] [CrossRef]
- Yakovleff, A.; Cabelguen, J.M.; Orsal, D.; Gimenez y Ribotta, M.; Rajaofetra, N.; Drian, M.J.; Bussel, B.; Privat, A. Fictive motor activities in adult chronic spinal rats transplanted with embryonic brainstem neurons. Exp. Brain Res. 1995, 106, 69–78. [Google Scholar] [CrossRef]
- Perrin, F.E.; Noristani, H.N. Serotonergic mechanisms in spinal cord injury. Exp. Neurol. 2019, 318, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Perrier, J.F.; Cotel, F. Serotonergic modulation of spinal motor control. Curr. Opin. Neurobiol. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Okaty, B.W.; Freret, M.E.; Rood, B.D.; Brust, R.D.; Hennessy, M.L.; de Bairos, D.; Kim, J.C.; Cook, M.N.; Dymecki, S.M. Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron 2015, 88, 774–791. [Google Scholar] [CrossRef] [Green Version]
- Middlemiss, D.N.; Palmer, A.M.; Edel, N.; Bowen, D.M. Binding of the novel serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin in normal and Alzheimer brain. J. Neurochem. 1986, 46, 993–996. [Google Scholar] [CrossRef]
- Luo, C.B.; Zheng, D.R.; Guan, Y.L.; Shen, W.Z.; Liu, Y.G.; Yew, D.T. Localization of acetylcholinesterase positive neurons and substance P and enkephalin positive fibers by histochemistry and immunohistochemistry in the sympathetic intermediate zone of the developing human spinal cord. Neuroscience 1990, 39, 97–102. [Google Scholar] [CrossRef]
- Wai, M.S.; Lorke, D.E.; Kwong, W.H.; Zhang, L.; Yew, D.T. Profiles of serotonin receptors in the developing human thalamus. Psychiatry Res. 2011, 185, 238–242. [Google Scholar] [CrossRef]
- Hayashi, Y.; Jacob-Vadakot, S.; Dugan, E.A.; McBride, S.; Olexa, R.; Simansky, K.; Murray, M.; Shumsky, J.S. 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp. Neurol. 2010, 221, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Holmes, G.M.; Van Meter, M.J.; Beattie, M.S.; Bresnahan, J.C. Serotonergic fiber sprouting to external anal sphincter motoneurons after spinal cord contusion. Exp. Neurol. 2005, 193, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Strittmatter, S.M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 4219–4227. [Google Scholar] [CrossRef]
- Wang, X.; Duffy, P.; McGee, A.W.; Hasan, O.; Gould, G.; Tu, N.; Harel, N.Y.; Huang, Y.; Carson, R.E.; Weinzimmer, D.; et al. Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann. Neurol. 2011, 70, 805–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirokawa, T.; Zou, Y.; Kurihara, Y.; Jiang, Z.; Sakakibara, Y.; Ito, H.; Funakoshi, K.; Kawahara, N.; Goshima, Y.; Strittmatter, S.M.; et al. Regulation of axonal regeneration by the level of function of the endogenous Nogo receptor antagonist LOTUS. Sci. Rep. 2017, 7, 12119. [Google Scholar] [CrossRef]
- Kim, J.E.; Liu, B.P.; Park, J.H.; Strittmatter, S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 2004, 44, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Camand, E.; Morel, M.P.; Faissner, A.; Sotelo, C.; Dusart, I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur. J. Neurosci. 2004, 20, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Chapter 11 Nervous System. In Human Embryology and Developmental Biology; Elsevier/Saunders: Philadelphia, PA, USA, 2014; pp. 233–237. [Google Scholar]
- Rajaofetra, N.; Passagia, J.G.; Marlier, L.; Poulat, P.; Pellas, F.; Sandillon, F.; Verschuere, B.; Gouy, D.; Geffard, M.; Privat, A. Serotoninergic, noradrenergic, and peptidergic innervation of Onuf’s nucleus of normal and transected spinal cords of baboons (Papio papio). J. Comp. Neurol. 1992, 318, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Privat, A.; Mansour, H.; Geffard, M. Transplantation of fetal serotonin neurons into the transected spinal cord of adult rats: Morphological development and functional influence. Prog. Brain Res. 1988, 78, 155–166. [Google Scholar] [CrossRef]
- Privat, A.; Mansour, H.; Rajaofetra, N.; Geffard, M. Intraspinal transplants of serotonergic neurons in the adult rat. Brain Res. Bull. 1989, 22, 123–129. [Google Scholar] [CrossRef]
- Brumovsky, P.R. Dorsal root ganglion neurons and tyrosine hydroxylase--an intriguing association with implications for sensation and pain. Pain 2016, 157, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, R.; Small, J.; Dixon, A.K.; Spanswick, D.; Lee, K. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci. Lett. 2003, 337, 119–122. [Google Scholar] [CrossRef]
