Developmental Inhibition of Long Intergenic Non-Coding RNA, HOTAIRM1, Impairs Dopamine Neuron Differentiation and Maturation
Abstract
:1. Introduction
2. Results
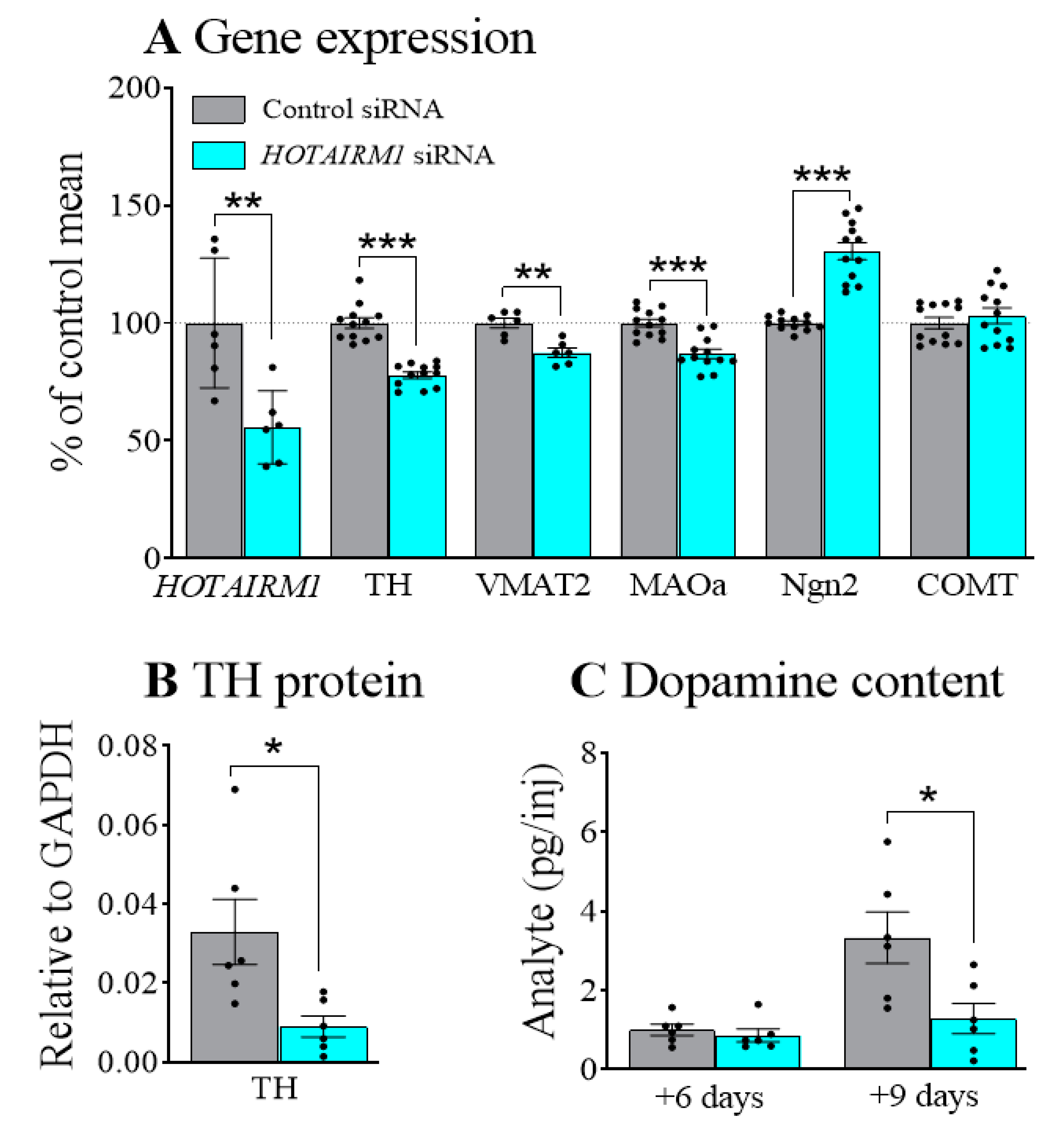
2.1. Decreasing HOTAIRM1 Reduced the Expression of DA Neuron Markers in SHSY5Y Cells
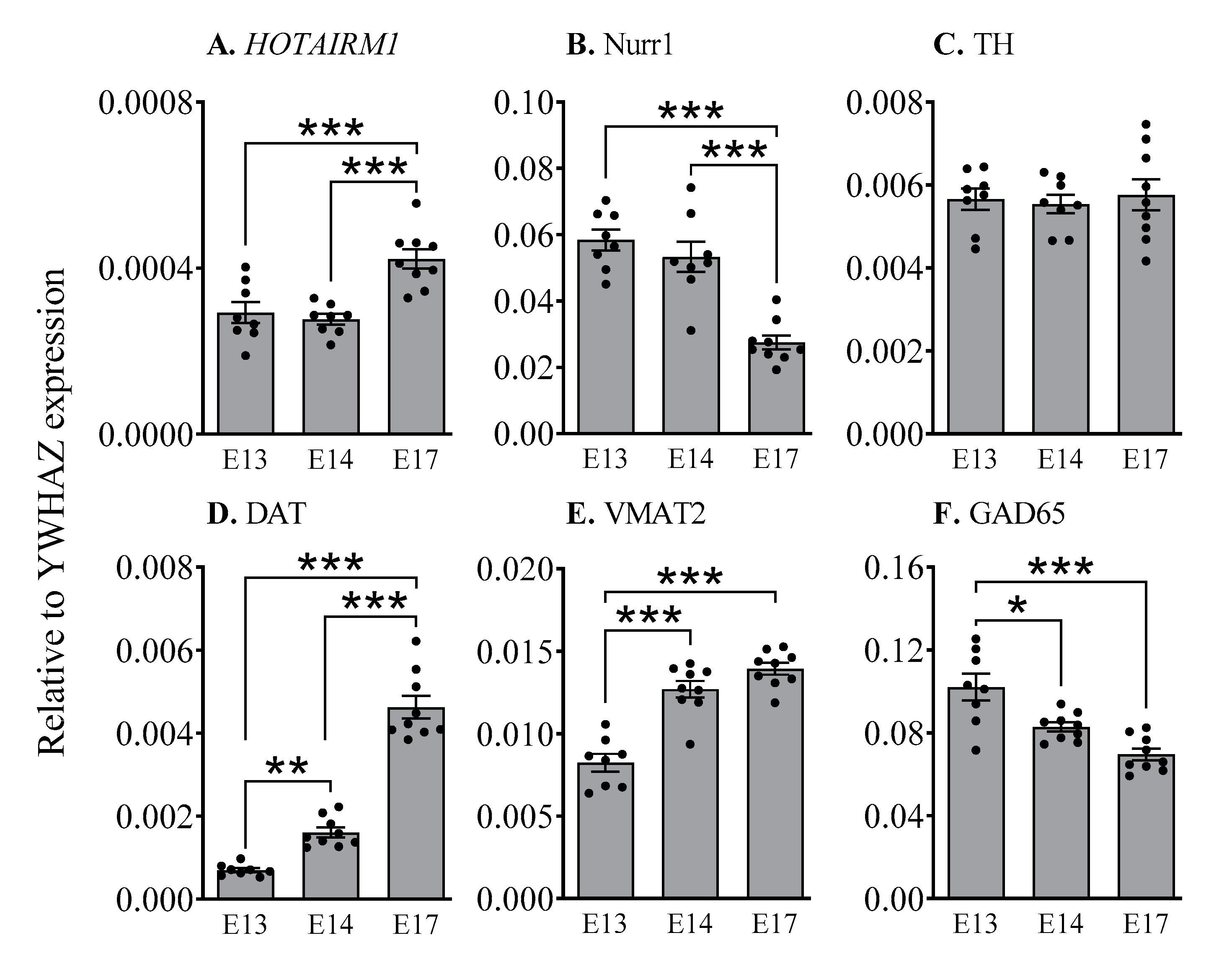
2.2. HOTAIRM1 Expression Increases during Early Development in the Ventral Midbrain
2.3. Electroporation of Mesencephalic Dopamine Progenitors in the Mouse
2.4. Inhibition of HOTAIRM1 alters Markers of DA Differentiation and Maturation in the Developing Mesencephalon
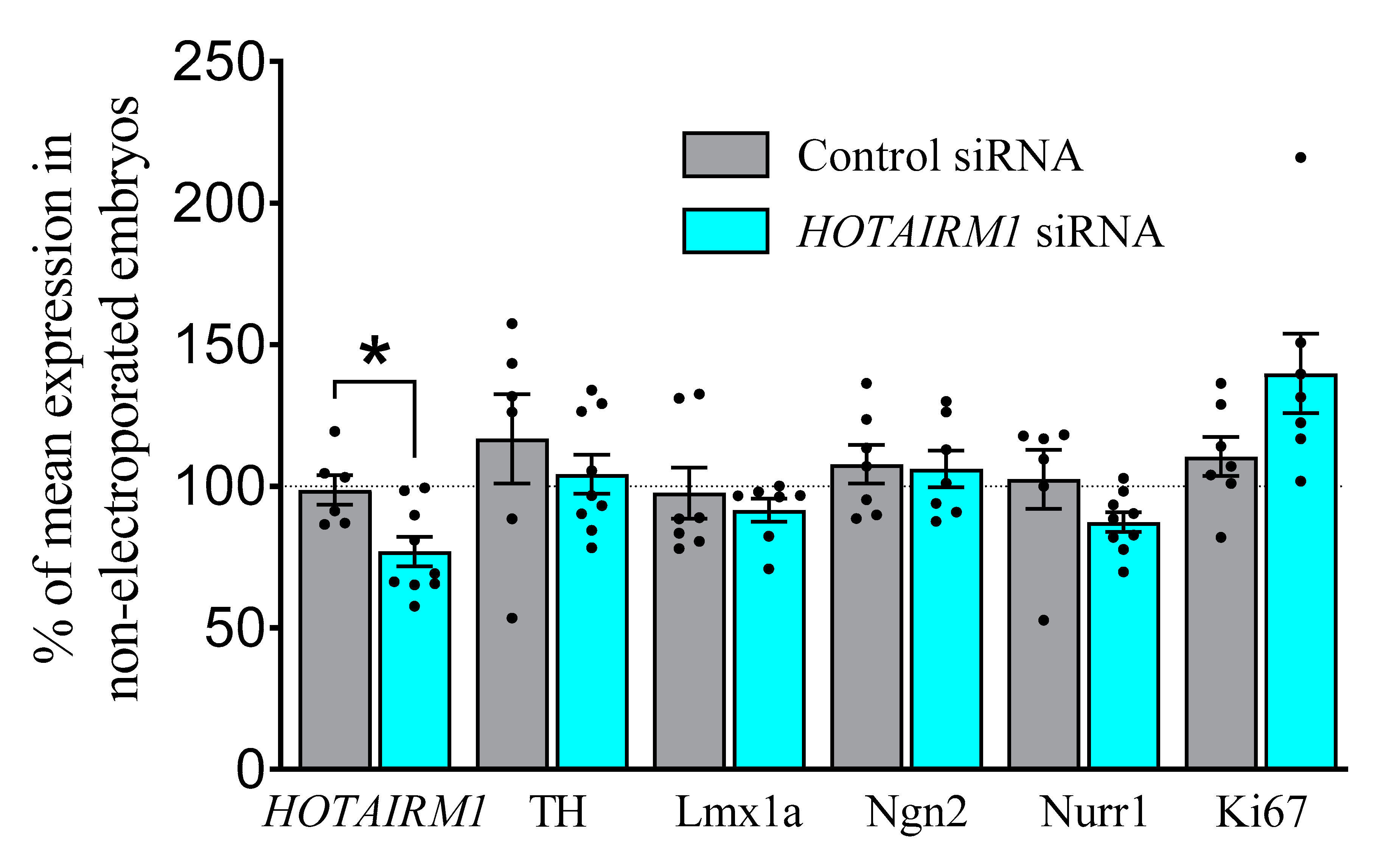
2.4.1. E13, Two-Days after Transfection
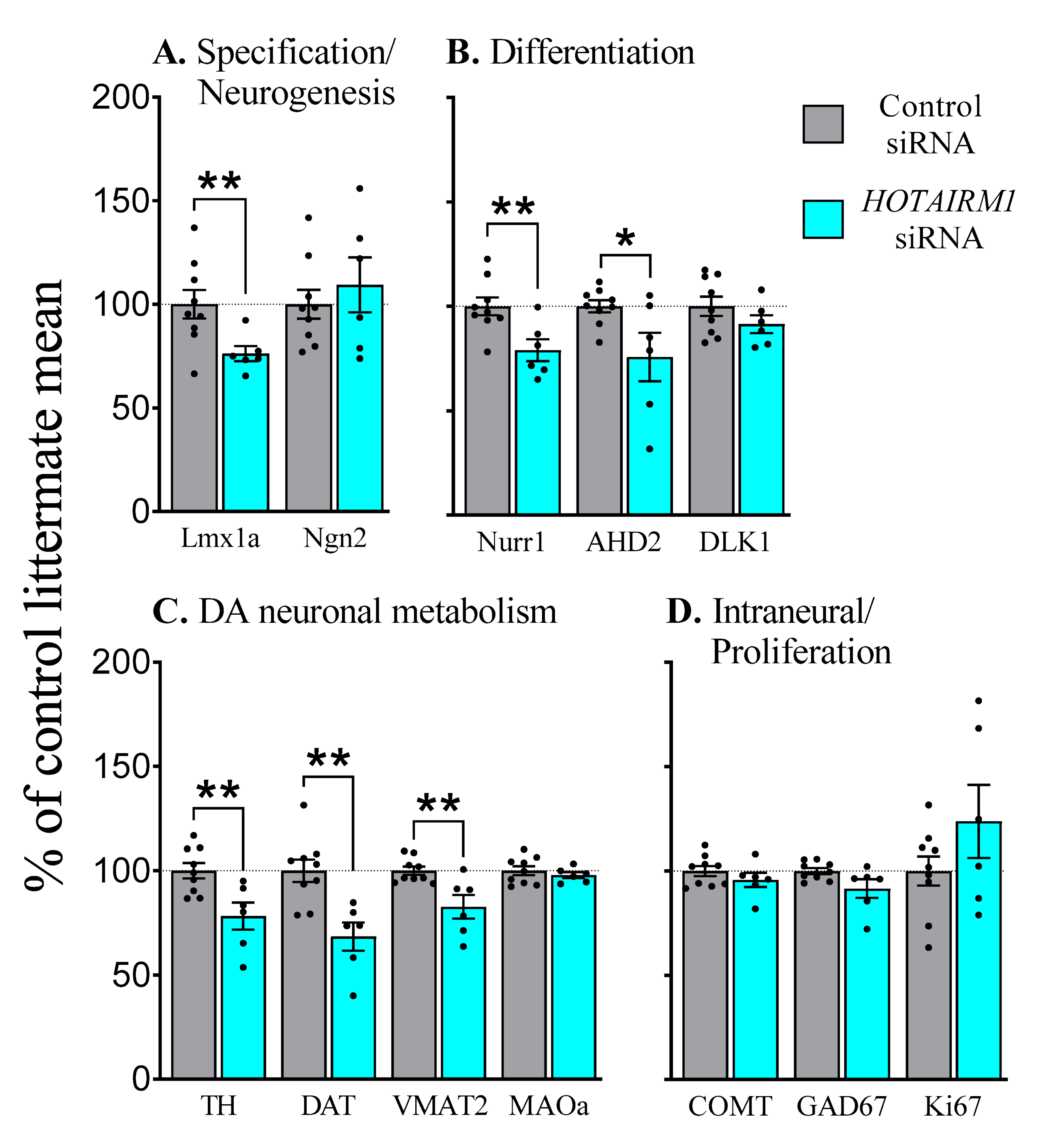
2.4.2. E17, Six-Days after Transfection
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture and siRNA Transfection
4.3. Western Blot
4.4. High Performance Liquid Chromatography
4.5. In Utero Electroporation
4.6. Tissue Collection
4.7. RNA Extraction and Real Time PCR
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldridge, J.W.; Berridge, K.C.; Rosen, A.R. Basal ganglia neural mechanisms of natural movement sequences. Can. J. Physiol. Pharmacol. 2004, 82, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Smith, Y.; Kieval, J.Z. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000, 23, S28–S33. [Google Scholar] [CrossRef]
- Conn, K.; Burne, T.H.J.; Kesby, J.P. Subcortical dopamine and cognition in schizophrenia: Looking beyond psychosis in preclinical models. Front Neurosci. 2020, 14, 542. [Google Scholar] [CrossRef]
- Smidt, M.P.; Burbach, J.P. How to make a mesodiencephalic dopaminergic neuron. Nat. Rev. Neurosci. 2007, 8, 21–32. [Google Scholar] [CrossRef]
