Sympathetic Denervation Alters the Inflammatory Response of Resident Muscularis Macrophages upon Surgical Trauma and Ameliorates Postoperative Ileus in Mice
Abstract
:1. Introduction
2. Results
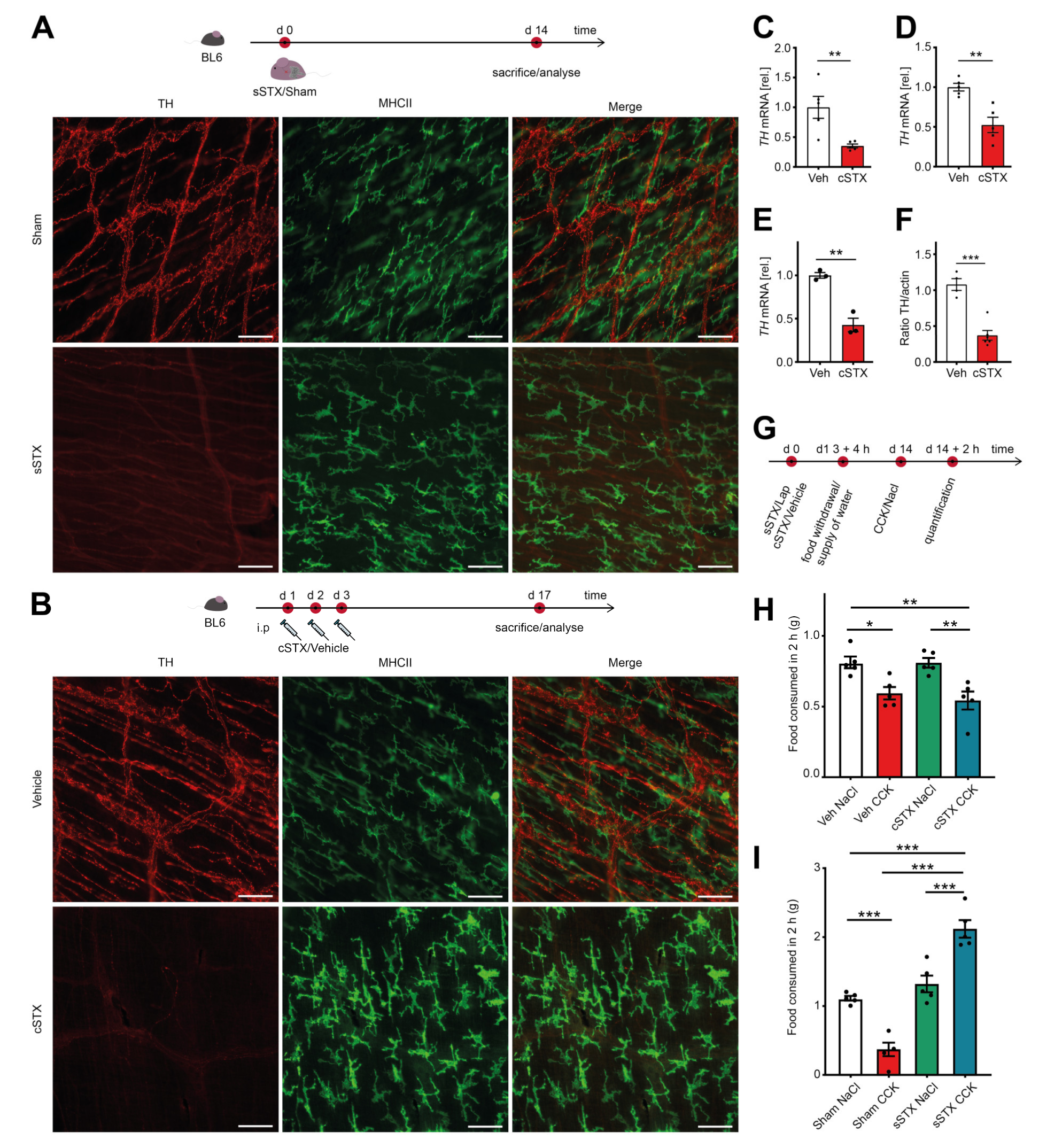
2.1. Chemical Depletion by 6-Hydroxydopamine (6-OHDA) Specifically Targets TH+ Neurons without Affecting the Vagal Innervation
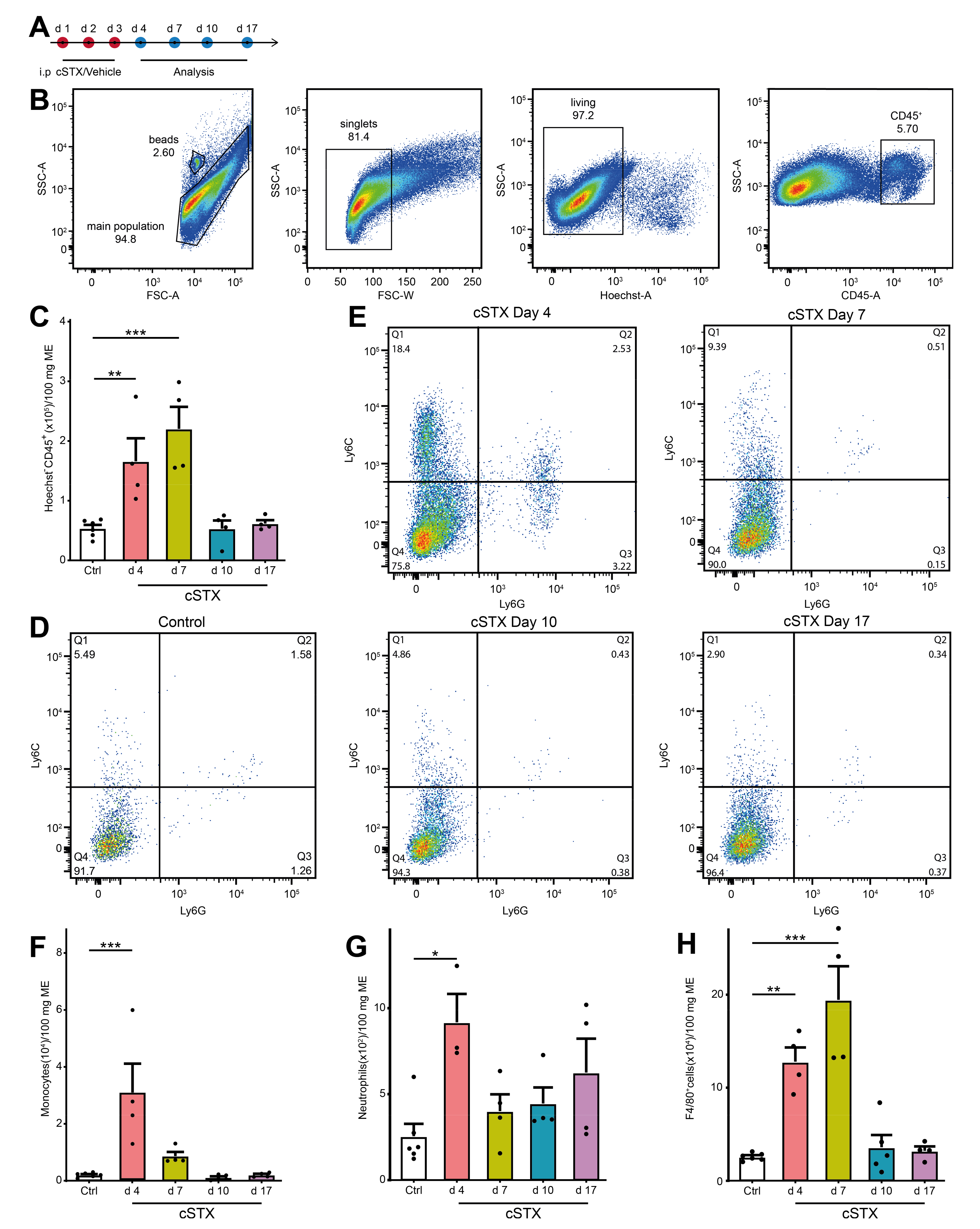
2.2. cSTX Induces a Transient Infiltration of Leukocytes into the Muscularis Externa
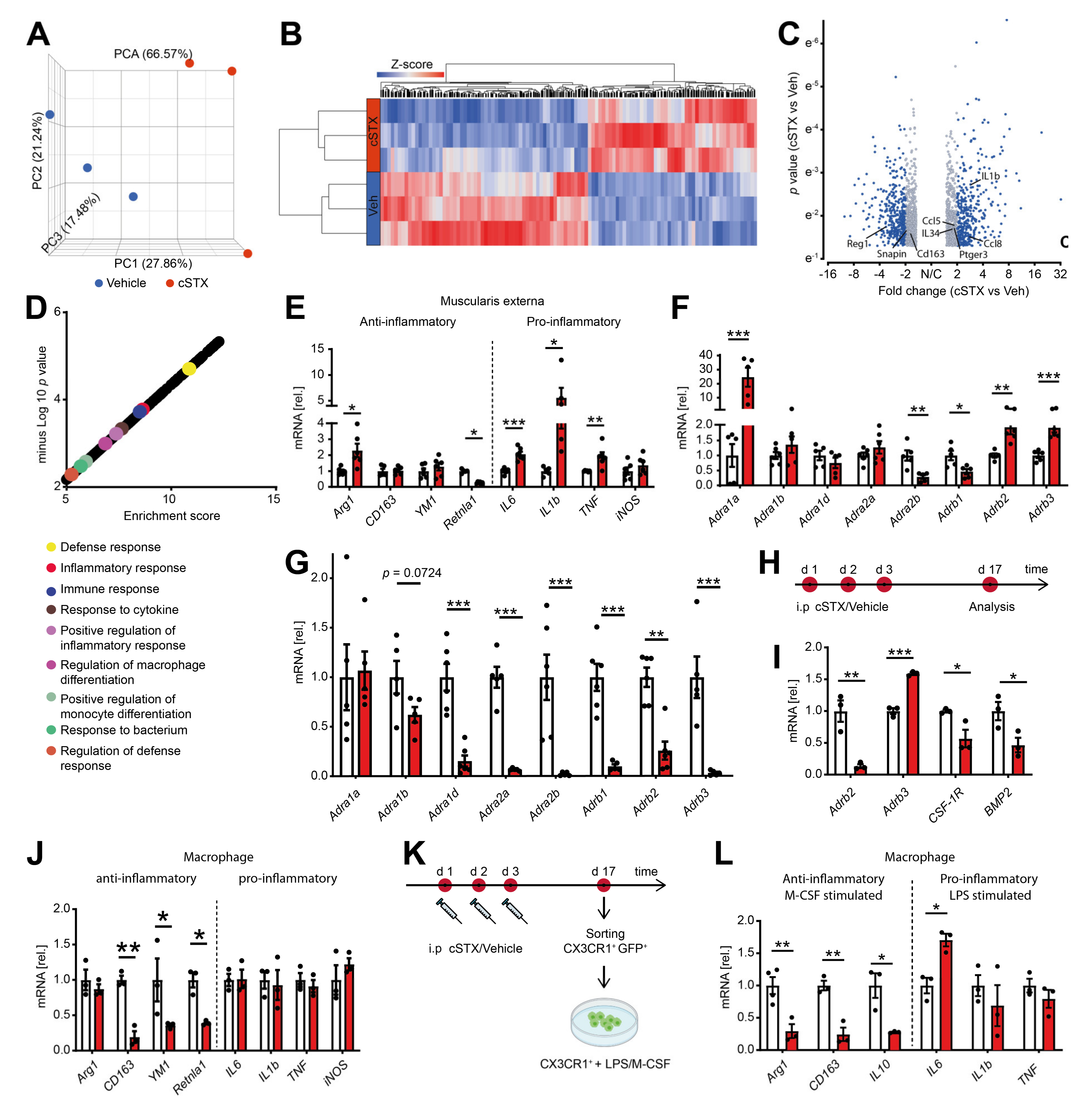
2.3. Sympathetic Denervation Alters the Inflammatory State of CX3CR1+ MMs in a Noninfectious Setting
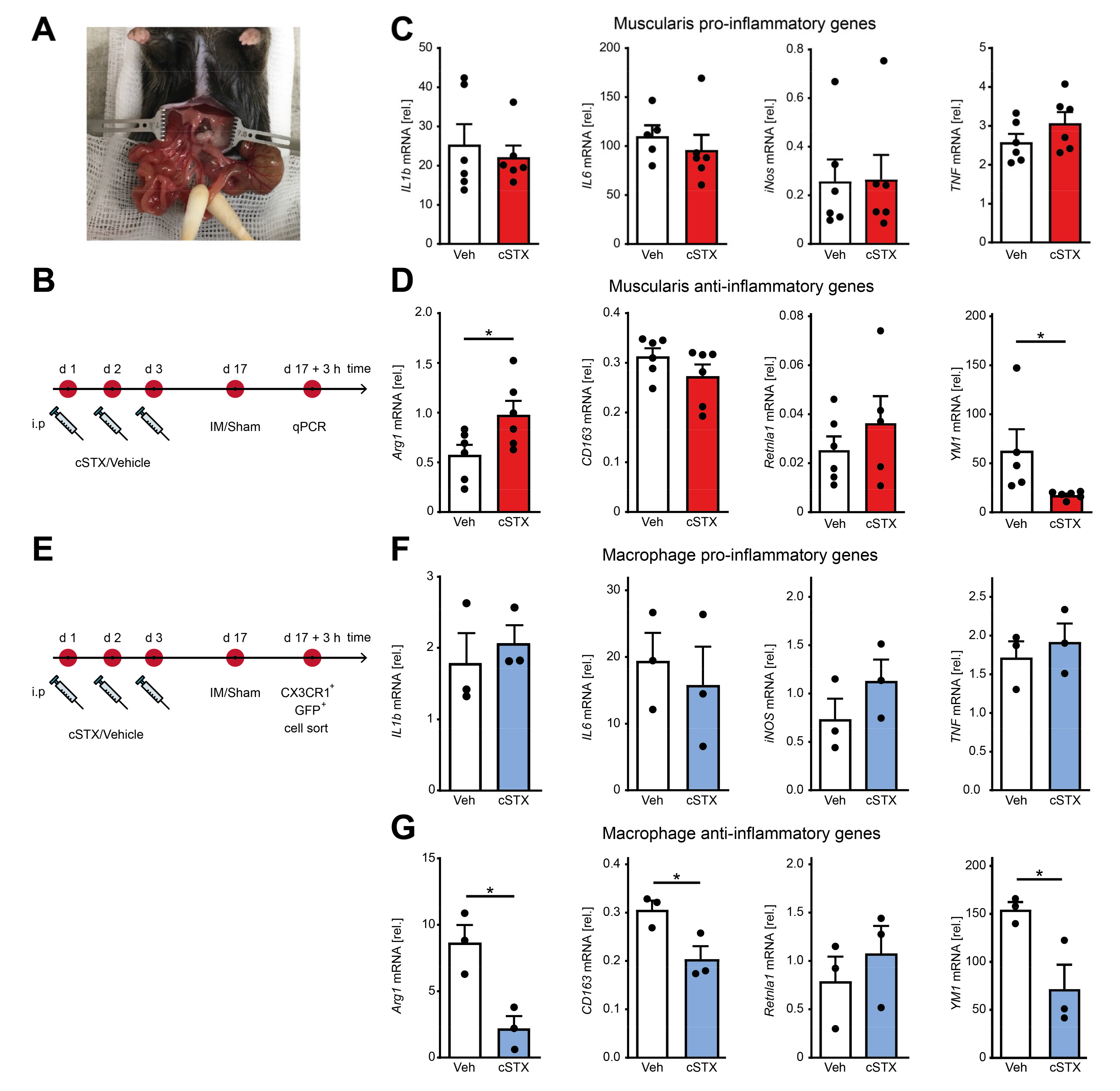
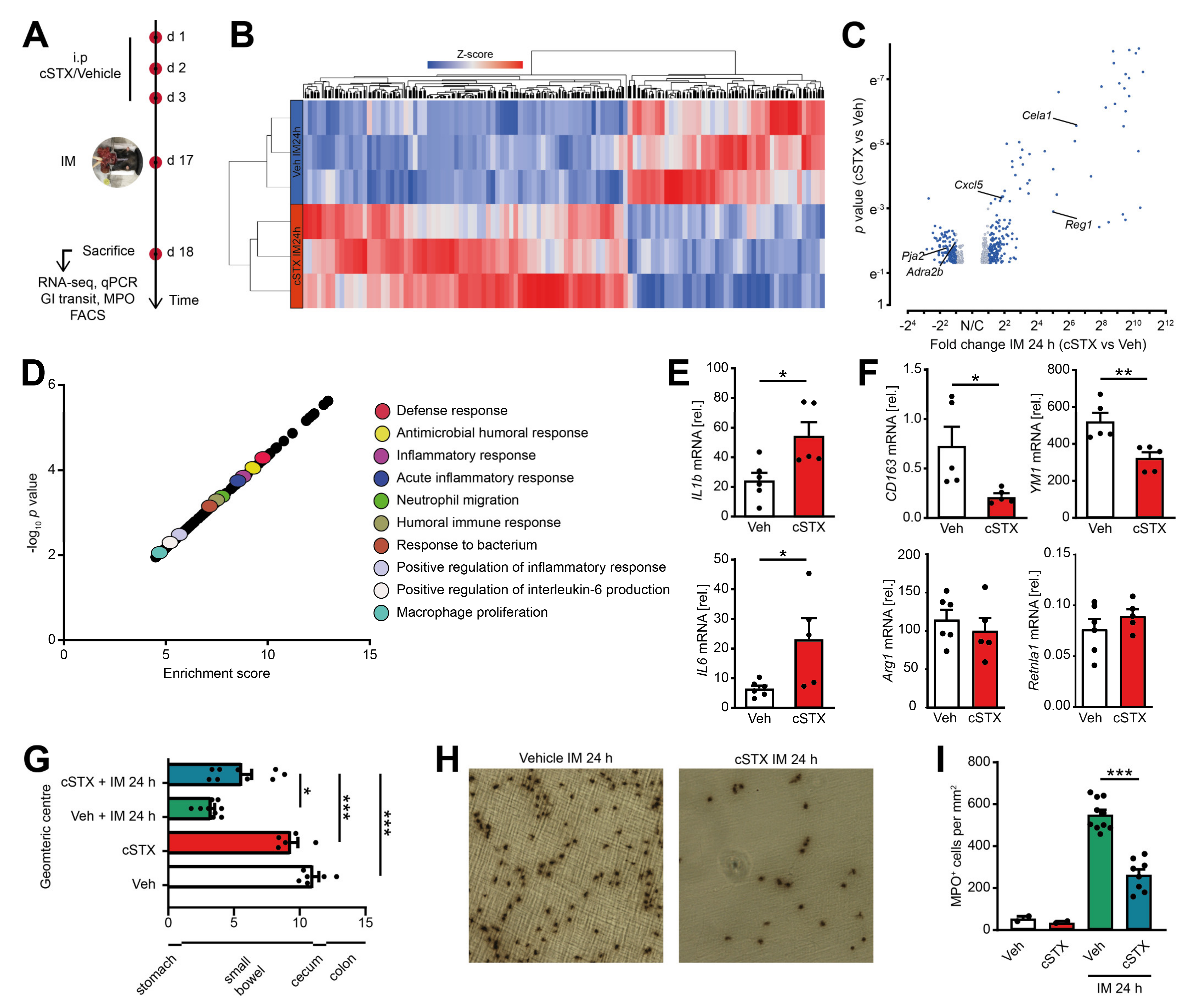
2.4. Sympathetic Denervation Reduced CX3CR1+ Muscularis Macrophage Anti-Inflammatory Response in an Acute Intestinal Postoperative Ileus Model
2.5. Sympathetic Denervation Reduced the Infiltration of Immune Cells into the Muscularis Externa and Led to an Accelerated GI Transit Time
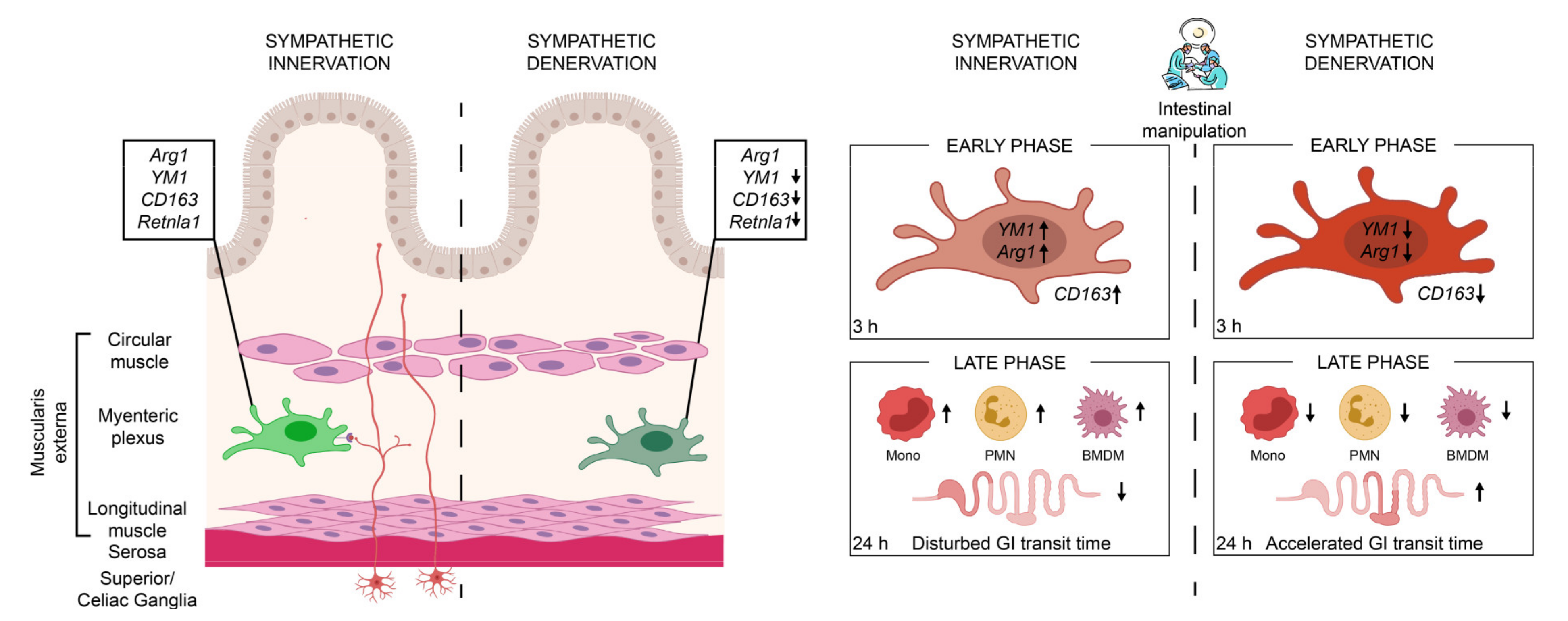
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Denervation
4.3. Chemical Denervation
4.4. Cholecystokinin (CCK) Test
4.5. Whole-Mounts Preparation and Immunofluorescence Staining
4.6. Intestinal Manipulation
4.7. Hanker Yates Staining
4.8. Gastrointestinal Transit Time Measurement
4.9. Flow Cytometry
4.10. Flow Cytometry-Based Cell Sorting
4.11. Ex Vivo Stimulation Assay
4.12. Western Blot
4.13. Quantitative RT-PCR
4.14. RNA Sequencing
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine hydrobromide |
| Adrb2 | Beta-adrenergic receptor 2 |
| Adrb3 | Beta-adrenergic receptor 3 |
| BMP-2 | bone morphogenic protein-2 |
| CCK | Cholecystokinin |
