Hsp70 Interacts with the TREM-1 Receptor Expressed on Monocytes and Thereby Stimulates Generation of Cytotoxic Lymphocytes Active against MHC-Negative Tumor Cells
Abstract
:1. Introduction
2. Results
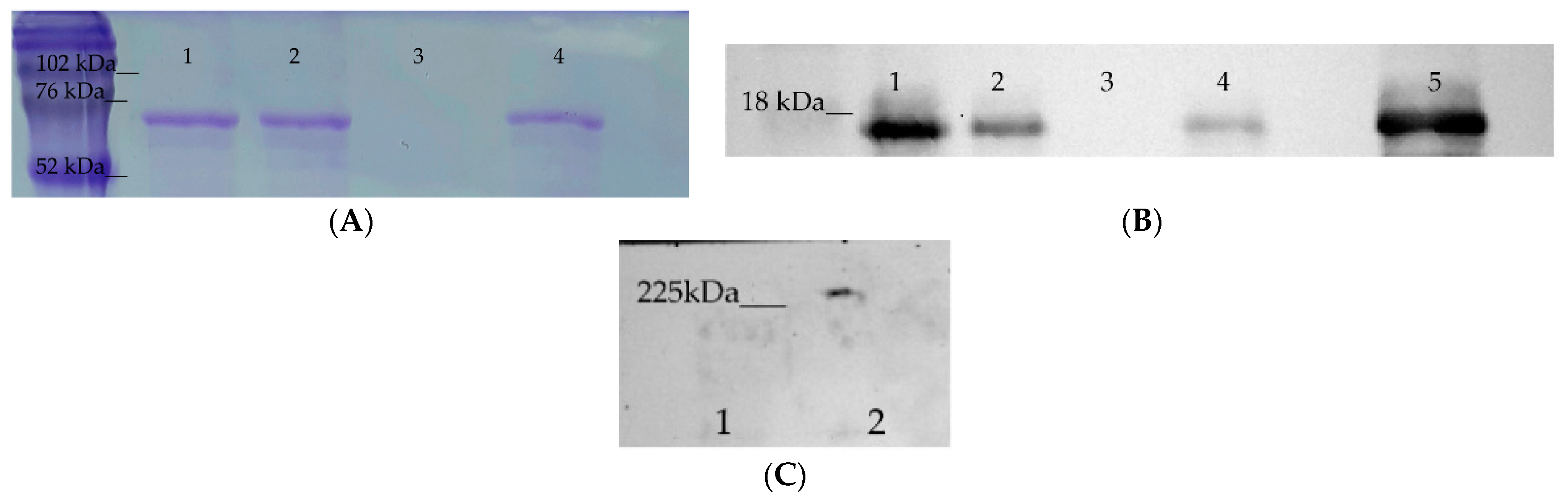
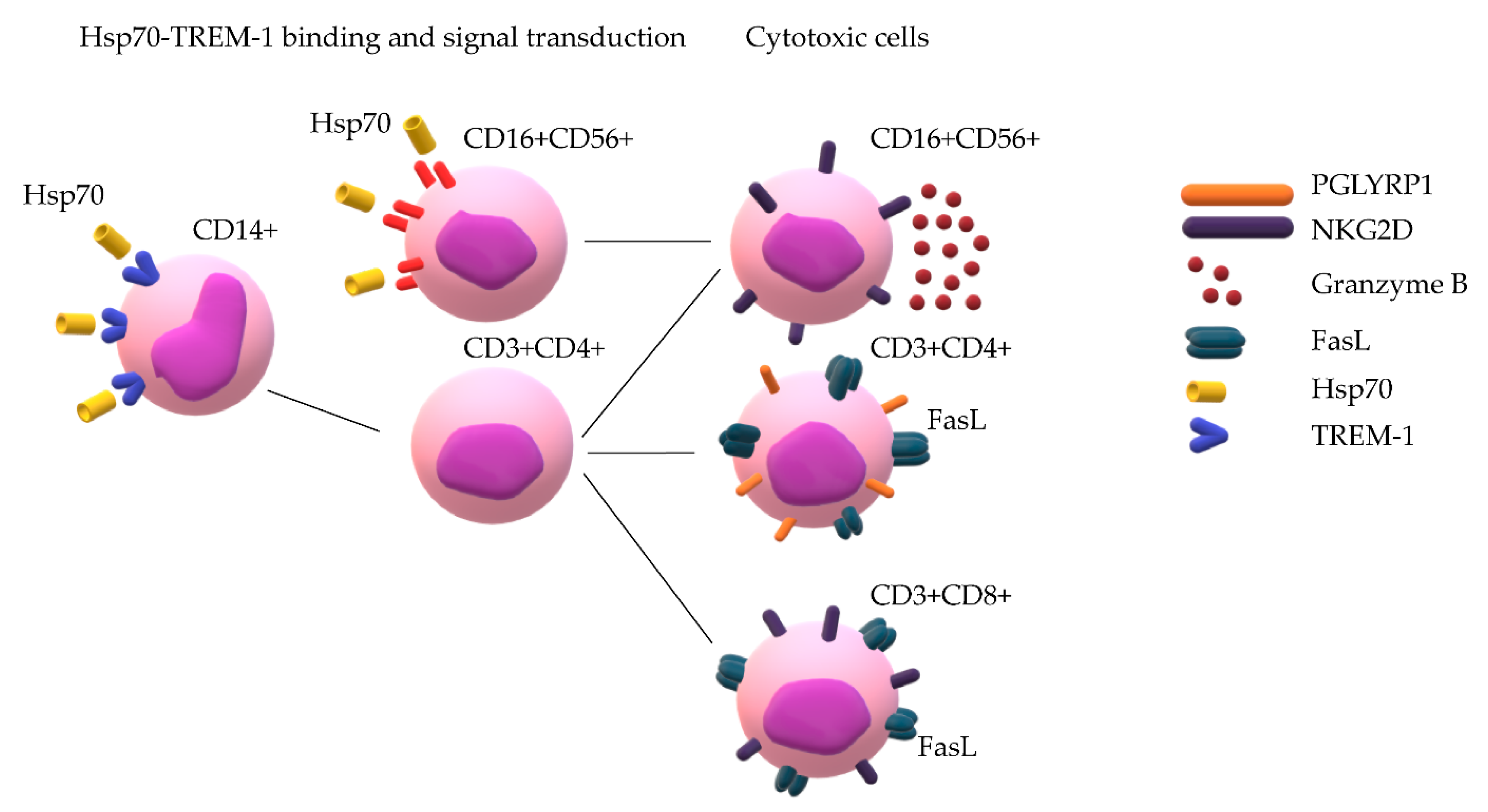
2.1. Hsp70 Binds to TREM-1
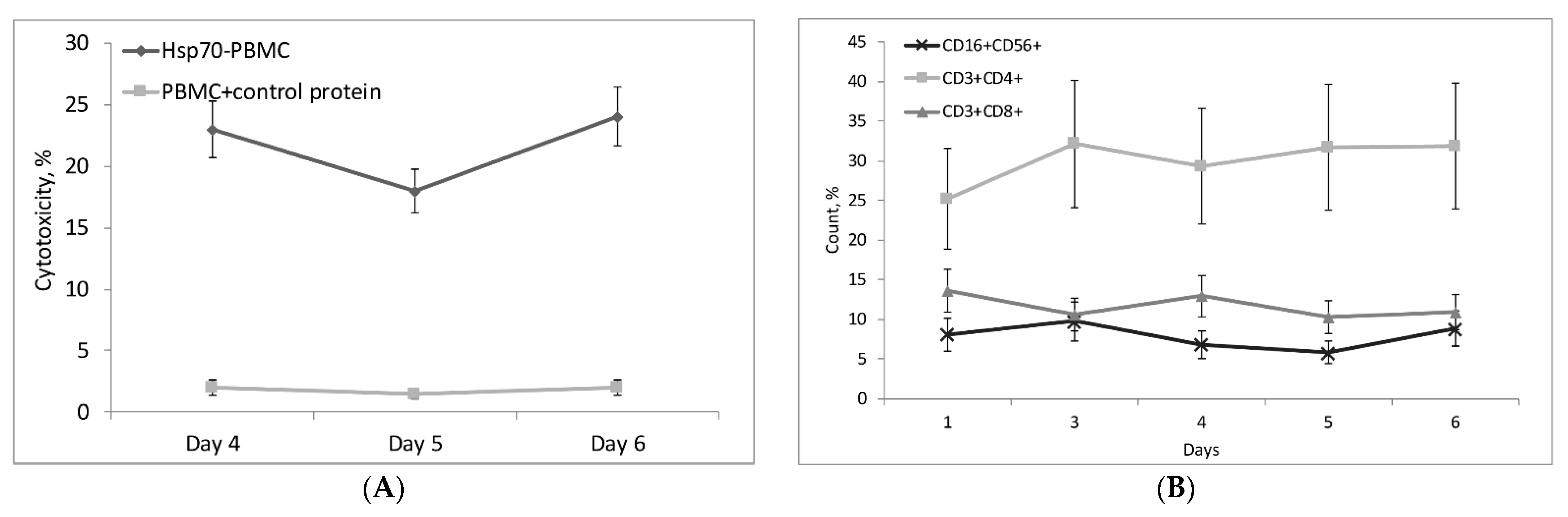
2.2. Hsp70 Activates Cytotoxic Lymphocytes Reacting against MHC-Negative Tumor Cells
2.3. CD14+ Monocytes and CD4+ T-Lymphocytes Are Involved in Activation of Hsp70-Dependent Cytotoxicity
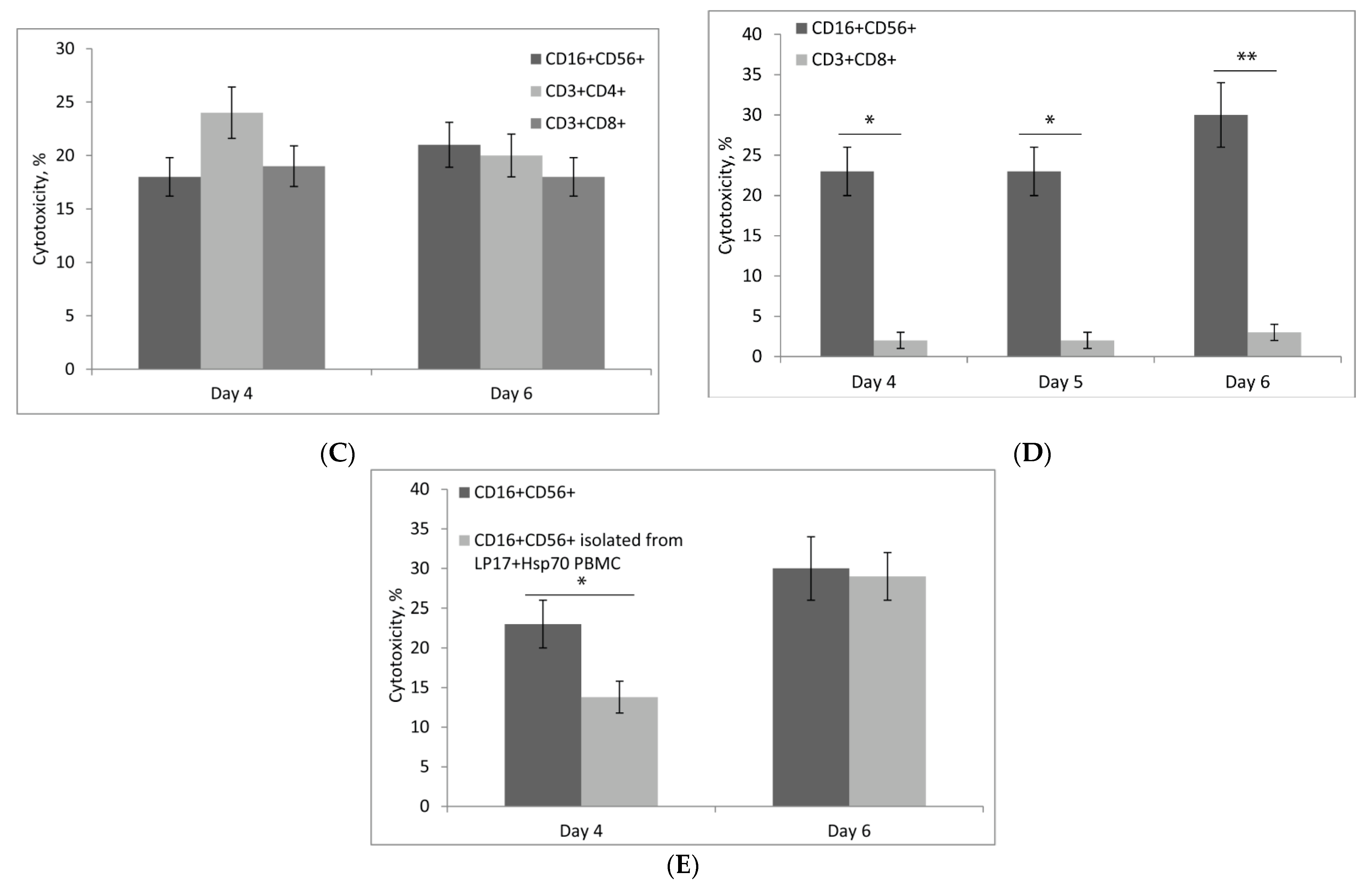
2.4. Hsp70 Stimulates Cytotoxic Activity of NK Cells, CD4+ T-Lymphocytes, and CD8+ T-Lymphocytes
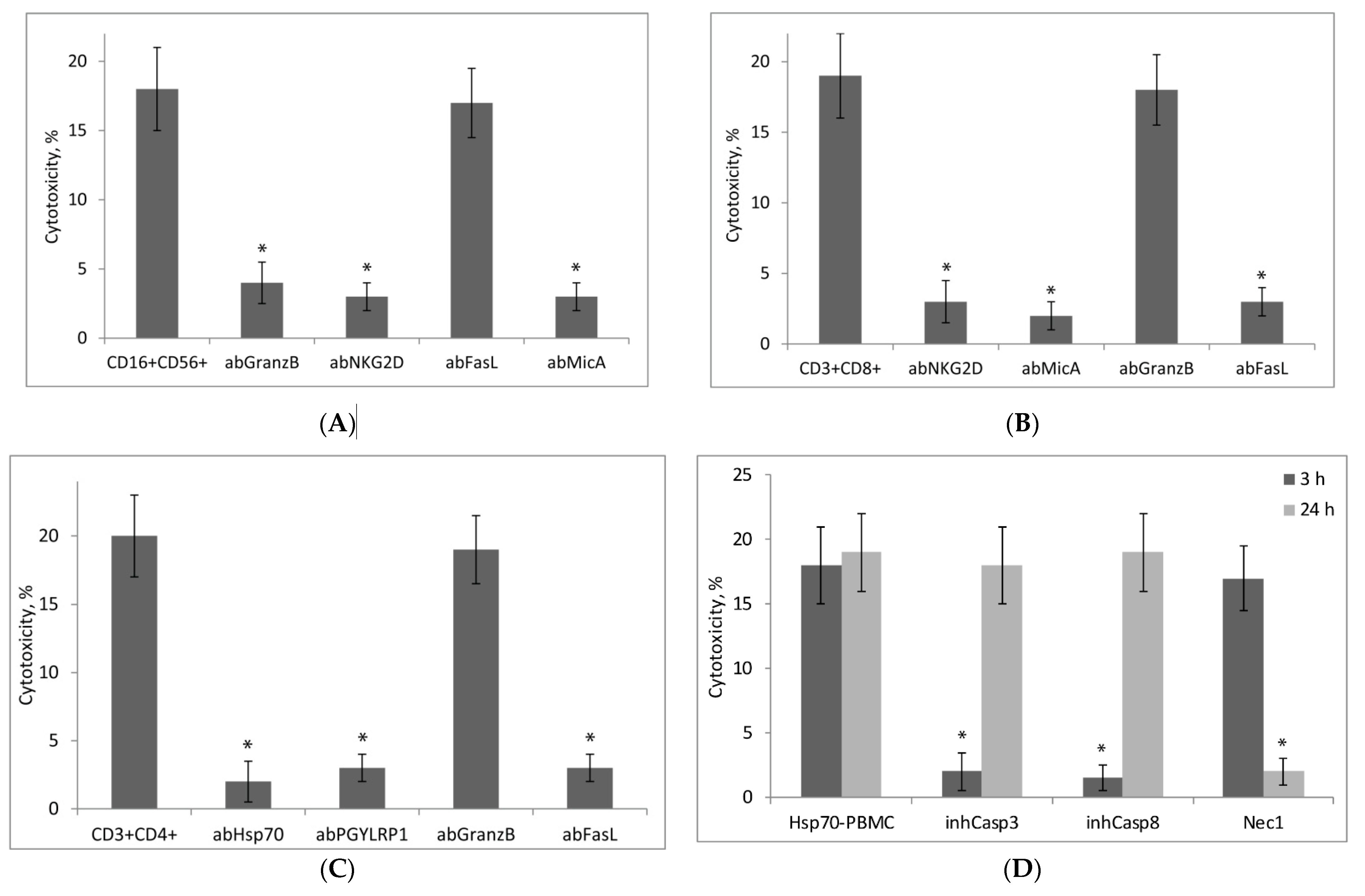
2.5. Hsp70-Activated NK Cells Secrete Granzymes, While Hsp70-Activated CD8+ and CD4+ T-Lymphocytes Kill Tumor Cells via the FasL–Fas Interaction
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Sorting
4.2. Proteins and Antibodies
4.3. Affinity Chromatography, Immunoadsorption, and Immunoblotting
4.4. Cytotoxicity Assays
4.5. Flow Cytometry
4.6. ELISA
4.7. qPCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like Receptors: Significance, Ligands, Signaling Pathways, and Functions in Mammals. Int. Rev. Immunol. 2018, 37, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jung, S.Y.; Hodgson, A.J.; Madden, C.R.; Qin, J.; Slagle, B.L. Hepatitis B Virus Regulatory HBx Protein Binds to Adaptor Protein IPS-1 and Inhibits the Activation of Beta Interferon. J. Virol. 2011, 85, 987–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.W.; McVicar, D.W. TREM and TREM-like Receptors in Inflammation and Disease. Curr. Opin. Immunol. 