The Cellular Choreography of Osteoblast Angiotropism in Bone Development and Homeostasis
Abstract
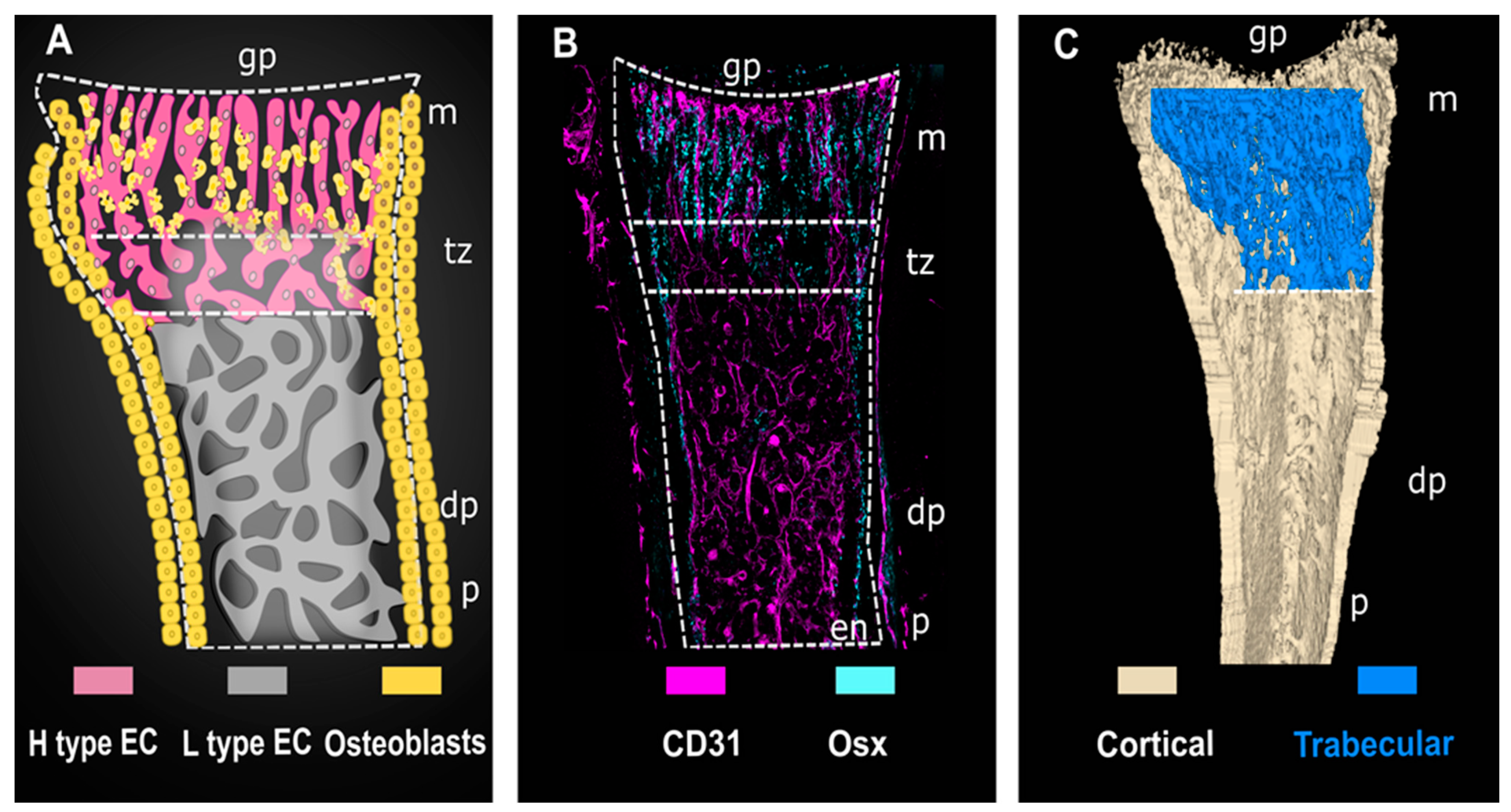
1. The Function, Anatomy, and Cellular Overview of the Skeletal System
2. Recent Advances in Bone Endothelial Cell Diversity
3. Bidirectional Cross Talk between Endothelial Cell and Osteoblast Regulates Bone Formation during Embryogenesis and Development
4. Osteoblast Differentiation and Angiotropism Control Bone Healing in Mature Tissues
5. The Extent of Osteocyte-Endothelial Cell Cross Talk Remains an Important, and Outstanding, Area for Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Kelly, N.H.; Schimenti, J.C.; Ross, F.P.; van der Meulen, M.C. Transcriptional profiling of cortical versus cancellous bone from mechanically-loaded murine tibiae reveals differential gene expression. Bone 2016, 86, 22–29. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone quality--the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef]
- Romeo, S.G.; Alawi, K.M.; Rodrigues, J.; Singh, A.; Kusumbe, A.P.; Ramasamy, S.K. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 2019, 21, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, K.; Qi, W. Osteoclast and its roles in calcium metabolism and bone development and remodeling. Biochem. Biophys. Res. Commun. 2006, 343, 345–350. [Google Scholar] [CrossRef]
- Rauch, A.; Mandrup, S. Transcriptional networks controlling stromal cell differentiation. Nat. Rev. Mol. Cell Biol. 2021, 22, 465–482. [Google Scholar] [CrossRef]
- Wolock, S.L.; Krishnan, I.; Tenen, D.E.; Matkins, V.; Camacho, V.; Patel, S.; Agarwal, P.; Bhatia, R.; Tenen, D.G.; Klein, A.M.; et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019, 28, 302–311.e305. [Google Scholar] [CrossRef] [PubMed]
- dela Paz, N.G.; D’Amore, P.A. Arterial versus venous endothelial cells. Cell Tissue Res. 2009, 335, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Itkin, T.; Gur-Cohen, S.; Lapidot, T.; Adams, R.H. Regulation of Hematopoiesis and Osteogenesis by Blood Vessel-Derived Signals. Annu. Rev. Cell Dev. Biol. 2016, 32, 649–675. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Schiller, M.; Zeuschner, D.; Bixel, M.G.; Milia, C.; Gamrekelashvili, J.; Limbourg, A.; Medvinsky, A.; Santoro, M.M.; et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 2016, 7, 13601. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; He, Y.; Li, Y.; He, X. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem Cell Res. Ther. 2020, 11, 537. [Google Scholar] [CrossRef]
- Stegen, S.; Carmeliet, G. The skeletal vascular system—Breathing life into bone tissue. Bone 2018, 115, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Pitulescu, M.E.; Kim, J.M.; Enriquez-Gasca, R.; Sivaraj, K.K.; Kusumbe, A.P.; Singh, A.; Di Russo, J.; Bixel, M.G.; Zhou, B.; et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat. Cell Biol. 2017, 19, 189–201. [Google Scholar] [CrossRef]