| CSF-1R | colony-stimulating factor 1 receptor |
| cSTX | chemical sympathectomy |
| ENS | enteric nervous system |
| FACS | fluorescence-activated cell sorting |
| GIT | gastrointestinal transit time |
| gSTX | genetic sympathectomy |
| IM | intestinal manipulation |
| i.p | Intraperitoneal |
| MMs | muscularis macrophages |
| MPO | myeloperoxidase positive cells |
| SNS | sympathetic nervous system |
| PNS | parasympathetic nervous system |
| POI | postoperative ileus |
| qPCR | quantitative polymerase chain reaction |
| STX | Sympathectomy |
| TH | tyrosine hydroxylase |
| sSTX | surgical sympathectomy |
References
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.C.; Furness, J.B.; Burns, O.; Sedo, A.; Hyakumura, T.; Shepherd, R.K.; Fallon, J.B. Anti-inflammatory Effects of Abdominal Vagus Nerve Stimulation on Experimental Intestinal Inflammation. Front. Neurosci. 2019, 13, 418. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.-S.; Zillekens, A.; Schneiker, B.; Pantelis, D.; de Jonge, W.J.; Schaefer, N.; Kalff, J.C.; Wehner, S. Non-invasive transcutaneous auricular vagus nerve stimulation prevents postoperative ileus and endotoxemia in mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13501. [Google Scholar] [CrossRef]
- Miksa, M.; Wu, R.; Zhou, M.; Wang, P. Sympathetic excitotoxicity in sepsis: Pro-inflammatory priming of macrophages by norepinephrine. Front. Biosci. A J. Virtual Libr. 2005, 10, 2217–2229. [Google Scholar] [CrossRef] [Green Version]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Willemze, R.A.; Welting, O.; van Hamersveld, P.; Verseijden, C.; Nijhuis, L.E.; Hilbers, F.W.; Meijer, S.L.; Heesters, B.A.; Folgering, J.H.A.; Darwinkel, H.; et al. Loss of intestinal sympathetic innervation elicits an innate immune driven colitis. Mol. Med. 2019, 25, 1. [Google Scholar] [CrossRef]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.