- Kesby, J.P.; Cui, X.; Burne, T.H.J.; Eyles, D.W. Altered dopamine ontogeny in the developmentally vitamin D deficient rat and its relevance to schizophrenia. Front. Cell Neurosci. 2013, 7, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyles, D.; Feldon, J.; Meyer, U. Schizophrenia: Do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl. Psychiatry 2012, 2, e81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Scott, J.G. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiatry 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Nelander, J.; Hebsgaard, J.B.; Parmar, M. Organization of the human embryonic ventral mesencephalon. Gene Expr. Patterns 2009, 9, 555–561. [Google Scholar] [CrossRef]
- Gates, M.A.; Torres, E.M.; White, A.; Fricker-Gates, R.A.; Dunnett, S.B. Re-examining the ontogeny of substantia nigra dopamine neurons. Eur. J. Neurosci. 2006, 23, 1384–1390. [Google Scholar] [CrossRef]
- Specht, L.A.; Pickel, V.M.; Joh, T.H.; Reis, D.J. Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. I. Early ontogeny. J. Comp. Neurol. 1981, 199, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Yotsumoto, I.; Segawa, T. Ontogenetic development of high potassium-induced and acetylcholine-induced release of dopamine from striatal slices of the rat. Dev. Brain Res. 1981, 1, 171–177. [Google Scholar] [CrossRef]
- Van den Heuvel, D.M.A.; Pasterkamp, R.J. Getting connected in the dopamine system. Prog. Neurobiol. 2008, 85, 75–93. [Google Scholar] [CrossRef]
- Veenvliet, J.V.; Smidt, M.P. Molecular mechanisms of dopaminergic subset specification: Fundamental aspects and clinical perspectives. Cell Mol. Life Sci. 2014, 71, 4703–4727. [Google Scholar] [CrossRef] [PubMed]
- Blaess, S.; Ang, S.L. Genetic control of midbrain dopaminergic neuron development. Wiley Interdiscip Rev. Dev. Biol. 2015, 4, 113–134. [Google Scholar] [CrossRef]