- Itkin, T.; Gur-Cohen, S.; Spencer, J.A.; Schajnovitz, A.; Ramasamy, S.K.; Kusumbe, A.P.; Ledergor, G.; Jung, Y.; Milo, I.; Poulos, M.G.; et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016, 532, 323–328. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, F.; Zhang, P.; Wang, H.; Qu, Z.; Jia, P.; Yao, Z.; Shen, G.; Li, G.; Zhao, G.; et al. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017, 8, e2760. [Google Scholar] [CrossRef]
- Kuhn, A.; Brachtendorf, G.; Kurth, F.; Sonntag, M.; Samulowitz, U.; Metze, D.; Vestweber, D. Expression of endomucin, a novel endothelial sialomucin, in normal and diseased human skin. J. Investig. Dermatol. 2002, 119, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.J.; Albelda, S.M. Cellular and molecular aspects of PECAM-1. Nouv. Rev. Fr. Hematol. 1992, 34, S9–S13. [Google Scholar]
- Yan, Z.Q.; Wang, X.K.; Zhou, Y.; Wang, Z.G.; Wang, Z.X.; Jin, L.; Yin, H.; Xia, K.; Tan, Y.J.; Feng, S.K.; et al. H-type blood vessels participate in alveolar bone remodeling during murine tooth extraction healing. Oral Dis. 2020, 26, 998–1009. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Itkin, T.; Mäe, M.A.; Langen, U.H.; Betsholtz, C.; Lapidot, T.; Adams, R.H. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 2016, 532, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, F.; Yang, Y.; Zhou, X.; Cheng, Y.; Wei, X.; Li, M. LIPUS promotes spinal fusion coupling proliferation of type H microvessels in bone. Sci. Rep. 2016, 6, 20116. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.J.; Xiao, Y.; Guo, Q.; Huang, Y.; Su, T.; Luo, X.H.; Jiang, T.J. Ophiopogonin D promotes bone regeneration by stimulating CD31(hi) EMCN(hi) vessel formation. Cell Prolif. 2020, 53, e12784. [Google Scholar] [CrossRef]
- Zhu, Y.; Ruan, Z.; Lin, Z.; Long, H.; Zhao, R.; Sun, B.; Cheng, L.; Tang, L.; Xia, Z.; Li, C.; et al. The association between CD31(hi)Emcn(hi) endothelial cells and bone mineral density in Chinese women. J. Bone Miner. Metab. 2019, 37, 987–995. [Google Scholar] [CrossRef]
- Böhm, A.M.; Dirckx, N.; Tower, R.J.; Peredo, N.; Vanuytven, S.; Theunis, K.; Nefyodova, E.; Cardoen, R.; Lindner, V.; Voet, T.; et al. Activation of Skeletal Stem and Progenitor Cells for Bone Regeneration Is Driven by PDGFRβ Signaling. Dev. Cell 2019, 51, 236–254.e212. [Google Scholar] [CrossRef]
- Grüneboom, A.; Hawwari, I.; Weidner, D.; Culemann, S.; Müller, S.; Henneberg, S.; Brenzel, A.; Merz, S.; Bornemann, L.; Zec, K.; et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 2019, 1, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Grüneboom, A.; Kling, L.; Christiansen, S.; Mill, L.; Maier, A.; Engelke, K.; Quick, H.H.; Schett, G.; Gunzer, M. Next-generation imaging of the skeletal system and its blood supply. Nat. Rev. Rheumatol. 2019, 15, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The skeleton: A multi-functional complex organ: The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334. [Google Scholar] [CrossRef]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Jonitz, A.; Lochner, K.; Lindner, T.; Hansmann, D.; Marrot, A.; Bader, R. Oxygen consumption, acidification and migration capacity of human primary osteoblasts within a three-dimensional tantalum scaffold. J. Mater. Sci. Mater. Med. 2011, 22, 2089–2095. [Google Scholar] [CrossRef]