-L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.-M.; Mucida, D.; et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014, 158, 300–313. [Google Scholar] [CrossRef] [Green Version]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; de Casterlé, I.D.; et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 2018, 175, 400–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheis, F.; Muller, P.A.; Graves, C.L.; Gabanyi, I.; Kerner, Z.J.; Costa-Borges, D.; Ahrends, T.; Rosenstiel, P.; Mucida, D. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 2020, 180, 64–78. [Google Scholar] [CrossRef]
- Wehner, S.; Behrendt, F.F.; Lyutenski, B.N.; Lysson, M.; Bauer, A.J.; Hirner, A.; Kalff, J.C. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 2007, 56, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Kalff, J.C.; Schraut, W.H.; Simmons, R.L.; Bauer, A.J. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann. Surg. 1998, 228, 652–663. [Google Scholar] [CrossRef]
- Snoek, S.A.; Dhawan, S.; van Bree, S.H.; Cailotto, C.; van Diest, S.A.; Duarte, J.M.; Stanisor, O.I.; Hilbers, F.W.; Nijhuis, L.; Koeman, A.; et al. Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2012, 24, 172–e91. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E.; de Jonge, W.J. Neuroimmune mechanisms in postoperative ileus. Gut 2009, 58, 1300–1311. [Google Scholar] [CrossRef] [Green Version]
