Lanthionine, a Novel Uremic Toxin, in the Vascular Calcification of Chronic Kidney Disease: The Role of Proinflammatory Cytokines
Abstract
:1. Introduction
2. Results
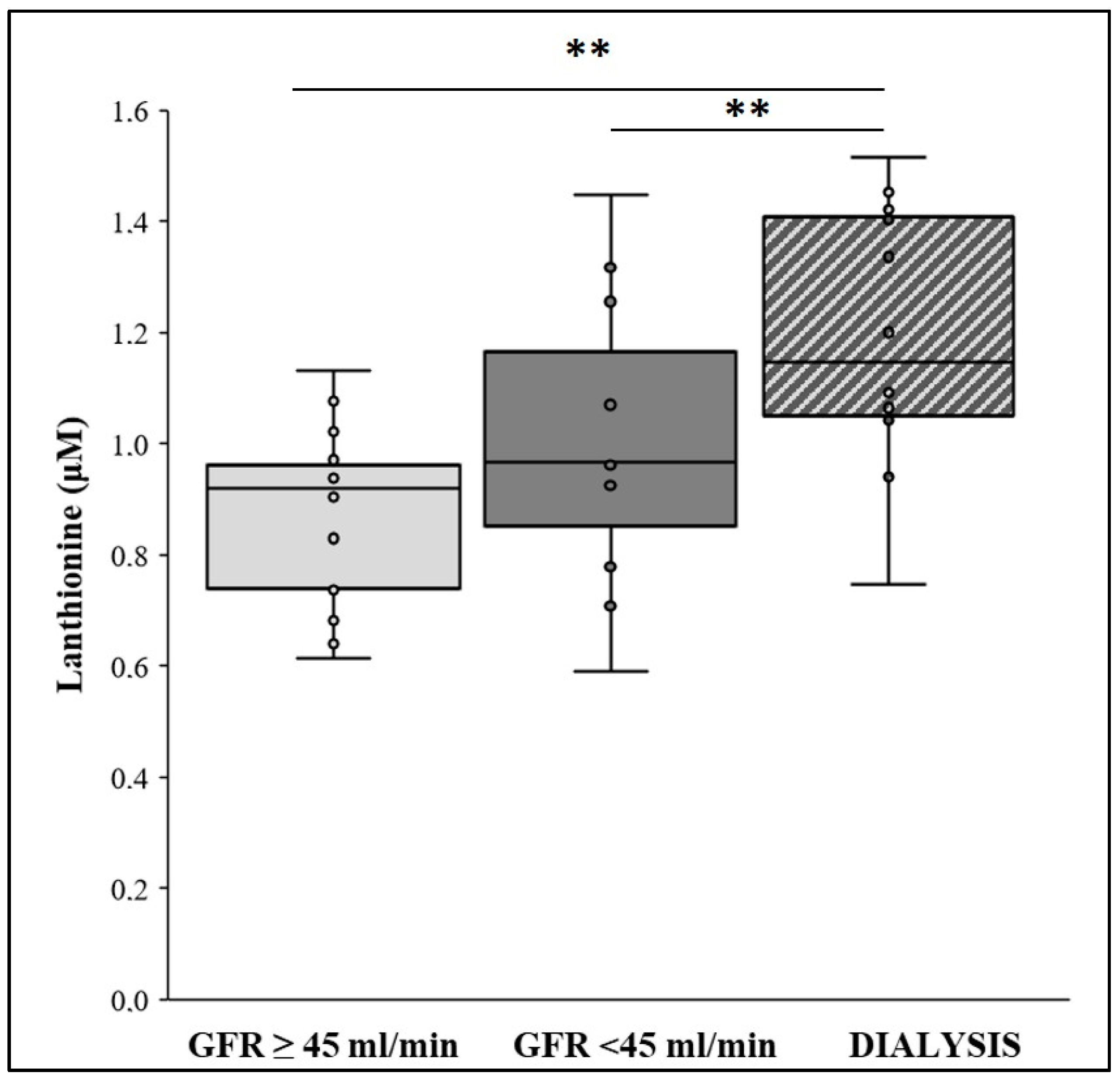
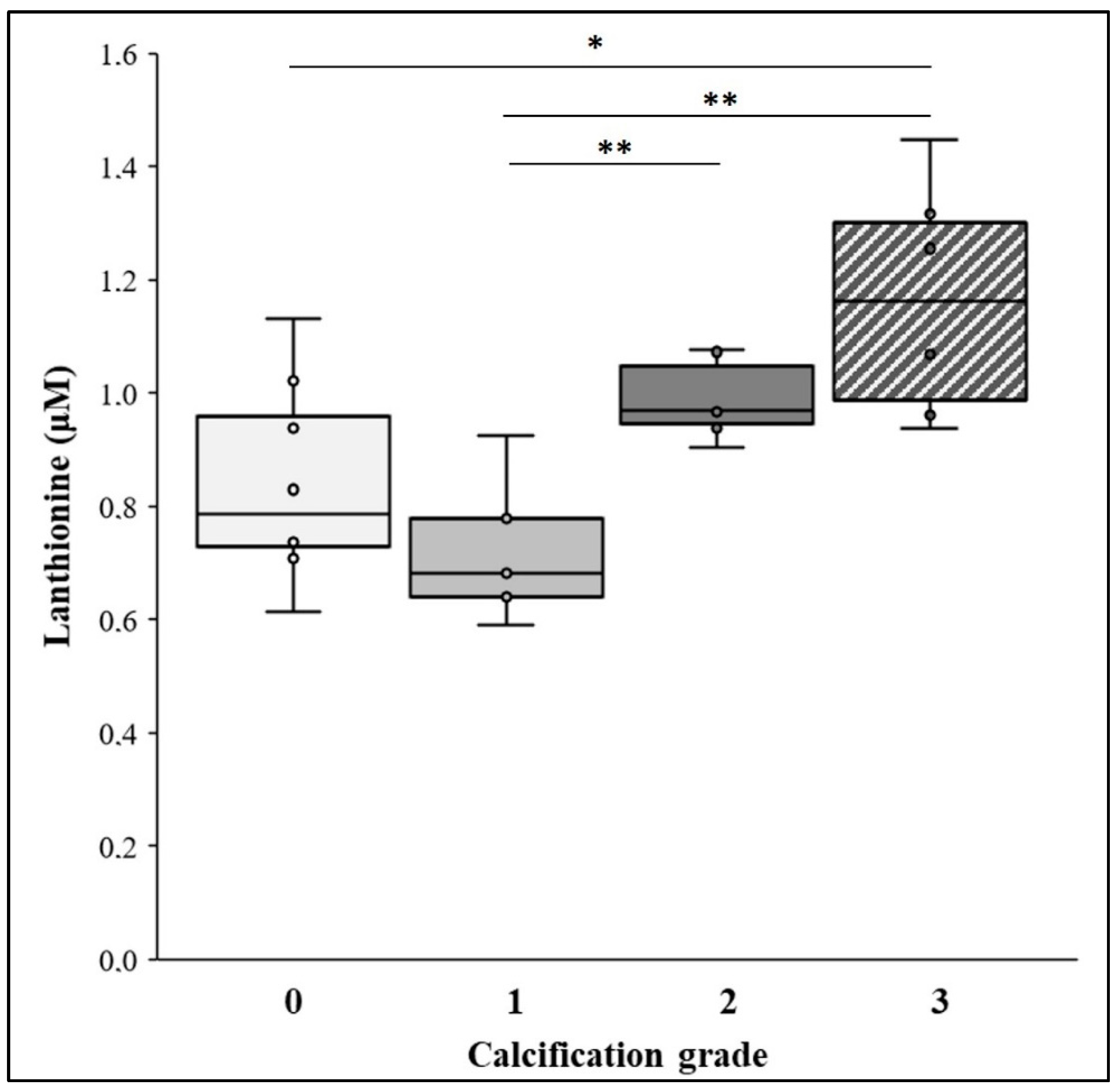
2.1. Lanthionine Concentration Is Increased in CKD and Hemodialysis Patients and in Higher VC Grades
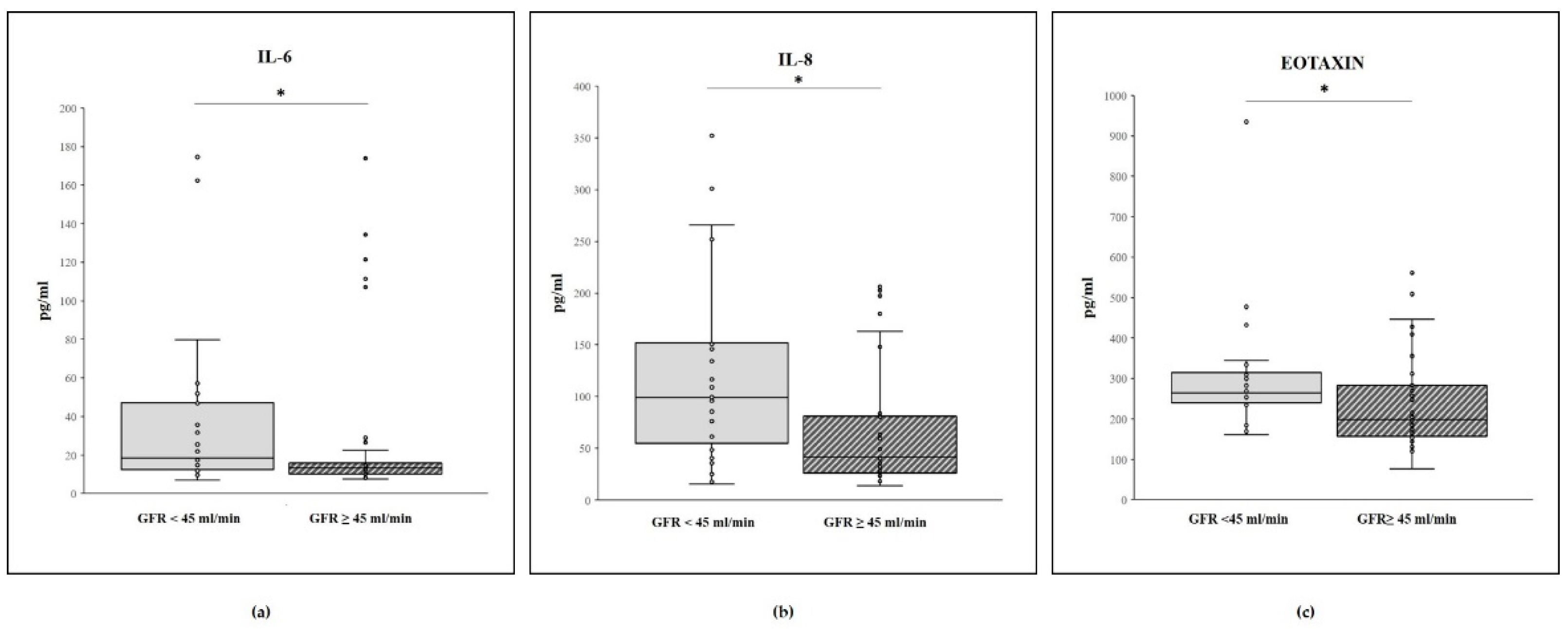
2.2. Cytokine Concentrations and GFR Level
2.3. Cytokine Concentrations and Calcification Score
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Lanthionine Analysis by UHPLC/MS Method
4.3. Cytokinome Analysis
4.4. Other Analytes
4.5. Calcification Assessment
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bover, J.; Evenepoel, P.; Vervloet, M.G.; Brandenburg, V.; Mazzaferro, S.; Covic, A.; Goldsmith, D.; Massy, Z.A.; Cozzolino, M.; on behalf of the CKD-MBD Working Group of ERA-EDTA. Pro: Cardiovascular calcifications are clinically relevant. Nephrol. Dial. Transplant. 2015, 30, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccali, C.; London, G.; Bover, J.; Evenepoel, P.; Ureña-Torres, P.; Vervloet, M.G.; Brandenburg, V.; Mazzaferro, S.; Covic, A.; Goldsmith, D.; et al. Con: Vascular calcification is a surrogate marker, but not the cause of ongoing vascular disease, and it is not a treatment target in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.; Cozzolino, M. Vascular calcification in chronic kidney disease: Different bricks in the wall? Kidney Int. 2017, 91, 808–817. [Google Scholar] [CrossRef]
- Cianciolo, G.; Galassi, A.; Capelli, I.; Schillaci, R.; La Manna, G.; Cozzolino, M. Klotho-FGF23, Cardiovascular Disease, and Vascular Calcification: Black or White? Curr. Vasc. Pharmacol. 2018, 16, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Di Lullo, L.; Russo, D.; Ciarcia, R.; Magnocavallo, M.; Lavalle, C.; Ratti, C.; Fusaro, M.; Cozzolino, M.; Di Iorio, B. Predictive Value of Measures of Vascular Calcification Burden and Progression for Risk of Death in Incident to Dialysis Patients. J. Clin. Med. 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Dusing, P.; Zietzer, A. Vascular pathologies in chronic kidney disease: Pathophysiological mechamisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef]
