Roles of Cytokines in the Temporal Changes of Microglial Membrane Currents and Neuronal Excitability and Synaptic Efficacy in ATP-Induced Cortical Injury Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs (Chemicals)
2.3. Stereotaxic Surgery for ATP Injection and BrdU Injection
2.4. Immunohistochemistry and Imaging
2.5. Confocal Microscope Imaging and Analysis
2.6. Slice Preparation and Electrophysiology
2.7. Statistical Analysis
3. Results
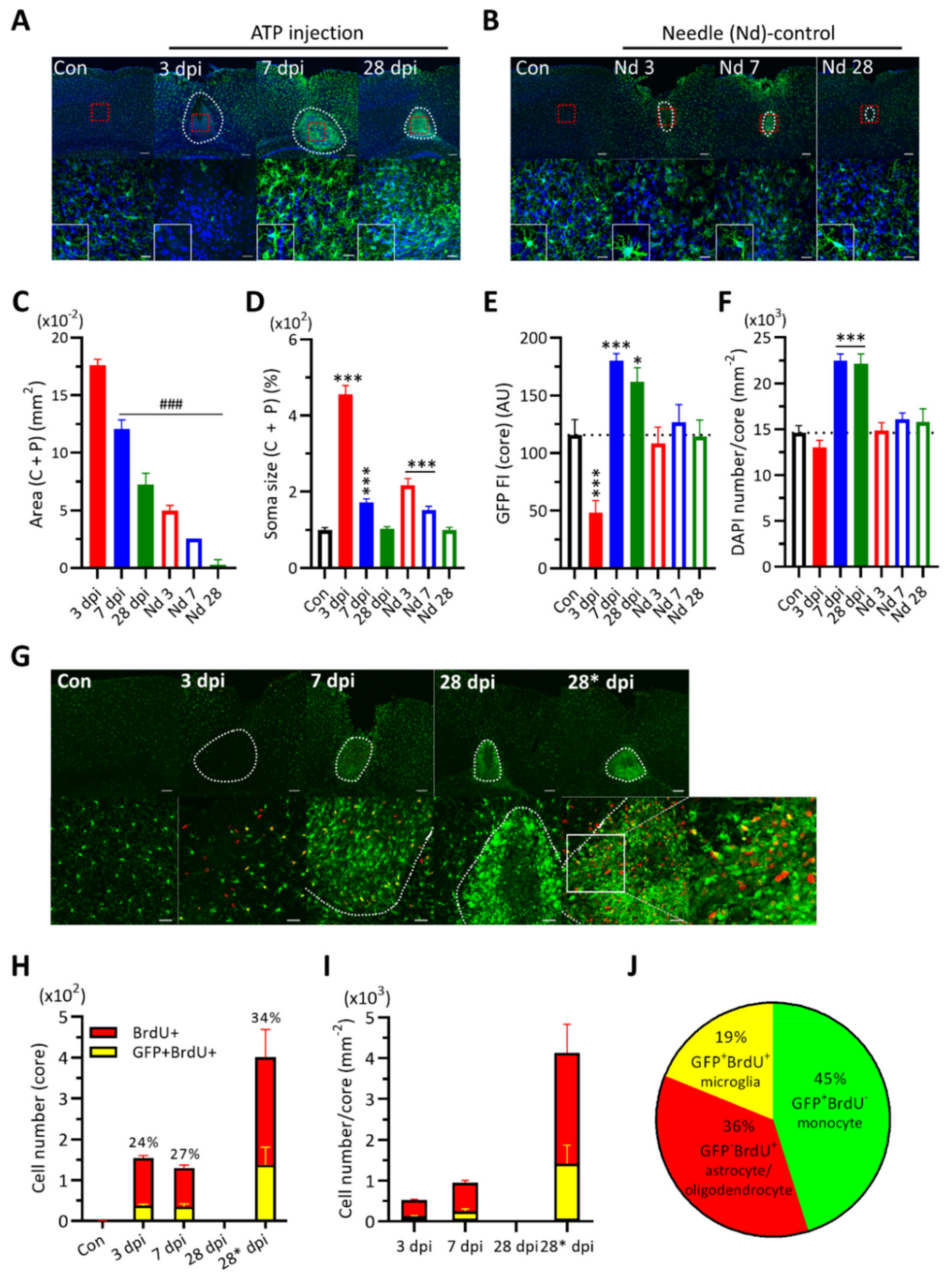
3.1. Changes in CX3CR1-GFP (+) Cell Morphology and Number in Damaged Cortical Area
3.2. Expression of Cytokines and Their Co-Labeling with Some Core CX3CR1 (+) Cells
3.3. Changes of Excitability and Synaptic Functions of Cortical Neurons after ATP Injection
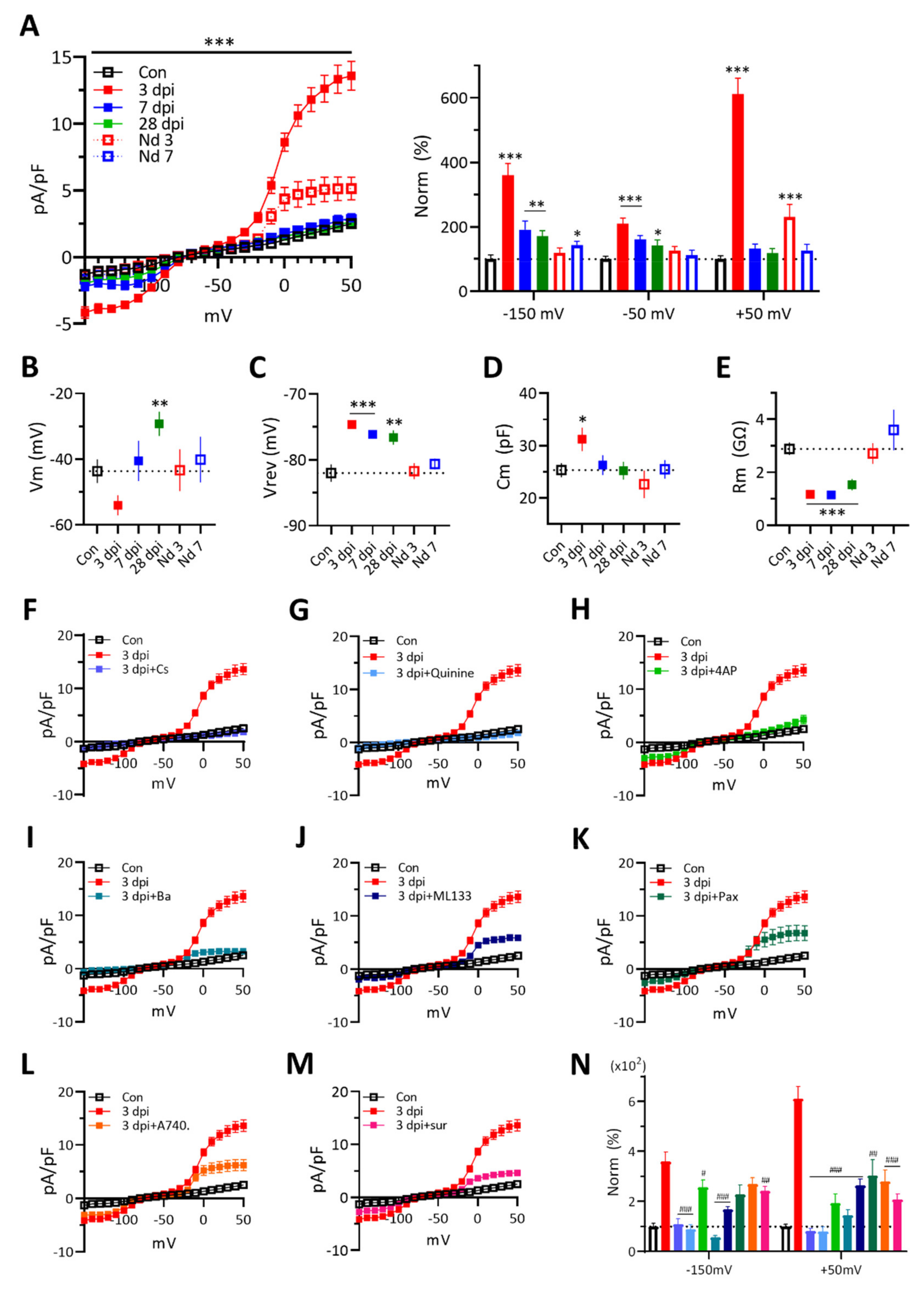
3.4. Changes of Microglial Membrane Currents in Response to Voltage Ramp Stimuli after ATP Injection
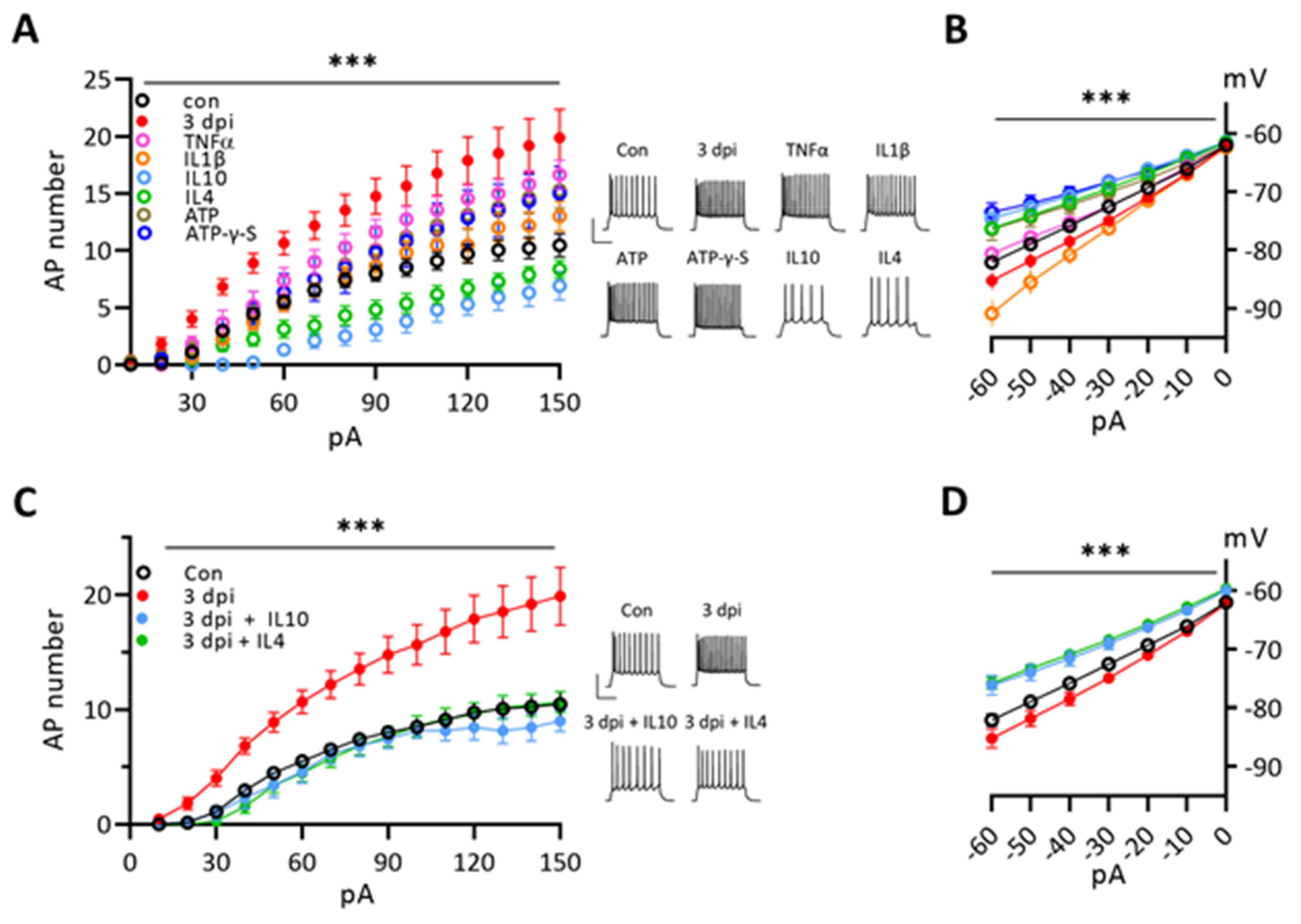
3.5. Cytokines Modulate the Excitability of Naïve Neurons and Anti-Inflammatory Cytokines Suppress the Enhanced Excitability of 3 dpi Neurons
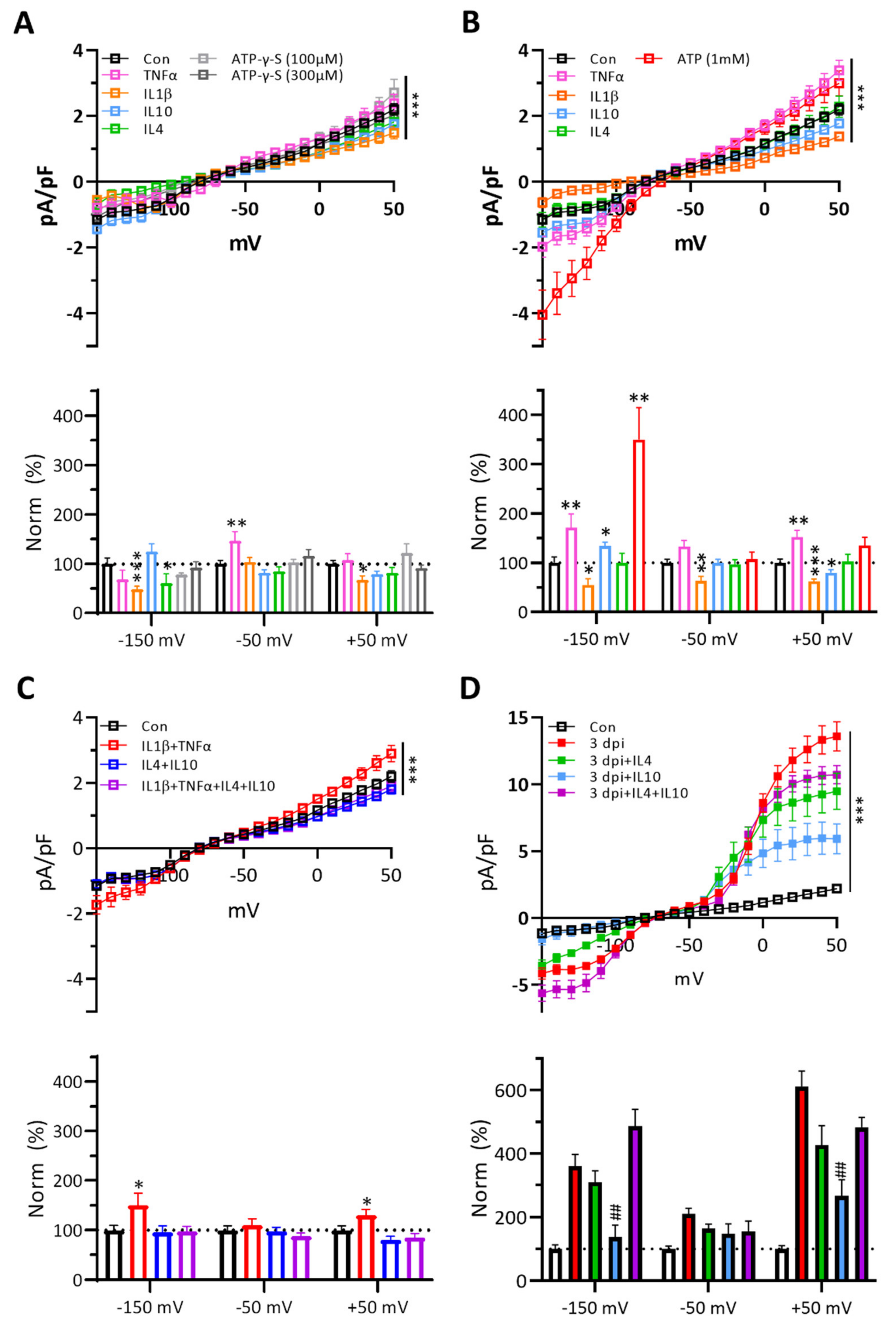
3.6. Cytokines Affect Naïve Microglial Membrane Properties Differently and IL-10 Effectively Suppresses the Membrane Currents of 3 dpi Microglia
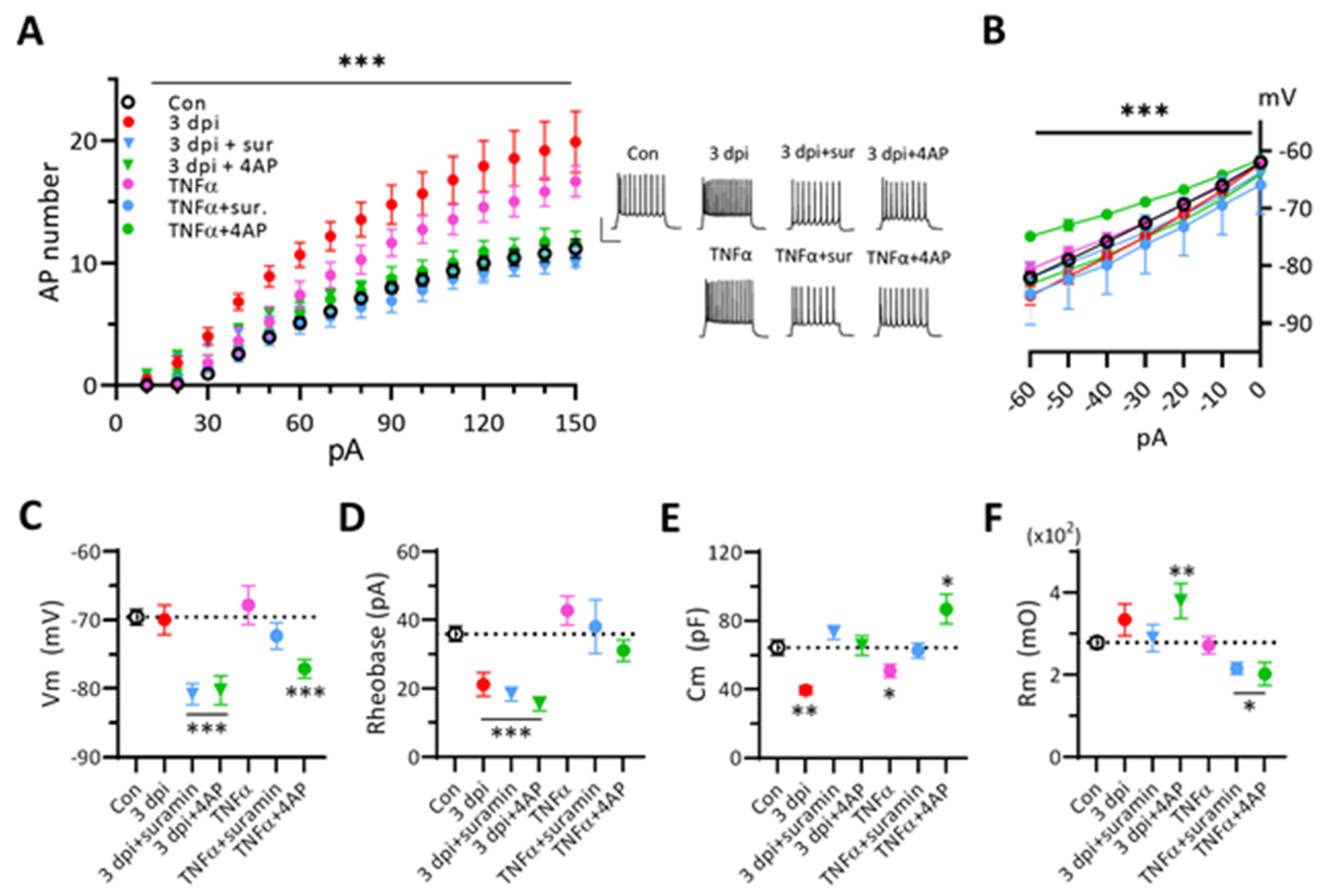
3.7. Blockers of Purinergic Receptors or K+ Channels Suppress the Excitability of 3 dpi Neurons and TNF-α-Treated Naïve Neurons
4. Discussion
4.1. ATP-Injection Injury Model Compared to Needle Stab-Wound Control
4.2. Cell Population Changes in the Core Region of ATP-Injected Cortical Injury Site
4.3. Interactions among P2 Receptors, K+ Ion Channels, and Cytokines of 3 dpi Microglia
4.4. Cytokine Modulation of Neuronal Excitability and Synaptic Inputs
4.5. Roles of TNF-α, IL-1β, IL-4, and IL-10 in ATP-Induced Cortical Brain Injury
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Hanisch, U.-K. Microglia as a source and target of cytokines. GLIA 2002, 40, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Beggs, S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef]