- Wehner, S.; Engel, D.R. Resident macrophages in the healthy and inflamed intestinal muscularis externa. Pflug. Arch. Eur. J. Physiol. 2017, 469, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, B.; Hupa, K.J.; Snoek, S.A.; van Bree, S.; Stein, K.; Schwandt, T.; Vilz, T.O.; Lysson, M.; Veer, C.V.; Kummer, M.P.; et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 2014, 146, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Leven, P.; Glowka, T.; Kuzmanov, I.; Lysson, M.; Schneiker, B.; Miesen, A.; Baqi, Y.; Spanier, C.; Grants, I.; et al. A novel P2X2-dependent purinergic mechanism of enteric gliosis in intestinal inflammation. EMBO Mol. Med. 2020, e12724. [Google Scholar] [CrossRef]
- Farro, G.; Stakenborg, M.; Gomez-Pinilla, P.J.; Labeeuw, E.; Goverse, G.; di Giovangiulio, M.; Stakenborg, N.; Meroni, E.; D’Errico, F.; Elkrim, Y.; et al. CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut 2017, 66, 2098–2109. [Google Scholar] [CrossRef] [Green Version]
- Stein, K.; Lysson, M.; Schumak, B.; Vilz, T.; Specht, S.; Heesemann, J.; Roers, A.; Kalff, J.C.; Wehner, S. Leukocyte-Derived Interleukin-10 Aggravates Postoperative Ileus. Front. Immunol. 2018, 9, 2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borden, P.; Houtz, J.; Leach, S.D.; Kuruvilla, R. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 2013, 4, 287–301. [Google Scholar] [CrossRef]
- Wang, F.-B.; Powley, T.L. Vagal innervation of intestines: Afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 2007, 329, 221–230. [Google Scholar] [CrossRef]
- Matteoli, G.; Boeckxstaens, G.E. The vagal innervation of the gut and immune homeostasis. Gut 2013, 62, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Ghia, J.-E.; Blennerhassett, P.; El-Sharkawy, R.T.; Collins, S.M. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G711–G718. [Google Scholar] [CrossRef]