2009, 21, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klesney-Tait, J.; Keck, K.; Li, X.; Gilfillan, S.; Otero, K.; Baruah, S.; Meyerholz, D.K.; Varga, S.M.; Knudson, C.J.; Moninger, T.O.; et al. Transepithelial Migration of Neutrophils into the Lung Requires TREM-1. J. Clin. Investig. 2013, 123, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Tammaro, A.; Derive, M.; Gibot, S.; Leemans, J.C.; Florquin, S.; Dessing, M.C. TREM-1 and Its Potential Ligands in Non-Infectious Diseases: From Biology to Clinical Perspectives. Pharm. Therapeutics 2017, 177, 81–95. [Google Scholar] [CrossRef]
- Allcock, R.J.N.; Barrow, A.D.; Forbes, S.; Beck, S.; Trowsdale, J. The Human TREM Gene Cluster at 6p21.1 Encodes Both Activating and Inhibitory Single IgV Domain Receptors and Includes NKp44. Eur. J. Immunol. 2003, 33, 567–577. [Google Scholar] [CrossRef]
- Matesanz-Isabel, J.; Sintes, J.; Llinàs, L.; de Salort, J.; Lázaro, A.; Engel, P. New B-Cell CD Molecules. Immunol. Lett. 2011, 134, 104–112. [Google Scholar] [CrossRef]
- Kelker, M.S.; Foss, T.R.; Peti, W.; Teyton, L.; Kelly, J.W.; Wüthrich, K.; Wilson, I.A. Crystal Structure of Human Triggering Receptor Expressed on Myeloid Cells 1 (TREM-1) at 1.47 A. J. Mol. Biol. 2004, 342, 1237–1248. [Google Scholar] [CrossRef]
- Radaev, S.; Kattah, M.; Rostro, B.; Colonna, M.; Sun, P.D. Crystal Structure of the Human Myeloid Cell Activating Receptor TREM-1. Structure 2003, 11, 1527–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessarz, A.S.; Cerwenka, A. The TREM-1/DAP12 Pathway. Immunol. Lett. 2008, 116, 111–116. [Google Scholar] [CrossRef]