- Kawabori, M.; Yenari, M.A. The role of the microglia in acute CNS injury. Metab. Brain Dis. 2014, 30, 381–392. [Google Scholar] [CrossRef]
- Arcuri, C.; Mecca, C.; Bianchi, R.; Giambanco, I.; Donato, R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Front. Mol. Neurosci. 2017, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2017, 18, 225–242. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Norris, G.T.; Kipnis, J. Immune cells and CNS physiology: Microglia and beyond. J. Exp. Med. 2018, 216, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome is Expressed and Functional in Mouse Brain Microglia but not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef]
- Becher, B.; Spath, B.B.S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2016, 17, 49–59. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Lukens, J.R.; Kipnis, J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron 2015, 87, 47–62. [Google Scholar] [CrossRef]
- Dugue, R.; Nath, M.; Dugue, A.; Barone, F.C. Roles of Pro- and Anti-inflammatory Cytokines in Traumatic Brain Injury and Acute Ischemic Stroke. In Mechanisms of Neuroinflammation; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, D.W.S.H.B.R.S.B.; Loane, D.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2016, 54, 8071–8089. [Google Scholar] [CrossRef]
- Illes, P.; Burnstock, G.; Tang, Y. Astroglia-Derived ATP Modulates CNS Neuronal Circuits. Trends Neurosci. 2019, 42, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J.; Sisodia, S.S.; Ransohoff, R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011, 14, 1227–1235. [Google Scholar] [CrossRef]
- Shechter, R.; Schwartz, M. CNS sterile injury: Just another wound healing? Trends Mol. Med. 2013, 19, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef]
- Fogal, B.; Hewett, S.J. Interleukin-1β: A bridge between inflammation and excitotoxicity? J. Neurochem. 2008, 106, 1–23. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Bowers, W.J. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J. Neuroimmune Pharmacol. 2011, 7, 42–59. [Google Scholar] [CrossRef]
- Kearney, C.J.; Martin, S.J. An Inflammatory Perspective on Necroptosis. Mol. Cell 2017, 65, 965–973. [Google Scholar] [CrossRef]