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jonge, W.J.; van den Wijngaard, R.M.; The, F.O.; ter Beek, M.-L.; Bennink, R.J.; Tytgat, G.N.J.; Buijs, R.M.; Reitsma, P.H.; van Deventer, S.J.; Boeckxstaens, G.E. Postoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in mice. Gastroenterology 2003, 125, 1137–1147. [Google Scholar] [CrossRef]
- Yoo, B.B.; Griffiths, J.A.; Thuy-Boun, P.; Cantu, V.; Weldon, K.; Challis, C.; Sweredoski, M.J.; Chan, K.Y.; Thron, T.M.; Sharon, G.; et al. Targeted Neuronal Activation of the Gastrointestinal Tract Shapes the Environment of the Gut in Mice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Olivier, B.J.; Cailotto, C.; van der Vliet, J.; Knippenberg, M.; Greuter, M.J.; Hilbers, F.W.; Konijn, T.; te Velde, A.A.; Nolte, M.A.; Boeckxstaens, G.E.; et al. Vagal innervation is required for the formation of tertiary lymphoid tissue in colitis. Eur. J. Immunol. 2016, 46, 2467–2480. [Google Scholar] [CrossRef] [Green Version]
- Bucinskaite, V.; Kurosawa, M.; Lundeberg, T. Exogenous cholecystokinin-8 reduces vagal efferent nerve activity in rats through CCK(A) receptors. Br. J. Pharmacol. 2000, 129, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Baltazar, D.; Zavala-Flores, L.M.; Villanueva-Olivo, A. The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model. Neurology 2017, 32, 533–539. [Google Scholar] [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [Google Scholar] [CrossRef]
- Calvani, M.; Dabraio, A.; Bruno, G.; de Gregorio, V.; Coronnello, M.; Bogani, C.; Ciullini, S.; La Marca, G.; Vignoli, M.; Chiarugi, P.; et al. β3-Adrenoreceptor Blockade Reduces Hypoxic Myeloid Leukemic Cells Survival and Chemoresistance. Int. J. Mol. Sci. 2020, 21, 4210. [Google Scholar] [CrossRef] [PubMed]