- Voelkl, J.; Egli-Spichtig, D. Inflammation: A putative link between phosphate metabolism and cardiovascular disease. Clin. Sci. 2021, 135, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Cobo, G.; Dai, L.; Lindholm, B.; Stenvinkel, P. Role of Uremic Toxins in Early Vascular Ageing and Calcification. Toxins 2021, 13, 26. [Google Scholar] [CrossRef]
- Di Lullo, L.; Gorini, A.; Bellasi, A.; Morrone, L.F.P.; Rivera, R.; Russo, L.; Santoboni, A.; Russo, M. Fibroblast growth factor 23 and parathyroid hormone predict extent of aortic valve calcifications in patients with mild to moderate chronic kidney disease. Clin. Kidney J. 2015, 8, 732–736. [Google Scholar] [CrossRef] [Green Version]
- Perna, A.F.; Pizza, A.; Di Nunzio, A.; Bellantone, R.; Raffaelli, M.; Cicchella, T.; Conzo, G.; Santini, L.; Zacchia, M.; Trepiccione, F.; et al. ADAM17, a New Player in the Pathogenesis of Chronic Kidney Disease—Mineral and Bone Disorder. J. Ren. Nutr. 2017, 27, 453–457. [Google Scholar] [CrossRef]
- Belmokhtar, K.; Ortillon, J.; Jaisson, S.; Massy, Z.A.; Rombi, C.B.; Doué, M.; Maurice, P.; Fritz, G.; Gillery, P.; Schmidt, A.M.; et al. Receptor for advanced glycation end products: A key molecule in the genesis of chronic kidney disease vascular calcification and a potential modulator of sodium phosphate co-transporter PIT-1 expression. Nephrol. Dial. Transplant. 2019, 34, 2018–2030. [Google Scholar] [CrossRef]
- Conzo, G.; Perna, A.F. Total parathyroidectomy without autotransplantation in the surgical treatment of secondary hyperparathyroidism of chronic kidney disease. J. Endocrinol. Investig. 2012, 35, 8–13. [Google Scholar]
- Conzo, G.; Perna, A.F. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. BMC Surg. 2013, 13, S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perna, A.F.; Ingrosso, D.; Satta, E.; Lombardi, C.; Acanfora, F.; De Santo, N.G. Homocysteine metabolism in renal failure. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D. Plasma protein aspartyl damage is increased in hemodialysis patients: Studies on causes and consequences. J. Am. Soc. Nephrol. 2004, 15, 2747–2754. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Corrao, S.; Battaglia, Y.; Andreucci, M.; Caiazza, A.; Carlomagno, A.; Lamberti, M.; Pezone, N.; Pota, A.; Russo, L.; et al. Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int. 2011, 80, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Perna, A.F.; Zacchia, M.; Trepiccione, F.; Ingrosso, D. The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin. Toxins 2017, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Perna, A.F.; Anishchenko, E.; Vigorito, C.; Zacchia, M.; Trepiccione, F.; D’Aniello, S.; Ingrosso, D. Zebrafish, a Novel Model System to Study Uremic Toxins: The Case for the Sulfur Amino Acid Lanthionine. Int. J. Mol. Sci. 2018, 19, 1323. [Google Scholar] [CrossRef] [Green Version]
- Vigorito, C.; Anishchenko, E.; Mele, L.; Capolongo, G.; Trepiccione, F.; Zacchia, M.; Lombari, P.; Capasso, R.; Ingrosso, D.; Perna, A.F. Uremic Toxin Lanthionine Interferes with the Transsulfuration Pathway, Angiogenetic Signaling and Increases Intracellular Calcium. Int. J. Mol. Sci. 2019, 20, 2269. [Google Scholar] [CrossRef] [Green Version]
- Perna, A.F.; Pane, F. Lanthionine and Other Relevant Sulfur Amino Acid Metabolites: Detection of Prospective Uremic Toxins in Serum by Multiple Reaction Monitoring Tandem Mass Spectrometry. Methods Mol. Biol. 2019, 2007, 9–17. [Google Scholar]
- Perna, A.F.; Di Nunzio, A.; Amoresano, A.; Pane, F.; Fontanarosa, C.; Pucci, P.; Vigorito, C.; Cirillo, G.; Zacchia, M.; Trepiccione, F.; et al. Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 2016, 126, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Golembiewska, E. End-Stage Renal Disease. Inflammation and Cardiovascular Outcomes. In Expanded Hemodialysis—Innovative Clinical Approach in Dialysis; Ronco, C., Contrib, N., Eds.; Karger: Basel, Switzerland, 2017; Volume 191, pp. 32–43. [Google Scholar]
- Benz, K.; Hilgers, K.-F.; Daniel, C.; Amann, K. Vascular Calcification in Chronic Kidney Disease: The Role of Inflammation. Int. J. Nephrol. 2018, 2018, 4310379. [Google Scholar] [CrossRef] [PubMed]
- Lanza, D.; Perna, A.F.; Oliva, A.; Vanholder, R.; Pletinck, A.; Guastafierro, S.; Di Nunzio, A.; Vigorito, C.; Capasso, G.; Jankowski, V.; et al. Impact of the Uremic Milieu on the Osteogenic Potential of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0116468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- Fuster, J.J.; Walsh, K. The Good, the Bad, and the Ugly of interleukin-6 signaling. EMBO J. 2014, 33, 1425–1427. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; Nahas, M.E.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Delanaye, P.; Jager, K.J.; Bökenkamp, A.; Christensson, A.; Dubourg, L.; Eriksen, B.O.; Gaillard, F.; Gambaro, G.; Van Der Giet, M.; Glassock, R.J.; et al. CKD: A Call for an Age-Adapted Definition. J. Am. Soc. Nephrol. 2019, 30, 1785–1805. [Google Scholar] [CrossRef]