- Spera, P.A.; Ellison, J.A.; Feuerstein, G.Z.; Barone, F.C. IL-10 reduces rat brain injury following focal stroke. Neurosci. Lett. 1998, 251, 189–192. [Google Scholar] [CrossRef]
- De Bilbao, F.; Arsenijevic, D.; Moll, T.; Garcia-Gabay, I.; Vallet, P.; Langhans, W.; Giannakopoulos, P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J. Neurochem. 2009, 110, 12–22. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Sun, G.; Zhang, J.; Edwards, N.J.; Aronowski, J. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J. Neurosci. 2015, 35, 11281–11291. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Zhao, S.; Zhang, H.; Cai, W.; Cai, M.; Ji, X.; Leak, R.; Gao, Y.; Chen, J.; et al. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke 2016, 47, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Bergher, J.P.; Swanson, R.A. ATP-induced ATP release from astrocytes. J. Neurochem. 2003, 88, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Akter, S.; Akundi, R.S. The two-hit hypothesis for neuroinflammation: Role of exogenous ATP in modulating inflammation in the brain. Front. Cell. Neurosci. 2014, 8, 260. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef]
- Medina, C.B.; Mehrotra, P.; Arandjelovic, S.; Perry, J.S.A.; Guo, Y.; Morioka, S.; Barron, B.; Walk, S.F.; Ghesquière, B.; Krupnick, A.S.; et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 2020, 580, 130–135. [Google Scholar] [CrossRef]
- Fields, R.D.; Burnstock, G. Purinergic signalling in neuron–glia interactions. Nat. Rev. Neurosci. 2006, 7, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.M.; Zychlinsky, A.; Liu, C.C.; Ojcius, D.M.; Young, J.D. Extracellular ATP as a trigger for apoptosis or programmed cell death. J. Cell Biol. 1991, 112, 279–288. [Google Scholar] [CrossRef]
- Franke, H.; Krügel, U.; Illes, P. P2 receptors and neuronal injury. Pflug. Arch. Eur. J. Physiol. 2006, 452, 622–644. [Google Scholar] [CrossRef]
- Rodrigues, R.; Tomé, A.R.; Cunha, R.A. ATP as a multi-target danger signal in the brain. Front. Neurosci. 2015, 9, 148. [Google Scholar] [CrossRef]