- Ağaç, D.; Estrada, L.D.; Maples, R.; Hooper, L.V.; Farrar, J.D. The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav. Immun. 2018, 74, 176–185. [Google Scholar] [CrossRef]
- Vasina, V.; Abu-Gharbieh, E.; Barbara, G.; de Giorgio, R.; Colucci, R.; Blandizzi, C.; Bernardini, N.; Croci, T.; del Tacca, M.; de Ponti, F. The beta3-adrenoceptor agonist SR58611A ameliorates experimental colitis in rats. Neurogastroenterol. Motil. 2008, 20, 1030–1041. [Google Scholar] [CrossRef]
- Hume, D.A. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol. 2008, 1, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; MacDonald, K.P.A. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Wager, C.L.; Wormley, F.L. Classical versus alternative macrophage activation: The Ying and the Yang in host defense against pulmonary fungal infections. Mucosal. Immunol. 2014, 7, 1023–1035. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, T.A.; Zhao, C.; Pavicic, P.G.; Datta, S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front. Immunol. 2014, 5, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The Role of Neuropeptide Y in Cardiovascular Health and Disease. Frontiers in Physiology, 9. Front. Physiol. 2018, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Schlegel, M.; Kaussen, T.; Gudernatsch, V.; Hansmann, G.; Schumacher, T.; Giera, M.; Mirakaj, V. Sympathetic nervous system controls resolution of inflammation via regulation of repulsive guidance molecule A. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- De Winter, B.Y.; Boeckxstaens, G.E.; de Man, J.G.; Moreels, T.G.; Herman, A.G.; Pelckmans, P.A. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br. J. Pharmacol. 1997, 120, 464–468. [Google Scholar] [CrossRef]