- Pisano, S.; Catone, G.; Coppola, G.; Carotenuto, M.; Iuliano, R.; Tiano, C.; Montesanto, A.R.; D’Esposito, V.; Del Giudice, E.M.; Formisano, P.; et al. Different Immune Signature in Youths Experiencing Antipsychotic-Induced Weight Gain Compared to Untreated Obese Patients. J. Child Adolesc. Psychopharmacol. 2017, 27, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Agharazii, M.; St-Louis, R. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, M.-P.; Sidibé, A.; Fortier, C.; Mac-Way, F.; Marquis, K.; De Serres, S.; Larivière, R.; Agharazii, M. Association of interleukin-6 with aortic stiffness in end-stage renal disease. J. Am. Soc. Hypertens. 2018, 12, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Peyster, E.; Chen, J.; Feldman, H.I.; Go, A.S.; Gupta, J.; Mitra, N.; Pan, Q.; Porter, A.; Rahman, M.; Raj, D.; et al. Inflammation and Arterial Stiffness in Chronic Kidney Disease: Findings from the CRIC Study. Am. J. Hypertens. 2017, 30, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreillon, J.J.; Bowden, R.G.; Deike, E.; Griggs, J.; Wilson, R.; Shelmadine, B.; Cooke, M.; Beaujean, A. The use of an anti-inflammatory supplement in patients with chronic kidney disease. J. Complement. Integr. Med. 2013, 10, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Mendelsohn, A.; Larrick, J.W. Interleukin-7 and Immunosenescence. J. Immunol. Res. 2017, 2017, 4807853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.; Jo, Y.; Lee, B.; Ali, L.A.; Lee, B.; Hong, C. The Potential Role of a Soluble γ-Chain Cytokine Receptor as a Regulator of IL-7-Induced Lymphoproliferative Disorders. Int. J. Mol. Sci. 2018, 19, 3375. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.; Zhao, L.; Zhang, Y.; Ma, Q. Interleukin-7 stimulation inhibits nephrin activation and induces podocyte injury. Biochem. Biophys. Res. Commun. 2018, 507, 100–105. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, F.; Bai, P.; Dong, N.; Chu, C. Identification of key genes and pathways contributing to artery tertiary lymphoid organ development in advanced mouse atherosclerosis. Mol. Med. Rep. 2019, 19, 3071–3086. [Google Scholar] [CrossRef] [Green Version]