- Bours, M.; Swennen, E.; Di Virgilio, F.; Cronstein, B.; Dagnelie, P. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef]
- Fivenson, E.M.; Lautrup, S.; Sun, N.; Scheibye-Knudsen, M.; Stevnsner, T.; Nilsen, H.; Bohr, V.A.; Fang, E.F. Mitophagy in neurodegeneration and aging. Neurochem. Int. 2017, 109, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Cai, Q.; Jeong, Y.Y. Mitophagy in Alzheimer’s Disease and Other Age-Related Neurodegenerative Diseases. Cells 2020, 9, 150. [Google Scholar] [CrossRef]
- Choo, A.M.; Miller, W.J.; Chen, Y.-C.; Nibley, P.; Patel, T.P.; Goletiani, C.; Morrison, B.; Kutzing, M.K.; Firestein, B.; Sul, J.-Y.; et al. Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain 2013, 136, 65–80. [Google Scholar] [CrossRef]
- Burnstock, G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 2016, 104, 4–17. [Google Scholar] [CrossRef]
- Eder, C. Regulation of microglial behavior by ion channel activity. J. Neurosci. Res. 2005, 81, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Ponulak, F.; Kasinski, A. Introduction to spiking neural networks: Information processing, learning and applications. Acta Neurobiol. Exp. 2011, 71, 409–433. [Google Scholar]
- Stebbing, M.J.; Cottee, J.M.; Erana, I. The Role of Ion Channels in Microglial Activation and Proliferation—A Complex Interplay between Ligand-Gated Ion Channels, K+ Channels, and Intracellular Ca2+. Front. Immunol. 2015, 6, 497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Blomster, L.V.; Christophersen, P.; Wulff, H. Potassium channel expression and function in microglia: Plasticity and possible species variations. Channels 2017, 11, 305–315. [Google Scholar] [CrossRef]
- Izquierdo, P.; Attwell, D.; Madry, C. Ion Channels and Receptors as Determinants of Microglial Function. Trends Neurosci. 2019, 42, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Song, S.; Ezenwukwa, C.C.; Jalali, S.; Sun, B.; Sun, D. Ion channels and transporters in microglial function in physiology and brain diseases. Neurochem. Int. 2021, 142, 104925. [Google Scholar] [CrossRef]