- Arenas, E.; Denham, M.; Villaescusa, J.C. How to make a midbrain dopaminergic neuron. Development 2015, 142, 1918–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodea, G.O.; Blaess, S. Establishing diversity in the dopaminergic system. FEBS Lett. 2015, 589, 3773–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eom, T.; Berardi, V.; Zhong, J.; Risuleo, G.; Tiedge, H. Dual nature of translational control by regulatory BC RNAs. Mol. Cell. Biol. 2011, 31, 4538–4549. [Google Scholar] [CrossRef] [Green Version]
- Raveendra, B.L.; Swarnkar, S.; Avchalumov, Y.; Liu, X.A.; Grinman, E.; Badal, K.; Reich, A.; Pascal, B.D.; Puthanveettil, S.V. Long noncoding RNA GM12371 acts as a transcriptional regulator of synapse function. Proc. Natl. Acad. Sci. USA 2018, 115, E10197–E10205. [Google Scholar] [CrossRef] [Green Version]
- Salta, E.; De Strooper, B. Non-coding RNAs with essential roles in neurodegenerative disorders. Lancet Neurol. 2012, 11, 189–200. [Google Scholar] [CrossRef]
- Ng, S.Y.; Bogu, G.K.; Soh, B.S.; Stanton, L.W. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol. Cell 2013, 51, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Bernard, D.; Prasanth, K.V.; Tripathi, V.; Colasse, S.; Nakamura, T.; Xuan, Z.; Zhang, M.Q.; Sedel, F.; Jourdren, L.; Coulpier, F.; et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010, 29, 3082–3093. [Google Scholar] [CrossRef] [Green Version]
- Bond, A.M.; Vangompel, M.J.; Sametsky, E.A.; Clark, M.F.; Savage, J.C.; Disterhoft, J.F.; Kohtz, J.D. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat. Neurosci. 2009, 12, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Gendron, J.; Colace-Sauty, C.; Beaume, N.; Cartonnet, H.; Guegan, J.; Ulveling, D.; Pardanaud-Glavieux, C.; Moszer, I.; Cheval, H.; Ravassard, P. Long non-coding RNA repertoire and open chromatin regions constitute midbrain dopaminergic neuron - specific molecular signatures. Sci. Rep. 2019, 9, 1409. [Google Scholar] [CrossRef]