- Maeshima, T.; Ito, R.; Hamada, S.; Senzaki, K.; Hamaguchi-Hamada, K.; Shutoh, F.; Okado, N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res. 1998, 797, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Juric, M.; Zeitler, J.; Vukojevic, K.; Bocina, I.; Grobe, M.; Kretzschmar, G.; Saraga-Babic, M.; Filipovic, N. Expression of Connexins 37, 43 and 45 in Developing Human Spinal Cord and Ganglia. Int. J. Mol. Sci. 2020, 21, 9356. [Google Scholar] [CrossRef]
- Vukojevic, K.; Raguz, F.; Saraga, M.; Filipovic, N.; Bocina, I.; Kero, D.; Glavina Durdov, M.; Martinovic, V.; Saraga-Babic, M. Glomeruli from patients with nephrin mutations show increased number of ciliated and poorly differentiated podocytes. Acta Histochem. 2018, 120, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kosovic, I.; Filipovic, N.; Benzon, B.; Bocina, I.; Glavina Durdov, M.; Vukojevic, K.; Saraga, M.; Saraga-Babic, M. Connexin Signaling in the Juxtaglomerular Apparatus (JGA) of Developing, Postnatal Healthy and Nephrotic Human Kidneys. Int. J. Mol. Sci. 2020, 21, 8349. [Google Scholar] [CrossRef] [PubMed]

| Developmental Weeks | Spinal Cord Parts | sr1 | sr2 | sr3 |
|---|---|---|---|---|
| 5 | vz | +/++ | +/++ | +/− |
| iz | +/++ | +/++ | +/− | |
| mz | +/− | +/++ | +/− | |
| 6 | vz | +/++ | +/++ | + |
| iz | ++ | +/++ | + | |
| mz | + | + | +/− | |
| rp | +/++ | ++ | +/++ | |
| fp | +/++ | ++ | +/++ | |
| 7 and 8 | vz | +/++ | +/++ | ++/+++ |
| iz–vh | ++ | + | ++ | |
| iz–ih | + | + | + | |
| iz–dh | + | + | + | |
| mz | + | + | + | |
| rp | ++ | ++/+++ | +++ | |
| fp | ++ | ++/+++ | +++ | |
| 9 and 10 | el | ++ | ++ | ++ |
| iz–vh | +++ | ++/+++ | ++ | |
| iz–ih | +++ | ++/+++ | ++ | |
| iz–dh | +++ | ++ | ++ | |
| mz | + | + | + | |
| rp | ++ | ++ | ++ | |
| fp | ++ | ++ | ++ |
| Developmental Weeks | sr1 | sr2 | sr3 |
|---|---|---|---|
| 5–6 | + | ++ | +/− |
| 7–8 | ++ | ++/+++ | +/++ |
| 9–10 | ++ | ++/+++ | ++ |
| 9 (cervical spina bifida) | +/++ | ++ | + |
| Antibody | Code No. | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-5HT1A Receptor antibody | ab227165 | Rabbit | 1:100 | Abcam |
| SR-2A (A-4) | sc-166775 | Mouse | 1:100 | Santa Cruz Biotechnology Inc. | |
| 5-HT3A receptor antibody | GTX54151 | Rabbit | 1:100 | GeneTex | |
| PGP9.5 Monoclonal Antibody (BH7) | 480012 | Mouse | 1:500 | Invitrogen | |
| anti-GFAP antibody | ab53554 | Goat | 1:100 | Abcam | |
| anti-GFAP (2E1) | sc-33673 | Mouse | 1:50 | Santa Cruz Biotechnology Inc. | |
| anti-Ki-67 | M7240 | Mouse | 1:100 | DAKO | |
| Caspase-3 antibody | AF835 | Rabbit | 1:1500 | R&D System, Inc. | |
| Secondary | Alexa Fluor®488 AffiniPure Anti-Mouse lgG (H+L) | 715-545-150 | Donkey | 1:400 | Jackson Immuno Research Laboratories |
| Alexa Fluor®488 AffiniPure Anti-Goat lgG (H+L) | 705-545-003 | Donkey | 1:400 | Jackson Immuno Research Laboratories | |
| Alexa Fluor®488 AffiniPure Anti-Rabbit lgG (H+L) | 711-545-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories | |
| Rhodamine Red™-X (RRX) AffiniPure Anti-Goat IgG (H+L) | 705-295-003 | Donkey | 1:400 | Jackson Immuno Research Laboratories | |
| Rhodamine Red™-X (RRX) AffiniPure Anti-Mouse IgG (H+L) | 715-295-151 | Donkey | 1:400 | Jackson Immuno Research Laboratories |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punda, H.; Mardesic, S.; Filipovic, N.; Kosovic, I.; Benzon, B.; Ogorevc, M.; Bocina, I.; Kolic, K.; Vukojevic, K.; Saraga-Babic, M. Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida. Int. J. Mol. Sci. 2021, 22, 7320. https://doi.org/10.3390/ijms22147320
Punda H, Mardesic S, Filipovic N, Kosovic I, Benzon B, Ogorevc M, Bocina I, Kolic K, Vukojevic K, Saraga-Babic M. Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida. International Journal of Molecular Sciences. 2021; 22(14):7320. https://doi.org/10.3390/ijms22147320
Chicago/Turabian StylePunda, Hrvoje, Snjezana Mardesic, Natalija Filipovic, Ivona Kosovic, Benjamin Benzon, Marin Ogorevc, Ivana Bocina, Kresimir Kolic, Katarina Vukojevic, and Mirna Saraga-Babic. 2021. "Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida" International Journal of Molecular Sciences 22, no. 14: 7320. https://doi.org/10.3390/ijms22147320
APA StylePunda, H., Mardesic, S., Filipovic, N., Kosovic, I., Benzon, B., Ogorevc, M., Bocina, I., Kolic, K., Vukojevic, K., & Saraga-Babic, M. (2021). Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida. International Journal of Molecular Sciences, 22(14), 7320. https://doi.org/10.3390/ijms22147320