- Seifert, S.; Pannell, M.; Uckert, W.; Färber, K.; Kettenmann, H. Transmitter- and hormone-activated Ca2+ responses in adult microglia/brain macrophages in situ recorded after viral transduction of a recombinant Ca2+ sensor. Cell Calcium 2011, 49, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Di Lucente, J.; Nguyen, H.M.; Wulff, H.; Jin, L.-W.; Maezawa, I. The voltage-gated potassium channel Kv1.3 is required for microglial pro-inflammatory activation in vivo. GLIA 2018, 66, 1881–1895. [Google Scholar] [CrossRef]
- Cayabyab, F.S.; Khanna, R.; Jones, O.T.; Schlichter, L.C. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur. J. Neurosci. 2000, 12, 1949–1960. [Google Scholar] [CrossRef]
- Yi, Y.D. Role of microglia in the process of inflammation in the hypoxic developing brain. Front. Biosci. 2011, 3, 884–900. [Google Scholar] [CrossRef]
- Avignone, E.; Ulmann, L.; Levavasseur, F.; Rassendren, F.; Audinat, E. Status Epilepticus Induces a Particular Microglial Activation State Characterized by Enhanced Purinergic Signaling. J. Neurosci. 2008, 28, 9133–9144. [Google Scholar] [CrossRef] [PubMed]
- Richter, N.; Wendt, S.; Georgieva, P.B.; Hambardzumyan, D.; Nolte, C.; Kettenmann, H. Glioma-associated microglia and macrophages/monocytes display distinct electrophysiological properties and do not communicate via gap junctions. Neurosci. Lett. 2014, 583, 130–135. [Google Scholar] [CrossRef][Green Version]
- Thei, L.; Imm, J.; Kaisis, E.; Dallas, M.L.; Kerrigan, T.L. Microglia in Alzheimer’s Disease: A Role for Ion Channels. Front. Neurosci. 2018, 12, 676. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Jou, I.; Joe, E.-H. Absence of Delayed Neuronal Death in ATP-Injected Brain: Possible Roles of Astrogliosis. Exp. Neurobiol. 2013, 22, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Microglial activation by purines and pyrimidines. GLIA 2002, 40, 156–163. [Google Scholar] [CrossRef]
- Nakazawa, K.; Inoue, K.; Inoue, K.; Inoue, K. ATP reduces voltage-activated K+ current in cultured rat hippocampal neurons. Pflug. Arch. 1994, 429, 143–145. [Google Scholar] [CrossRef]