- Guttman, M.; Donaghey, J.; Carey, B.W.; Garber, M.; Grenier, J.K.; Munson, G.; Young, G.; Lucas, A.B.; Ach, R.; Bruhn, L.; et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 2011, 477, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, W.T.; Huang, W.; Fang, K.; Sun, Y.M.; Liu, S.R.; Luo, X.Q.; Chen, Y.Q. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017, 24, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K.I.; Clohessy, J.G.; Pandolfi, P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017, 541, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Bannon, M.J.; Savonen, C.L.; Jia, H.; Dachet, F.; Halter, S.D.; Schmidt, C.J.; Lipovich, L.; Kapatos, G. Identification of long noncoding RNAs dysregulated in the midbrain of human cocaine abusers. J. Neurochem. 2015, 135, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pedrosa, E.; Shah, A.; Hrabovsky, A.; Maqbool, S.; Zheng, D.; Lachman, H.M. RNA-Seq of human neurons derived from iPS cells reveals candidate long non-coding RNAs involved in neurogenesis and neuropsychiatric disorders. PLoS ONE 2011, 6, e23356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lian, Z.; Padden, C.; Gerstein, M.B.; Rozowsky, J.; Snyder, M.; Gingeras, T.R.; Kapranov, P.; Weissman, S.M.; Newburger, P.E. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 2009, 113, 2526–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, F.M.; Veenvliet, J.V.; Almirza, W.H.; Hoekstra, E.J.; von Oerthel, L.; van der Linden, A.J.; Neijts, R.; Koerkamp, M.G.; van Leenen, D.; Holstege, F.C.; et al. Retinoic acid-dependent and -independent gene-regulatory pathways of Pitx3 in meso-diencephalic dopaminergic neurons. Development 2011, 138, 5213–5222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dal Maschio, M.; Ghezzi, D.; Bony, G.; Alabastri, A.; Deidda, G.; Brondi, M.; Sato, S.S.; Zaccaria, R.P.; Di Fabrizio, E.; Ratto, G.M.; et al. High-performance and site-directed in utero electroporation by a triple-electrode probe. Nat. Commun. 