- Parisi, V.; Petraglia, L.; Cabaro, S.; D’Esposito, V.; Bruzzese, D.; Ferraro, G.; Urbani, A.; Grieco, F.V.; Conte, M.; Caruso, A.; et al. Imbalance Between Interleukin-1β and Interleukin-1 Receptor Antagonist in Epicardial Adipose Tissue Is Associated With Non ST-Segment Elevation Acute Coronary Syndrome. Front. Physiol. 2020, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.J.; Wu, Z.F. Effects of interleukin-7/interleukin-7 receptor on RANKL-mediated osteoclast differentiation and ovari-ectomy-induced bone loss by regulating c-Fos/c-Jun pathway. J. Cell Physiol. 2018, 233, 7182–7194. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Debnath, B. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef]
- Bouabdallah, J.; Zibara, K.; Issa, H.; Lenglet, G.; Kchour, G.; Caus, T.; Six, I.; Choukroun, G.; Kamel, S.; Bennis, Y. Endothelial cells exposed to phosphate and indoxyl sulphate promote vascular calcification through interleukin-8 secretion. Nephrol. Dial. Transplant. 2019, 34, 1125–1134. [Google Scholar] [CrossRef]
- Watford, W.T.; Moriguchi, M.; Morinobu, A.; O’Shea, J.J. The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003, 14, 361–368. [Google Scholar] [CrossRef]
- Yong, K.; Ooi, E.M. Elevated interleukin-12 and interleukin-18 in chronic kidney disease are not associated with arterial stiffness. Cytokine 2013, 64, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.E.; Hamm, L.L.; Batuman, G.; Kumbala, D.R.; Chen, C.-S.; Kallu, S.G.; Siriki, R.; Gadde, S.; Kleinpeter, M.A.; Krane, N.K.; et al. The association of angiogenic factors and chronic kidney disease. BMC Nephrol. 2018, 19, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, T.J. Eotaxin-1 (CCL11). Front. Immunol. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, A.; Baffi, J.Z.; Kleinman, M.E.; Cho, W.G.; Nozaki, M.; Yamada, K.; Kaneko, H.; Albuquerque, R.J.C.; Dridi, S.; Saito, K.; et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nat. Cell Biol. 2009, 460, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF23 and Phosphate–Cardiovascular Toxins in CKD. Toxins 2019, 11, 647. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Giachelli, C.M. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017, 100, 87–93. [Google Scholar] [CrossRef]