- Gómez-Piña, V.; Soares-Schanoski, A.; Rodríguez-Rojas, A.; del Fresno, C.; García, F.; Vallejo-Cremades, M.T.; Fernández-Ruiz, I.; Arnalich, F.; Fuentes-Prior, P.; López-Collazo, E. Metalloproteinases Shed TREM-1 Ectodomain from Lipopolysaccharide-Stimulated Human Monocytes. J. Immunol. 2007, 179, 4065–4073. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, J.; Salcedo, R.; Mivechi, N.F.; Trinchieri, G.; Horuzsko, A. The Proinflammatory Myeloid Cell Receptor TREM-1 Controls Kupffer Cell Activation and Development of Hepatocellular Carcinoma. Cancer Res. 2012, 72, 3977–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mezayen, R.; El Gazzar, M.; Seeds, M.C.; McCall, C.E.; Dreskin, S.C.; Nicolls, M.R. Endogenous Signals Released from Necrotic Cells Augment Inflammatory Responses to Bacterial Endotoxin. Immunol. Lett. 2007, 111, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, C.B.; Kuijper, J.L.; Hjorth, S.A.; Heipel, M.D.; Tang, X.; Fleetwood, A.J.; Dantzler, J.L.; Grell, S.N.; Kastrup, J.; Wang, C.; et al. Cutting Edge: Identification of Neutrophil PGLYRP1 as a Ligand for TREM-1. J. Immunol. 2015, 194, 1417–1421. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Romanova, E.A.; Sashchenko, L.P.; Gnuchev, N.V.; Yashin, D.V. Innate Immune Protein Tag7 Stimulates the Appearance of Cytotoxic NK Cells after Incubation with Lymphocytes. Dokl. Biochem. Biophys. 2019, 484, 92–94. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Romanova, E.A.; Ivanova, O.K.; Sashchenko, L.P.; Yashin, D.V. Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes. Cells 2020, 9, 2602. [Google Scholar] [CrossRef]