- Burke, D.; Dishowitz, M.; Sweetwyne, M.; Miedel, E.; Hankenson, K.D.; Kelly, D.J. The role of oxygen as a regulator of stem cell fate during fracture repair in TSP2-null mice. J. Orthop. Res. 2013, 31, 1585–1596. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chen, C.T.; Wei, Y.H. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2013, 31, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, M.; Eliseev, R.A.; Jonason, J.H.; Mills, B.N.; O’Keefe, R.J. PGE2 Receptor Subtype 1 (EP1) Regulates Mesenchymal Stromal Cell Osteogenic Differentiation by Modulating Cellular Energy Metabolism. J. Cell Biochem. 2017, 118, 4383–4393. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Zhou, Z.; Xing, Y.; Zhong, Y.; Zou, X.; Tian, W.; Zhang, C. Synergistic inhibition of Wnt pathway by HIF-1α and osteoblast-specific transcription factor osterix (Osx) in osteoblasts. PLoS ONE 2012, 7, e52948. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhou, Z.; Wu, C.; Xing, Y.; Zou, X.; Tian, W.; Zhang, C. HIF-1α inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS ONE 2013, 8, e65940. [Google Scholar] [CrossRef] [PubMed]
- van Gastel, N.; Carmeliet, G. Metabolic regulation of skeletal cell fate and function in physiology and disease. Nat. Metab. 2021, 3, 11–20. [Google Scholar] [CrossRef]
- van Gastel, N.; Stegen, S.; Eelen, G.; Schoors, S.; Carlier, A.; Daniëls, V.W.; Baryawno, N.; Przybylski, D.; Depypere, M.; Stiers, P.J.; et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 2020, 579, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, H.; Khan, M.T.; Sharan, K. Hyperglycemia impairs osteoblast cell migration and chemotaxis due to a decrease in mitochondrial biogenesis. Mol. Cell Biochem. 2020, 469, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef]
- Maes, C.; Carmeliet, P.; Moermans, K.; Stockmans, I.; Smets, N.; Collen, D.; Bouillon, R.; Carmeliet, G. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech. Dev. 2002, 111, 61–73. [Google Scholar] [CrossRef]
- Maes, C.; Goossens, S.; Bartunkova, S.; Drogat, B.; Coenegrachts, L.; Stockmans, I.; Moermans, K.; Nyabi, O.; Haigh, K.; Naessens, M.; et al. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 2010, 29, 424–441. [Google Scholar] [CrossRef]
- Gerber, H.P.; Ferrara, N. Angiogenesis and bone growth. Trends Cardiovasc. Med. 2000, 10, 223–228. [Google Scholar] [CrossRef]
- Fruttiger, M. Development of the mouse retinal vasculature: Angiogenesis versus vasculogenesis. Investig. Ophthalmol. Vis. Sci. 2002, 43, 522–527. [Google Scholar]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Bicknell, R. Angiogenic signalling pathways. Methods Mol. Biol. 2009, 467, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Herbert, S.P.; Stainier, D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol 2011, 12, 551–564. [Google Scholar] [CrossRef]