2012, 3, 960. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, A.; Wang, X.; Zhang, M.; Zhang, Z.; Tan, Z.; Qiu, M. Identifying suitable reference genes for developing and injured mouse CNS tissues. Dev. Neurobiol. 2018, 78, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, F.; Perrone-Capano, C.; Da Pozzo, P.; Colucci-D’Amato, L.; di Porzio, U. Modulation of nurr1 gene expression in mesencephalic dopaminergic neurones. J. Neurochem. 2004, 88, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Hammond, L.A.; Cotter, E.; Osborne, G.W.; Alexander, S.A.; Nink, V.; Cui, X.; Eyles, D.W. Developmental Vitamin D (DVD) Deficiency Reduces Nurr1 and TH Expression in Post-mitotic Dopamine Neurons in Rat Mesencephalon. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 2001, 240, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, T.R.; Dinger, M.E.; Sunkin, S.M.; Mehler, M.F.; Mattick, J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. USA 2008, 105, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Washietl, S.; Kellis, M.; Garber, M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014, 24, 616–628. [Google Scholar] [CrossRef] [Green Version]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016. [Google Scholar] [CrossRef]
- Aprea, J.; Prenninger, S.; Dori, M.; Ghosh, T.; Monasor, L.S.; Wessendorf, E.; Zocher, S.; Massalini, S.; Alexopoulou, D.; Lesche, M.; et al. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013, 32, 3145–3160. [Google Scholar] [CrossRef] [PubMed]