- Martin-Nuñez, E.; Donate-Correa, J.; Muros-De-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Implications of Klotho in vascular health and disease. World J. Cardiol. 2014, 6, 1262–1269. [Google Scholar] [CrossRef]
- Xie, J.; Cha, S.-K.; An, S.-W.; Kuro-O, M.; Birnbaumer, L.; Huang, C.-L. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waziri, B.; Duarte, R.; Naicker, S. Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD): Current Perspectives. Int. J. Nephrol. Renov. Dis. 2019, 12, 263–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease. Kidney Dis. 2017, 3, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Solari, R.; Pease, J.E. Chemokine receptors as therapeutic targets: Why aren’t there more drugs? Eur. J. Pharmacol. 2015, 746, 363–367. [Google Scholar] [CrossRef]
- Laurentius, T.; Raffetseder, U.; Fellner, C.; Kob, R.; Nourbakhsh, M.; Floege, J.; Bertsch, T.; Bollheimer, L.C.; Ostendorf, T. High-fat diet-induced obesity causes an inflammatory microenvironment in the kidneys of aging Long-Evans rats. J. Inflamm. 2019, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Carrero, J.J.K.; Lindholm, B.; Stenvinkel, P. Therapeutics targeting persistent inflammation in chronic kidney dis-ease. Transl. Res. 2016, 167, 204–213. [Google Scholar] [CrossRef]

| Variable (pg/mL) | GFR < 45 mL/min/1.73 m2 (n = 28; 37.8%) | GFR ≥ 45 mL/min/1.73 m2 (n = 46; 62.2%) | p-Value (Mann–Whitney U Test) |
|---|---|---|---|
| Hu PDGF-bb | 7157 [4929; 8710] | 7492 [5802; 9657] | 0.382 |
| Hu IL-1b | 2.3 [1.7; 4.3] | 1.8 [1.5; 3.5] | 0.206 |
| Hu IL-1ra | 164.9 [119.8; 210.3] | 148.8 [127.8; 197.3] | 0.895 |
| Hu IL-4 | 18.2 [15.7; 21] | 19.7 [18.2; 21.5] | 0.092 |
| Hu IL-5 | 1.9 [1.1; 2.8] | 1.9 [1.4; 2.6] | 0.972 |
| Hu IL-6 | 18.4 [12; 48.1] | 13.3 [10.1; 20.9] | 0.033 |
| Hu IL-7 | 9.1 [5; 11.2] | 8.1 [6.9; 10.3] | 0.834 |
| Hu IL-8 | 99.4 [48.8; 153.1] | 41.7 [25.9; 83] | 0.010 |
| Hu IL-9 | 36.6 [26.4; 46.7] | 33.2 [27.2; 44.3] | 0.572 |
| Hu IL-10 | 25.4 [12.4; 39.2] | 22.4 [15.1; 30.1] | 0.281 |
| Hu IL-12 (P70) | 112.7 [89; 184.7] | 111.6 [72.4; 137.4] | 0.286 |
| Hu IL-13 | 12.8 [8.9; 16.8] | 11.8 [9.3; 14.2] | 0.582 |
| Hu IL-17 | 160.2 [125.4; 185.7] | 163.7 [128; 205.6] | 0.569 |
| Hu Eotaxin | 264.7 [237.5; 327.4] | 198.7 [159.1; 286.7] | 0.009 |
| Hu FGF basic | 36.2 [23.8; 42.1] | 30.4 [25.4; 36.7] | 0.173 |
| Hu G-CSF | 74.1 [54.8; 95.7] | 73.6 [66.4; 84.3] | 0.599 |
| Hu IFN-g | 94.3 [64.2; 127.3] | 99.1 [82; 122.7] | 0.158 |