- Filippov, A.K.; Choi, R.C.Y.; Simon, J.; Barnard, E.A.; Brown, D.A. Activation of P2Y1 Nucleotide Receptors Induces Inhibition of the M-Type K+ Current in Rat Hippocampal Pyramidal Neurons. J. Neurosci. 2006, 26, 9340–9348. [Google Scholar] [CrossRef]
- Siebold, L.; Obenaus, A.; Goyal, R. Criteria to define mild, moderate, and severe traumatic brain injury in the mouse controlled cortical impact model. Exp. Neurol. 2018, 310, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol. Neurobiol. 2019, 56, 5332–5345. [Google Scholar] [CrossRef]
- Ryu, J.K.; Kim, J.; Choi, S.-H.; Oh, Y.J.; Lee, Y.B.; Kim, S.U.; Jin, B.K. ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. Neuroreport 2002, 13, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-K.; Ji, K.-M.; Kim, B.; Kim, J.; Jou, I.; Joe, E.-H. Inflammatory Responses Are Not Sufficient to Cause Delayed Neuronal Death in ATP-Induced Acute Brain Injury. PLoS ONE 2010, 5, e13756. [Google Scholar] [CrossRef]
- Franke, H.; Grummich, B.; Hartig, W.; Grosche, J.; Regenthal, R.; Edwards, R.H.; Illes, P.; Krugel, U. Changes in purinergic signaling after cerebral injury—Involvement of glutamatergic mechanisms? Int. J. Dev. Neurosci. 2006, 24, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Kohsaka, S. Dynamic motility of microglia: Purinergic modulation of microglial movement in the normal and pathological brain. GLIA 2011, 59, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Wu, H.-J.; Li, H.-Q.; Qin, S.; Wang, Y.-E.; Li, J.; Lou, H.-F.; Chen, Z.; Li, X.-M.; Luo, Q.-M.; et al. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012, 22, 1022–1033. [Google Scholar] [CrossRef]
- Schelper, R.L.; Adrian, E.K. Monocytes Become Macrophages; They Do Not Become Microglia. J. Neuropathol. Exp. Neurol. 1986, 45, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; A Ling, E.; Wong, W.C. Origin and fate of neural macrophages in a stab wound of the brain of the young rat. J. Anat. 1987, 154, 215–227. [Google Scholar]