- Rea, J.; Menci, V.; Tollis, P.; Santini, T.; Armaos, A.; Garone, M.G.; Iberite, F.; Cipriano, A.; Tartaglia, G.G.; Rosa, A.; et al. HOTAIRM1 regulates neuronal differentiation by modulating NEUROGENIN 2 and the downstream neurogenic cascade. Cell Death Dis. 2020, 11, 527. [Google Scholar] [CrossRef]
- Wang, L.; Schuster, G.U.; Hultenby, K.; Zhang, Q.; Andersson, S.; Gustafsson, J.A. Liver X receptors in the central nervous system: From lipid homeostasis to neuronal degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 13878–13883. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.H.; Andersson, E.; Jensen, J.B.; Barraud, P.; Guillemot, F.; Parmar, M.; Bjorklund, A. Neurogenin2 identifies a transplantable dopamine neuron precursor in the developing ventral mesencephalon. Exp. Neurol. 2006, 198, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Yoon, K.J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.S.; Zhu, Y.; Zheng, L.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell 2017, 171, 877–889.e817. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhao, G.; Yang, Y.; Jiang, Z.; Cai, J.; Zhang, Z.; Hu, H. Long noncoding RNA HOTAIRM1 inhibits cell progression by regulating miR-17-5p/ PTEN axis in gastric cancer. J. Cell. Biochem. 2019, 120, 4952–4965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, L.; Xuan, Y.; Gao, C.; Wang, Y.H.; Ming, H.X.; Liu, J. LncRNA HOTAIRM1 inhibits the progression of hepatocellular carcinoma by inhibiting the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4861–4868. [Google Scholar] [CrossRef]
- Zhang, X.; Weissman, S.M.; Newburger, P.E. Long intergenic non-coding RNA HOTAIRM1 regulates cell cycle progression during myeloid maturation in NB4 human promyelocytic leukemia cells. RNA Biol. 2014, 11, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Q.; Dostie, J. Reciprocal regulation of chromatin state and architecture by HOTAIRM1 contributes to temporal collinear HOXA gene activation. Nucleic Acids Res. 2017, 45, 1091–1104. [Google Scholar] [CrossRef]