- Sashchenko, L.P.; Dukhanina, E.A.; Shatalov, Y.V.; Yashin, D.V.; Lukyanova, T.I.; Kabanova, O.D.; Romanova, E.A.; Khaidukov, S.V.; Galkin, A.V.; Gnuchev, N.V.; et al. Cytotoxic T Lymphocytes Carrying a Pattern Recognition Protein Tag7 Can Detect Evasive, HLA-Negative but Hsp70-Exposing Tumor Cells, Thereby Ensuring FasL/Fas-Mediated Contact Killing. Blood 2007, 110, 1997–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakeeva, I.R.; Berezhnoĭ, A.E.; Gnuchev, N.V.; Georgiev, G.P.; Lapin, S.S. Inhibitory receptors of lymphocytes and their role in antitumor immunity. Vopr. Onkol. 2007, 53, 140–149. [Google Scholar]
- Johnson, D.R. Locus-Specific Constitutive and Cytokine-Induced HLA Class I Gene Expression. J. Immunol. 2003, 170, 1894–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, N.; Suzuki, Y.; Yonezu, T.; Nakagawa, Y.; Shiina, T.; Hirayama, N.; Inokuchi, S.; Inoue, S. A Cell-Based High-Throughput Screening Assay System for Inhibitor Compounds of Antigen Presentation by HLA Class II Molecule. Sci. Rep. 2017, 7, 6798. [Google Scholar] [CrossRef]
- Multhoff, G. Heat Shock Protein 70 (Hsp70) Stimulates Proliferation and Cytolytic Activity of Natural Killer Cells. Exp. Hematol. 1999, 27, 1627–1636. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat Shock Protein 72 on Tumor Cells: A Recognition Structure for Natural Killer Cells. J. Immunol. 