- Jeong, H.-K.; Ji, K.; Min, K.; Joe, E.-H. Brain Inflammation and Microglia: Facts and Misconceptions. Exp. Neurobiol. 2013, 22, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Krabbe, G.; Du, F.; Jones, I.; Reichert, M.C.; Telpoukhovskaia, M.; Kodama, L.; Wang, C.; Cho, S.-H.; Sayed, F.; et al. Proximal recolonization by self-renewing microglia re-establishes microglial homeostasis in the adult mouse brain. PLoS Biol. 2019, 17, e3000134. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Bruttger, J.; Karram, K.; Wörtge, S.; Regen, T.; Marini, F.; Hoppmann, N.; Klein, M.; Blank, T.; Yona, S.; Wolf, Y.; et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 2015, 43, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Frik, J.; Merl-Pham, J.; Plesnila, N.; Mattugini, N.; Kjell, J.; Kraska, J.; Gomez, R.; Hauck, S.M.; Sirko, S.; Götz, M. Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Rep. 2018, 19, e45294. [Google Scholar] [CrossRef]
- Wonderlin, W.F.; Strobl, J.S. Potassium channels, proliferation, and G1 progression. J. Memb. Biol. 1996, 154, 91–107. [Google Scholar] [CrossRef]
- Andrejew, R.; Oliveira-Giacomelli, A.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Ikeda, M.; Tsuno, S.; Sugiyama, T.; Hashimoto, A.; Yamoto, K.; Takeuchi, K.; Kishi, H.; Mizuguchi, H.; Kohsaka, S.-I.; Yoshioka, T. Ca 2+ spiking activity caused by the activation of store-operated Ca 2+ channels mediates TNF-α release from microglial cells under chronic purinergic stimulation. Biochim. Biophys. Acta 2013, 1833, 2573–2585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eder, C. Ion channels in monocytes and microglia / brain macrophages: Promising therapeutic targets for neurological diseases. J. Neuroimmunol. 2010, 224, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, M.; Sorkin, L. Effect of cytokines on neuronal excitability. Neurosci. Lett. 2008, 437, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Volterra, A. TNFα in synaptic function: Switching gears. Trends Neurosci. 2012, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Gustafson-Vickers, S.L.; Van Lu, B.; Lai, A.Y.; Todd, K.G.; Ballanyi, K.; A Smith, P. Long-Term Actions of Interleukin-1β on Delay and Tonic Firing Neurons in Rat Superficial Dorsal Horn and Their Relevance to Central Sensitization. Mol. Pain 2008, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Nenov, M.N.; Konakov, M.V.; Teplov, I.Y.; Levin, S.G. Interleukin-10 Facilitates Glutamatergic Synaptic Transmission and Homeostatic Plasticity in Cultured Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 3375. [Google Scholar] [CrossRef] [PubMed]