- Fukuda, H.; Tsuchida, D.; Koda, K.; Miyazaki, M.; Pappas, T.N.; Takahashi, T. Inhibition of sympathetic pathways restores postoperative ileus in the upper and lower gastrointestinal tract. J. Gastroenterol. Hepatol. 2007, 22, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Zittel, T.T.; Reddy, S.N.; Plourde, V.; Raybould, H.E. Role of spinal afferents and calcitonin gene-related peptide in the postoperative gastric ileus in anesthetized rats. Ann. Surg. 1994, 219, 79–87. [Google Scholar] [CrossRef]
- Kalff, J.C.; Carlos, T.M.; Schraut, W.H.; Billiar, T.R.; Simmons, R.L.; Bauer, A.J. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 1999, 117, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wan, Y.; Liu, Y.; Yang, Y.; Tang, J.; Huang, W.; Cheng, B. Sympathetic Denervation Accelerates Wound Contraction but Inhibits Reepithelialization and Pericyte Proliferation in Diabetic Mice. J. Diabetes Res. 2017, 2017, 7614685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Ippolito, C.; Segnani, C.; Dolfi, A.; Errede, M.; Virgintino, D.; Fornai, M.; Antonioli, L.; Garelli, F.; Nericcio, A.; et al. Pathological remodelling of colonic wall following dopaminergic nigrostriatal neurodegeneration. Neurobiol. Dis. 2020, 139, 104821. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Dunbrasky, D.L.; Taishi, P.; Souza, G.; Krueger, J.M. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-α and lipopolysaccharide in mice. Sleep 2013, 36, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Vilz, T.O.; Overhaus, M.; Stoffels, B.; von Websky, M.; Kalff, J.C.; Wehner, S. Functional assessment of intestinal motility and gut wall inflammation in rodents: Analyses in a standardized model of intestinal manipulation. JoVE 2012, e4086. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallesh, S.; Schneider, R.; Schneiker, B.; Lysson, M.; Efferz, P.; Lin, E.; de Jonge, W.J.; Wehner, S. Sympathetic Denervation Alters the Inflammatory Response of Resident Muscularis Macrophages upon Surgical Trauma and Ameliorates Postoperative Ileus in Mice. Int. J. Mol. Sci. 2021, 22, 6872. https://doi.org/10.3390/ijms22136872
Mallesh S, Schneider R, Schneiker B, Lysson M, Efferz P, Lin E, de Jonge WJ, Wehner S. Sympathetic Denervation Alters the Inflammatory Response of Resident Muscularis Macrophages upon Surgical Trauma and Ameliorates Postoperative Ileus in Mice. International Journal of Molecular Sciences. 2021; 22(13):6872. https://doi.org/10.3390/ijms22136872
Chicago/Turabian StyleMallesh, Shilpashree, Reiner Schneider, Bianca Schneiker, Mariola Lysson, Patrik Efferz, Eugene Lin, Wouter J de Jonge, and Sven Wehner. 2021. "Sympathetic Denervation Alters the Inflammatory Response of Resident Muscularis Macrophages upon Surgical Trauma and Ameliorates Postoperative Ileus in Mice" International Journal of Molecular Sciences 22, no. 13: 6872. https://doi.org/10.3390/ijms22136872
APA StyleMallesh, S., Schneider, R., Schneiker, B., Lysson, M., Efferz, P., Lin, E., de Jonge, W. J., & Wehner, S. (2021). Sympathetic Denervation Alters the Inflammatory Response of Resident Muscularis Macrophages upon Surgical Trauma and Ameliorates Postoperative Ileus in Mice. International Journal of Molecular Sciences, 22(13), 6872. https://doi.org/10.3390/ijms22136872