1997, 158, 4341–4350. [Google Scholar] [PubMed]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Müller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A Stress-Inducible 72-KDa Heat-Shock Protein (HSP72) Is Expressed on the Surface of Human Tumor Cells, but Not on Normal Cells. Int J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Issels, R. The Role of Heat Shock Proteins in the Stimulation of an Immune Response. Biol. Chem. 1998, 379, 295–300. [Google Scholar] [PubMed]
- Gehrmann, M.; Liebisch, G.; Schmitz, G.; Anderson, R.; Steinem, C.; Maio, A.D.; Pockley, G.; Multhoff, G. Tumor-Specific Hsp70 Plasma Membrane Localization Is Enabled by the Glycosphingolipid Gb3. PLoS ONE 2008, 3, e1925. [Google Scholar] [CrossRef] [Green Version]
- Gehrmann, M.; Schmetzer, H.; Eissner, G.; Haferlach, T.; Hiddemann, W.; Multhoff, G. Membrane-Bound Heat Shock Protein 70 (Hsp70) in Acute Myeloid Leukemia: A Tumor Specific Recognition Structure for the Cytolytic Activity of Autologous NK Cells. Haematologica 2003, 88, 474–476. [Google Scholar]
- Böttger, E.; Multhoff, G.; Kun, J.F.J.; Esen, M. Plasmodium Falciparum-Infected Erythrocytes Induce Granzyme B by NK Cells through Expression of Host-Hsp70. PLoS ONE 2012, 7, e33774. [Google Scholar] [CrossRef]
- Gehrmann, M.; Specht, H.M.; Bayer, C.; Brandstetter, M.; Chizzali, B.; Duma, M.; Breuninger, S.; Hube, K.; Lehnerer, S.; van Phi, V.; et al. Hsp70--a Biomarker for Tumor Detection and Monitoring of Outcome of Radiation Therapy in Patients with Squamous Cell Carcinoma of the Head and Neck. Radiat Oncol. 2014, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Multhoff, G. Activation of Natural Killer Cells by Heat Shock Protein 70. 2002. Int. J. Hyperth. 