| Hu IP-10 | 1186 [863; 1623] | 1003 [764; 1302] | 0.057 |
| Hu MCP-1 (MCAF) | 95.1 [64.7; 150.3] | 106.3 [67.7; 162.5] | 0.598 |
| Hu MIP-1a | 7.2 [4.9; 11.4] | 5 [4.1; 7.4] | 0.067 |
| Hu MIP-1b | 158.6 [104.2; 297.8] | 155.4 [101.7; 210.7] | 0.613 |
| Hu RANTES | 31,075 [16,841; 37,995] | 31,100 [24,342; 36,450] | 0.828 |
| Hu TNF- | 39.8 [29.2; 55.5] | 45.7 [33.6; 68.2] | 0.149 |
| Hu VEGF | 293.4 [182.2; 453.2] | 232.4 [139; 371.3] | 0.179 |
| Variable (pg/mL) | No Calcification (n = 34; 45.9%) | Calcification (n = 40; 54.1%) | p-Value (Mann–Whitney U Test) |
|---|---|---|---|
| Hu PDGF-bb | 7530 [5620; 8484] | 7282 [5540; 10,722] | 0.638 |
| Hu IL-1b | 1.8 [1.5; 2.2] | 2.3 [1.7; 5] | 0.025 |
| Hu IL-1ra | 148.8 [126.2; 194.1] | 174.6 [119.8; 211.1] | 0.508 |
| Hu IL-4 | 18.7 [16.9; 21.2] | 19.6 [18.2; 21.8] | 0.344 |
| Hu IL-5 | 1.9 [1.1; 2.7] | 1.9 [1.4; 2.6] | 0.876 |
| Hu IL-6 | 13.3 [10.1; 24] | 16.5 [10.8; 44.1] | 0.114 |
| Hu IL-7 | 7.4 [6; 9.2] | 9.2 [7.7; 11.1] | 0.009 |
| Hu IL-8 | 43.2 [26.1; 90] | 80.1 [35.8; 159.8] | 0.046 |
| Hu IL-9 | 33.9 [27.4; 48.5] | 34.5 [26.5; 44.4] | 0.719 |
| Hu IL-10 | 19.8 [12.7; 30.1] | 24 [16.6; 34.7] | 0.269 |
| Hu IL-12 (P70) | 104.8 [62.5; 120.2] | 124.2 [89.6; 184.7] | 0.018 |
| Hu IL-13 | 11.6 [8.6; 14.6] | 12.4 [9.6; 15.8] | 0.364 |
| Hu IL-17 | 163.7 [120.4; 182.8] | 161.4 [134.4; 220.5] | 0.285 |
| Hu Eotaxin | 208 [160.9; 263.8] | 265.8 [184.3; 317.5] | 0.031 |
| Hu FGF basic | 32.1 [23.3; 36] | 36.2 [27.7; 43.3] | 0.120 |
| Hu G-CSF | 71.7 [60.1; 80.3] | 77.4 [65.9; 96.4] | 0.154 |
| Hu IFN-g | 99.1 [75.7; 118.1] | 99.1 [73.2; 127.3] | 0.791 |
| Hu IP-10 | 972 [789; 1317] | 1112 [819; 1585] | 0.235 |
| Hu MCP-1 (MCAF) | 110.6 [72.9; 158.9] | 93.4 [62; 179.6] | 0.429 |
| Hu MIP-1a | 5.4 [4.3; 10] | 5.8 [4.1; 10.2] | 0.870 |
| Hu MIP-1b | 158 [110; 210.5] | 149.3 [101.4; 303.2] | 0.827 |
| Hu RANTES | 30,480 [22,736; 35,399] | 31,453 [22,328; 37,531] | 0.576 |
| Hu TNF- | 43.2 [31.1; 64] | 46.5 [33.6; 68.2] | 0.335 |
| Hu VEGF | 174 [99.2; 353.9] | 298.3 [203.6; 467.8] | 0.012 |
| Variable | Non-Calcified | Calcified |
|---|---|---|
| AGE (YEARS) | 53.6 ± 12.2 | 67.8 ± 8.7 |
| SEX (M; %) | 66 | 85 |
| DIABETES (%) | 8 | 25 |
| HYPERTENSION (%) | 87 | 100 |
| AGATSTON SCORE (AU) | 0 | 248 (2–734) |
| BMI (KG/M2) | 27.8 ± 5.9 | 28.9 ± 5.4 |