- Sivan, U.; De Angelis, J.; Kusumbe, A.P. Role of angiocrine signals in bone development, homeostasis and disease. Open Biol. 2019, 9, 190144. [Google Scholar] [CrossRef]
- Wrotnowski, U.; Mills, S.E.; Cooper, P.H. Malignant angioendotheliomatosis. An angiotropic lymphoma? Am. J. Clin. Pathol. 1985, 83, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Clarkin, C.E.; Emery, R.J.; Pitsillides, A.A.; Wheeler-Jones, C.P. Evaluation of VEGF-mediated signaling in primary human cells reveals a paracrine action for VEGF in osteoblast-mediated crosstalk to endothelial cells. J. Cell Physiol. 2008, 214, 537–544. [Google Scholar] [CrossRef]
- Riehl, B.D.; Lee, J.S.; Ha, L.; Kwon, I.K.; Lim, J.Y. Flowtaxis of osteoblast migration under fluid shear and the effect of RhoA kinase silencing. PLoS ONE 2017, 12, e0171857. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. Targeted and nontargeted bone remodeling: Relationship to basic multicellular unit origination and progression. Bone 2002, 30, 5–7. [Google Scholar] [CrossRef]
- Liang, Y.; Diehn, M.; Bollen, A.W.; Israel, M.A.; Gupta, N. Type I collagen is overexpressed in medulloblastoma as a component of tumor microenvironment. J. Neurooncol. 2008, 86, 133–141. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Clarkin, C.E.; Garonna, E.; Pitsillides, A.A.; Wheeler-Jones, C.P. Heterotypic contact reveals a COX-2-mediated suppression of osteoblast differentiation by endothelial cells: A negative modulatory role for prostanoids in VEGF-mediated cell: Cell communication? Exp. Cell Res. 2008, 314, 3152–3161. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tian, W.; Li, Y.; Tang, W.; Zhang, C. Osteoblast-specific transcription factor Osterix (Osx) and HIF-1α cooperatively regulate gene expression of vascular endothelial growth factor (VEGF). Biochem. Biophys. Res. Commun. 2012, 424, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yallowitz, A.; Qin, A.; Wu, Z.; Shin, D.Y.; Kim, J.M.; Debnath, S.; Ji, G.; Bostrom, M.P.; Yang, X.; et al. Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 2018, 24, 823–833. [Google Scholar] [CrossRef]
- Bergmann, P.; Body, J.J.; Boonen, S.; Boutsen, Y.; Devogelaer, J.P.; Goemaere, S.; Kaufman, J.; Reginster, J.Y.; Rozenberg, S. Loading and skeletal development and maintenance. J. Osteoporos. 2010, 2011, 786752. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef]
- Popsuishapka, O.K.; Ashukina, N.O.; Litvishko, V.O.; Grigorjev, V.V.; Pidgaiska, O.O.; Popsuishapka, K.O. Fibrin-blood clot as an initial stage of formation of bone regeneration after a bone fracture. Regul. Mech. Biosyst. 2018, 9. [Google Scholar] [CrossRef]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef]
- Yuasa, M.; Mignemi, N.A.; Nyman, J.S.; Duvall, C.L.; Schwartz, H.S.; Okawa, A.; Yoshii, T.; Bhattacharjee, G.; Zhao, C.; Bible, J.E.; et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Investig. 2015, 125, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Damsaz, M.; Castagnoli, C.Z.; Eshghpour, M.; Alamdari, D.H.; Alamdari, A.H.; Noujeim, Z.E.F.; Haidar, Z.S. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front. Surg. 2020, 7, 537138. [Google Scholar] [CrossRef]