2009, 25, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sashchenko, L.P.; Romanova, E.A.; Ivanova, O.K.; Sharapova, T.N.; Yashin, D.V. FasL and the NKG2D Receptor Are Required for the Secretion of the Tag7/PGRP-S-Hsp70 Complex by the Cytotoxic CD8+ Lymphocytes. Iubmb Life 2017, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yashin, D.V.; Sashchenko, L.P.; Dukhanina, E.A.; Romanova, E.A.; Luk’yanova, T.I.; Kabanova, O.D.; Sorokin, V.A.; Gnuchev, N.V. LAK Cells Kill Fas- Cancer Cells Using the Tag7/Hsp70 Protein Complex Secreted from the Golgi Apparatus. Dokl. Biol. Sci. 2004, 395, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Sashchenko, L.P.; Dukhanina, E.A.; Yashin, D.V.; Shatalov, Y.V.; Romanova, E.A.; Korobko, E.V.; Demin, A.V.; Lukyanova, T.I.; Kabanova, O.D.; Khaidukov, S.V.; et al. Peptidoglycan Recognition Protein Tag7 Forms a Cytotoxic Complex with Heat Shock Protein 70 in Solution and in Lymphocytes*. J. Biol. Chem. 2004, 279, 2117–2124. [Google Scholar] [CrossRef] [Green Version]
- Yashin, D.V.; Ivanova, O.K.; Soshnikova, N.V.; Sheludchenkov, A.A.; Romanova, E.A.; Dukhanina, E.A.; Tonevitsky, A.G.; Gnuchev, N.V.; Gabibov, A.G.; Georgiev, G.P.; et al. Tag7 (PGLYRP1) in Complex with Hsp70 Induces Alternative Cytotoxic Processes in Tumor Cells via TNFR1 Receptor. J. Biol. Chem. 2015, 290, 21724–21731. [Google Scholar] [CrossRef] [Green Version]
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 Stimulates Cytokine Production through a CD14-Dependant Pathway, Demonstrating Its Dual Role as a Chaperone and Cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef]