- S-Rózsa, K.; Rubakhin, S.S.; Szücs, A.; Hughes, T.K.; Stefano, G.B. Opposite effects of interleukin-2 and interleukin-4 on GABA-induced inward currents of dialysed Lymnaea neurons. Gen. Pharmacol. Vasc. Syst. 1997, 29, 73–77. [Google Scholar] [CrossRef]
- Suryanarayanan, A.; Carter, J.; Landin, J.; Morrow, A.; Werner, D.; Spigelman, I. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology 2016, 107, 181–188. [Google Scholar] [CrossRef]
- Anderson, W.D.; Greenhalgh, A.D.; Takwale, A.; David, S.; Vadigepalli, R. Novel Influences of IL-10 on CNS Inflammation Revealed by Integrated Analyses of Cytokine Networks and Microglial Morphology. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Lee, S.-J.; Kim, C.-H. Roles of Cytokines in the Temporal Changes of Microglial Membrane Currents and Neuronal Excitability and Synaptic Efficacy in ATP-Induced Cortical Injury Model. Int. J. Mol. Sci. 2021, 22, 6853. https://doi.org/10.3390/ijms22136853
Song B, Lee S-J, Kim C-H. Roles of Cytokines in the Temporal Changes of Microglial Membrane Currents and Neuronal Excitability and Synaptic Efficacy in ATP-Induced Cortical Injury Model. International Journal of Molecular Sciences. 2021; 22(13):6853. https://doi.org/10.3390/ijms22136853
Chicago/Turabian StyleSong, Bokyung, Sung-Joong Lee, and Chong-Hyun Kim. 2021. "Roles of Cytokines in the Temporal Changes of Microglial Membrane Currents and Neuronal Excitability and Synaptic Efficacy in ATP-Induced Cortical Injury Model" International Journal of Molecular Sciences 22, no. 13: 6853. https://doi.org/10.3390/ijms22136853
APA StyleSong, B., Lee, S.-J., & Kim, C.-H. (2021). Roles of Cytokines in the Temporal Changes of Microglial Membrane Currents and Neuronal Excitability and Synaptic Efficacy in ATP-Induced Cortical Injury Model. International Journal of Molecular Sciences, 22(13), 6853. https://doi.org/10.3390/ijms22136853