- Kesby, J.P.; Turner, K.; Alexander, S.; Eyles, D.W.; McGrath, J.J.; Burne, T.H.J. Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int. J. Dev. Neurosci. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Cui, X.; Ko, P.; McGrath, J.J.; Burne, T.H.; Eyles, D.W. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci. Lett. 2009, 461, 155–158. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Pertile, R.A.N.; Du, Z.; Wei, W.; Sun, Z.; Eyles, D.W.; Kesby, J.P. Developmental Inhibition of Long Intergenic Non-Coding RNA, HOTAIRM1, Impairs Dopamine Neuron Differentiation and Maturation. Int. J. Mol. Sci. 2021, 22, 7268. https://doi.org/10.3390/ijms22147268
Cui X, Pertile RAN, Du Z, Wei W, Sun Z, Eyles DW, Kesby JP. Developmental Inhibition of Long Intergenic Non-Coding RNA, HOTAIRM1, Impairs Dopamine Neuron Differentiation and Maturation. International Journal of Molecular Sciences. 2021; 22(14):7268. https://doi.org/10.3390/ijms22147268
Chicago/Turabian StyleCui, Xiaoying, Renata Ap. Nedel Pertile, Zilong Du, Wei Wei, Zichun Sun, Darryl W. Eyles, and James P. Kesby. 2021. "Developmental Inhibition of Long Intergenic Non-Coding RNA, HOTAIRM1, Impairs Dopamine Neuron Differentiation and Maturation" International Journal of Molecular Sciences 22, no. 14: 7268. https://doi.org/10.3390/ijms22147268
APA StyleCui, X., Pertile, R. A. N., Du, Z., Wei, W., Sun, Z., Eyles, D. W., & Kesby, J. P. (2021). Developmental Inhibition of Long Intergenic Non-Coding RNA, HOTAIRM1, Impairs Dopamine Neuron Differentiation and Maturation. International Journal of Molecular Sciences, 22(14), 7268. https://doi.org/10.3390/ijms22147268