- Guzhova, I.V.; Shevtsov, M.A.; Abkin, S.V.; Pankratova, K.M.; Margulis, B.A. Intracellular and Extracellular Hsp70 Chaperone as a Target for Cancer Therapy. Int J. Hyperth. 2013, 29, 399–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharapova, T.N.; Romanova, E.A.; Ivanova, O.K.; Yashin, D.V.; Sashchenko, L.P. Hsp70 Interacts with the TREM-1 Receptor Expressed on Monocytes and Thereby Stimulates Generation of Cytotoxic Lymphocytes Active against MHC-Negative Tumor Cells. Int. J. Mol. Sci. 2021, 22, 6889. https://doi.org/10.3390/ijms22136889
Sharapova TN, Romanova EA, Ivanova OK, Yashin DV, Sashchenko LP. Hsp70 Interacts with the TREM-1 Receptor Expressed on Monocytes and Thereby Stimulates Generation of Cytotoxic Lymphocytes Active against MHC-Negative Tumor Cells. International Journal of Molecular Sciences. 2021; 22(13):6889. https://doi.org/10.3390/ijms22136889
Chicago/Turabian StyleSharapova, Tatiana N., Elena A. Romanova, Olga K. Ivanova, Denis V. Yashin, and Lidia P. Sashchenko. 2021. "Hsp70 Interacts with the TREM-1 Receptor Expressed on Monocytes and Thereby Stimulates Generation of Cytotoxic Lymphocytes Active against MHC-Negative Tumor Cells" International Journal of Molecular Sciences 22, no. 13: 6889. https://doi.org/10.3390/ijms22136889
APA StyleSharapova, T. N., Romanova, E. A., Ivanova, O. K., Yashin, D. V., & Sashchenko, L. P. (2021). Hsp70 Interacts with the TREM-1 Receptor Expressed on Monocytes and Thereby Stimulates Generation of Cytotoxic Lymphocytes Active against MHC-Negative Tumor Cells. International Journal of Molecular Sciences, 22(13), 6889. https://doi.org/10.3390/ijms22136889