| E-GFR (ML/MIN) | 75 ± 41 | 71.7 ± 25 |
| DURATION CKD (MONTHS) | 24.5 ± 43 | 17 ± 25 |
| PROTEINURIA (MG/24H) | 303 ± 577 | 255 ± 476 |
| CALCEMIA (MG/DL) | 9.5 ± 1.3 | 9.6 ± 0.5 |
| PHOSPHOREMIA (MG/DL) | 3.7 ± 1.4 | 3.7 ± 0.4 |
| PHOSPHATURIA (MG/24H) | 858 ± 370 | 807 ± 290 |
| PTH (PG/ML) | 58 ± 20 | 53 ± 35 |
| HOMOCYSTEINE (µMOLI/L) | 19 ± 9 | 19 ± 4 |
| CRP (MG/L) | 0.4 ± 0.2 | 0.3 ± 0.1 |
| URIC ACID (MG/DL) | 6.0 ± 1.3 | 6.2 ± 1.1 |
| HEMOGLOBIN (G/DL) | 14.2 ± 1.1 | 14.9 ± 1.9 |
| CHOLESTEROL (MG/DL) | 183 ± 48 | 195 ± 39 |
| HDL-CHOLESTEROL (MG/DL) | 51 ± 14 | 40 ± 11 |
| TRIGLYCERIDES (MG/DL) | 130 ± 52 | 182 ± 80 |
| LDL-CHOLESTEROL (MG/DL) | 103 ± 41 | 110 ± 45 |
| FIBRINOGEN (MG/DL) | 315 ± 79 | 366 ± 78 |
| SAP (MMHG) | 127 ± 13 | 138 ± 8 |
| DAP (MMHG) | 80 ± 13 | 82 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perna, A.F.; Russo, L.; D’Esposito, V.; Formisano, P.; Bruzzese, D.; Vigorito, C.; Coppola, A.; Lombari, P.; Russo, D.; Ingrosso, D. Lanthionine, a Novel Uremic Toxin, in the Vascular Calcification of Chronic Kidney Disease: The Role of Proinflammatory Cytokines. Int. J. Mol. Sci. 2021, 22, 6875. https://doi.org/10.3390/ijms22136875
Perna AF, Russo L, D’Esposito V, Formisano P, Bruzzese D, Vigorito C, Coppola A, Lombari P, Russo D, Ingrosso D. Lanthionine, a Novel Uremic Toxin, in the Vascular Calcification of Chronic Kidney Disease: The Role of Proinflammatory Cytokines. International Journal of Molecular Sciences. 2021; 22(13):6875. https://doi.org/10.3390/ijms22136875
Chicago/Turabian StylePerna, Alessandra Fortunata, Luigi Russo, Vittoria D’Esposito, Pietro Formisano, Dario Bruzzese, Carmela Vigorito, Annapaola Coppola, Patrizia Lombari, Domenico Russo, and Diego Ingrosso. 2021. "Lanthionine, a Novel Uremic Toxin, in the Vascular Calcification of Chronic Kidney Disease: The Role of Proinflammatory Cytokines" International Journal of Molecular Sciences 22, no. 13: 6875. https://doi.org/10.3390/ijms22136875
APA StylePerna, A. F., Russo, L., D’Esposito, V., Formisano, P., Bruzzese, D., Vigorito, C., Coppola, A., Lombari, P., Russo, D., & Ingrosso, D. (2021). Lanthionine, a Novel Uremic Toxin, in the Vascular Calcification of Chronic Kidney Disease: The Role of Proinflammatory Cytokines. International Journal of Molecular Sciences, 22(13), 6875. https://doi.org/10.3390/ijms22136875