- Franklin, S.P.; Burke, E.E.; Holmes, S.P. The effect of platelet-rich plasma on osseous healing in dogs undergoing high tibial osteotomy. PLoS ONE 2017, 12, e0177597. [Google Scholar] [CrossRef]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Nagata, M.; Kozloff, K.M.; Welch, J.D.; Mizuhashi, K.; Tokavanich, N.; Hallett, S.A.; Link, D.C.; Nagasawa, T.; Ono, W.; et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 2020, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Coenegrachts, L.; Stockmans, I.; Daci, E.; Luttun, A.; Petryk, A.; Gopalakrishnan, R.; Moermans, K.; Smets, N.; Verfaillie, C.M.; et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J. Clin. Investig. 2006, 116, 1230–1242. [Google Scholar] [CrossRef]
- Kristensen, H.B.; Andersen, T.L.; Marcussen, N.; Rolighed, L.; Delaisse, J.M. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J. Bone Miner. Res. 2013, 28, 574–585. [Google Scholar] [CrossRef]
- Mann, F.A.; Payne, J.T. Bone healing. Semin Vet. Med. Surg Small Anim 1989, 4, 312–321. [Google Scholar]
- Hausman, M.R.; Schaffler, M.B.; Majeska, R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 2001, 29, 560–564. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Silva, M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; de Guzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Karsdal, M.; Delaisse, J.M.; Engsig, M.T. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. J. Biol. Chem. 2003, 278, 48745–48753. [Google Scholar] [CrossRef]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.C.; Cummins, J.; Huard, J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Investig. 2002, 110, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.P.; Carr, L.; Woodley, P.K.; Barry, R.W.; Drake, L.K.; Mindos, T.; Roberts, S.L.; Lloyd, A.C.; Parkinson, D.B. Macrophage-Derived Slit3 Controls Cell Migration and Axon Pathfinding in the Peripheral Nerve Bridge. Cell Rep. 2019, 26, 1458–1472.e1454. [Google Scholar] [CrossRef] [PubMed]
- Geutskens, S.B.; Hordijk, P.L.; van Hennik, P.B. The chemorepellent Slit3 promotes monocyte migration. J. Immunol. 2010, 185, 7691–7698. [Google Scholar] [CrossRef]
- Gorski, J.P.; Hankenson, K.D. Secreted Noncollagenous Proteins of Bone, 4th ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol. 2016, 28, 24–30. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Bonewald, L.F. Generation and function of osteocyte dendritic processes. J. Musculoskelet. Neuronal Interact. 2005, 5, 321–324. [Google Scholar]
- Prasadam, I.; Zhou, Y.; Du, Z.; Chen, J.; Crawford, R.; Xiao, Y. Osteocyte-induced angiogenesis via VEGF-MAPK-dependent pathways in endothelial cells. Mol. Cell Biochem. 2014, 386, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kogianni, G.; Mann, V.; Noble, B.S. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J. Bone Miner. Res. 2008, 23, 915–927. [Google Scholar] [CrossRef]
- Oranger, A.; Brunetti, G.; Colaianni, G.; Tamma, R.; Carbone, C.; Lippo, L.; Mori, G.; Pignataro, P.; Cirulli, N.; Zerlotin, R.; et al. Sclerostin stimulates angiogenesis in human endothelial cells. Bone 2017, 101, 26–36. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Liu, C.; Tonelli-Zasarsky, R.M.; Simmons, C.A.; You, L. Osteocyte apoptosis is mechanically regulated and induces angiogenesis in vitro. J. Orthop. Res. 2011, 29, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, M.B.; Cheung, W.Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Kindle, L.; Rothe, L.; Kriss, M.; Osdoby, P.; Collin-Osdoby, P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J. Bone Miner. Res. 2006, 21, 193–206. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Simmons, C.A.; You, L. Osteocyte apoptosis regulates osteoclast precursor adhesion via osteocytic IL-6 secretion and endothelial ICAM-1 expression. Bone 2012, 50, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.V.; Xu, L.; Mei, X.; Middleton, K.; You, L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell Biochem. 2018. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Wu, X.; Gou, M.; Shen, J.; Wang, H. Advanced glycation end products induced IL-6 and VEGF-A production and apoptosis in osteocyte-like MLO-Y4 cells by activating RAGE and ERK1/2, P38 and STAT3 signalling pathways. Int. Immunopharmacol. 2017, 52, 143–149. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Shanbhogue, V.V.; Mitchell, D.M.; Rosen, C.J.; Bouxsein, M.L. Type 2 diabetes and the skeleton: New insights into sweet bones. Lancet Diabetes Endocrinol. 2016, 4, 159–173. [Google Scholar] [CrossRef]
- Heidari, F.; Rabizadeh, S.; Rajab, A.; Heidari, F.; Mouodi, M.; Mirmiranpour, H.; Esteghamati, A.; Nakhjavani, M. Advanced glycation end-products and advanced oxidation protein products levels are correlates of duration of type 2 diabetes. Life Sci. 2020, 260, 118422. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Cannata, F.; Strollo, R.; Pedone, C.; Leanza, G.; Russo, F.; Greto, V.; Isgrò, C.; Quattrocchi, C.C.; Massaroni, C.; et al. Sclerostin Regulation, Microarchitecture, and Advanced Glycation End-Products in the Bone of Elderly Women With Type 2 Diabetes. J. Bone Miner. Res. 2020, 35, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S.; Alfonso, H.; Norman, P.E.; Davis, T.M.E.; Forbes, J.; Müench, G.; Irrgang, F.; Almeida, O.P.; Golledge, J.; Hankey, G.J.; et al. Advanced Glycation End Products and esRAGE Are Associated With Bone Turnover and Incidence of Hip Fracture in Older Men. J. Clin. Endocrinol. Metab. 2018, 103, 4224–4231. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, S.Y.; Kim, J.H.; Choi, Y.H.; Yang, D.W.; Kang, J.H.; Ko, H.M.; Cho, J.H.; Koh, J.T.; Kim, W.J.; et al. Synergistic alveolar bone resorption by diabetic advanced glycation end products and mechanical forces. J. Periodontol. 2019, 90, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.A.; Bhattacharya, R.; Mukhopadhyay, D.; Kumar, R. Sclerostin binds and regulates the activity of cysteine-rich protein 61. Biochem. Biophys. Res. Commun. 2010, 392, 36–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; Bakker, A.D.; Klein-Nulend, J.; Bravenboer, N. Studies on Osteocytes in Their 3D Native Matrix Versus 2D In Vitro Models. Curr. Osteoporos. Rep. 2019, 17, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mabilleau, G.; Mieczkowska, A.; Edmonds, M.E. Thiazolidinediones induce osteocyte apoptosis and increase sclerostin expression. Diabet. Med. 2010, 27, 925–932. [Google Scholar] [CrossRef]
- Papanicolaou, S.E.; Phipps, R.J.; Fyhrie, D.P.; Genetos, D.C. Modulation of sclerostin expression by mechanical loading and bone morphogenetic proteins in osteogenic cells. Biorheology 2009, 46, 389–399. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Iordachescu, A.; Amin, H.D.; Rankin, S.M.; Williams, R.L.; Yapp, C.; Bannerman, A.; Pacureanu, A.; Addison, O.; Hulley, P.A.; Grover, L.M. An In Vitro Model for the Development of Mature Bone Containing an Osteocyte Network. Adv. Biosyst. 2018, 2, 1700156. [Google Scholar] [CrossRef]
- Akiva, A.; Melke, J.; Ansari, S.; Liv, N.; van der Meijden, R.; van Erp, M.; Zhao, F.; Stout, M.; Nijhuis, W.H.; de Heus, C.; et al. An Organoid for Woven Bone. Adv. Funct. Mater. 2021, 31, 2010524. [Google Scholar] [CrossRef]
- Chen, J.; Lippo, L.; Labella, R.; Tan, S.L.; Marsden, B.D.; Dustin, M.L.; Ramasamy, S.K.; Kusumbe, A.P. Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system. Embo J. 2021, 40, e105242. [Google Scholar] [CrossRef] [PubMed]

| Protein | Role |
|---|---|
| NOTCH4 SEMA6 | Angiogenesis-regulators of stalk/tip cell phenotype balance |
| FLT1 FLT4 | Angiogenesis-VEGF receptors |
| Roundabout family receptor 1 (ROBO1) | Angiogenesis-VEGF signaling mediator |
| Claudin5 (CLD5) | Vascular permeability-cell-motility and vascular permeability mediator EC tight junction protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neag, G.; Finlay, M.; Naylor, A.J. The Cellular Choreography of Osteoblast Angiotropism in Bone Development and Homeostasis. Int. J. Mol. Sci. 2021, 22, 7253. https://doi.org/10.3390/ijms22147253
Neag G, Finlay M, Naylor AJ. The Cellular Choreography of Osteoblast Angiotropism in Bone Development and Homeostasis. International Journal of Molecular Sciences. 2021; 22(14):7253. https://doi.org/10.3390/ijms22147253
Chicago/Turabian StyleNeag, Georgiana, Melissa Finlay, and Amy J. Naylor. 2021. "The Cellular Choreography of Osteoblast Angiotropism in Bone Development and Homeostasis" International Journal of Molecular Sciences 22, no. 14: 7253. https://doi.org/10.3390/ijms22147253
APA StyleNeag, G., Finlay, M., & Naylor, A. J. (2021). The Cellular Choreography of Osteoblast Angiotropism in Bone Development and Homeostasis. International Journal of Molecular Sciences, 22(14), 7253. https://doi.org/10.3390/ijms22147253






