GMI, an Immunomodulatory Peptide from Ganoderma microsporum, Restrains Periprosthetic Joint Infections via Modulating the Functions of Myeloid-Derived Suppressor Cells and Effector T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. GMI, S. aureus and Biofilm Preparation
2.3. Isolating MDSCs from Mouse Bone Marrow by Cell Sorting
2.4. Analysis of MDSC Expansion
2.5. Analysis of Tc, TH, Treg Cells by Flow Cytometry
2.6. Preparation of Blood Samples for Flow Cytometry Analysis
2.7. Analysis of IL-6 and TNF-α in Culture Medium and Plasma
2.8. Measurement of ROS
2.9. GMI Treatment of Mice Infected by S. aureus
2.10. Fluorescence Molecular Tomography
2.11. Statistical Analysis
3. Results
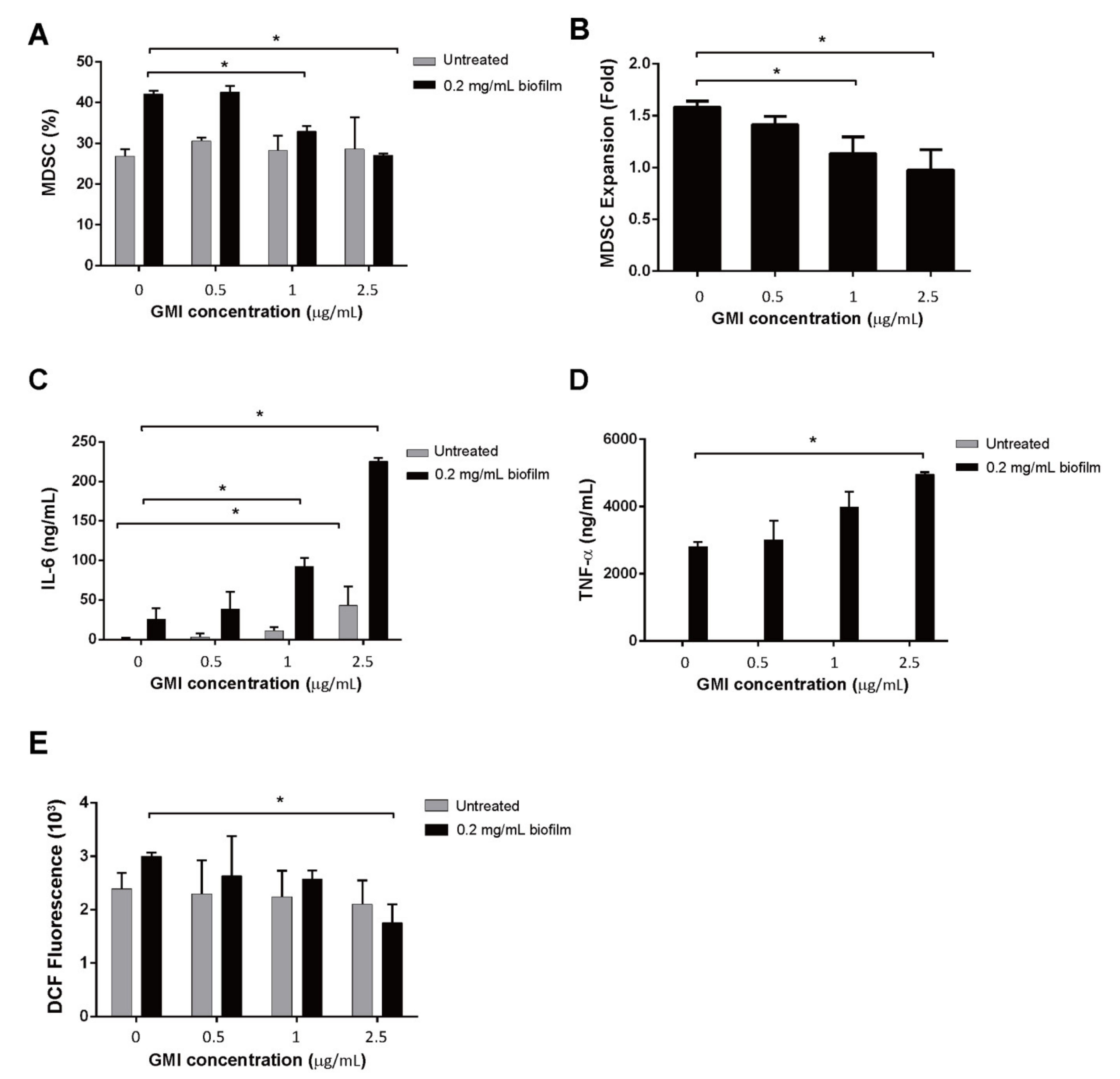
3.1. Influence of GMI on S. aureus Biofilm-Mediated MDSC Expansion
3.2. Activation of IL-6 and TNF-α Expression by GMI
3.3. Reduction in ROS Expression by GMI
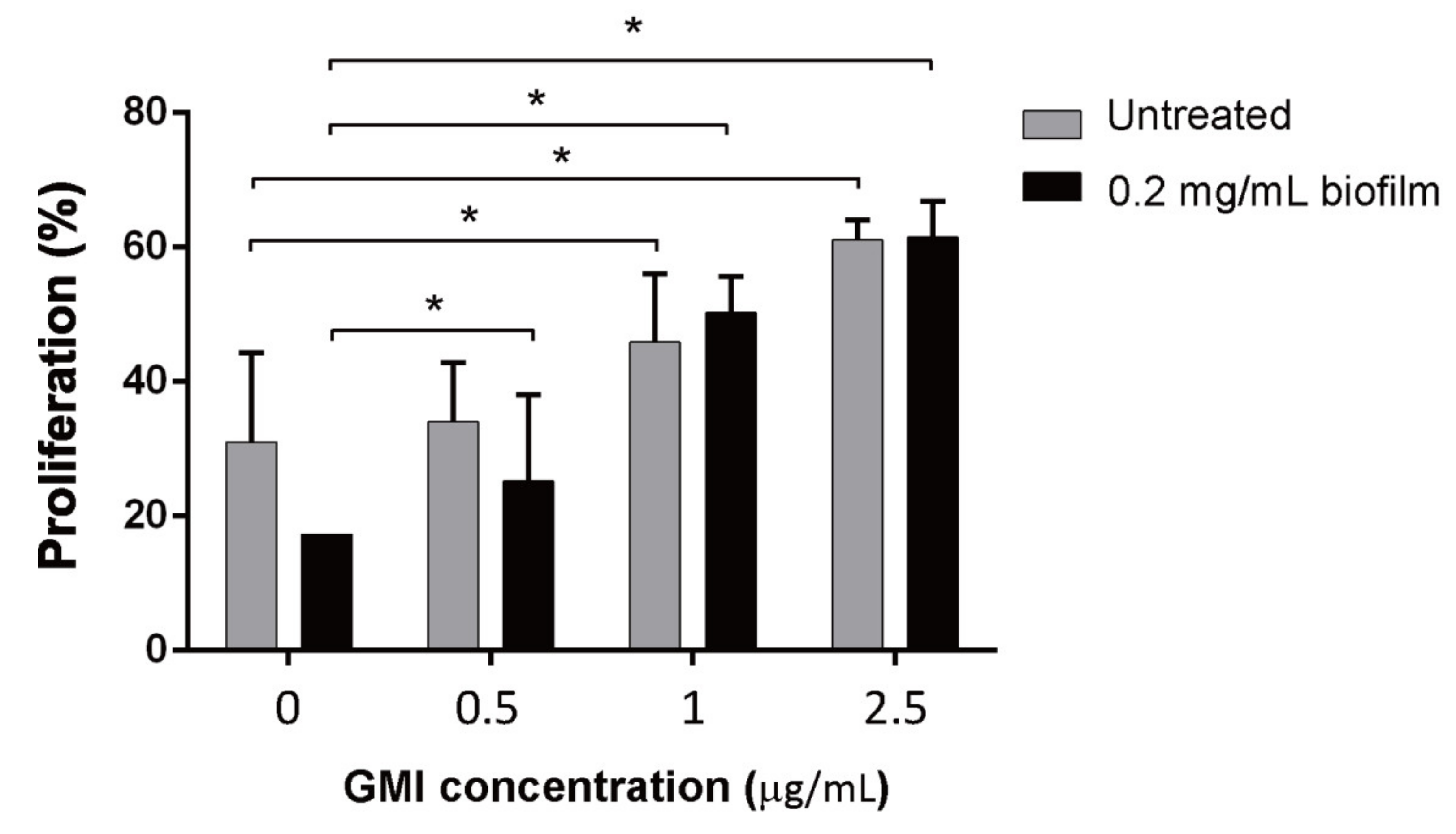
3.4. Effects of GMI on the Suppression of T Cell Proliferation by MDSCs
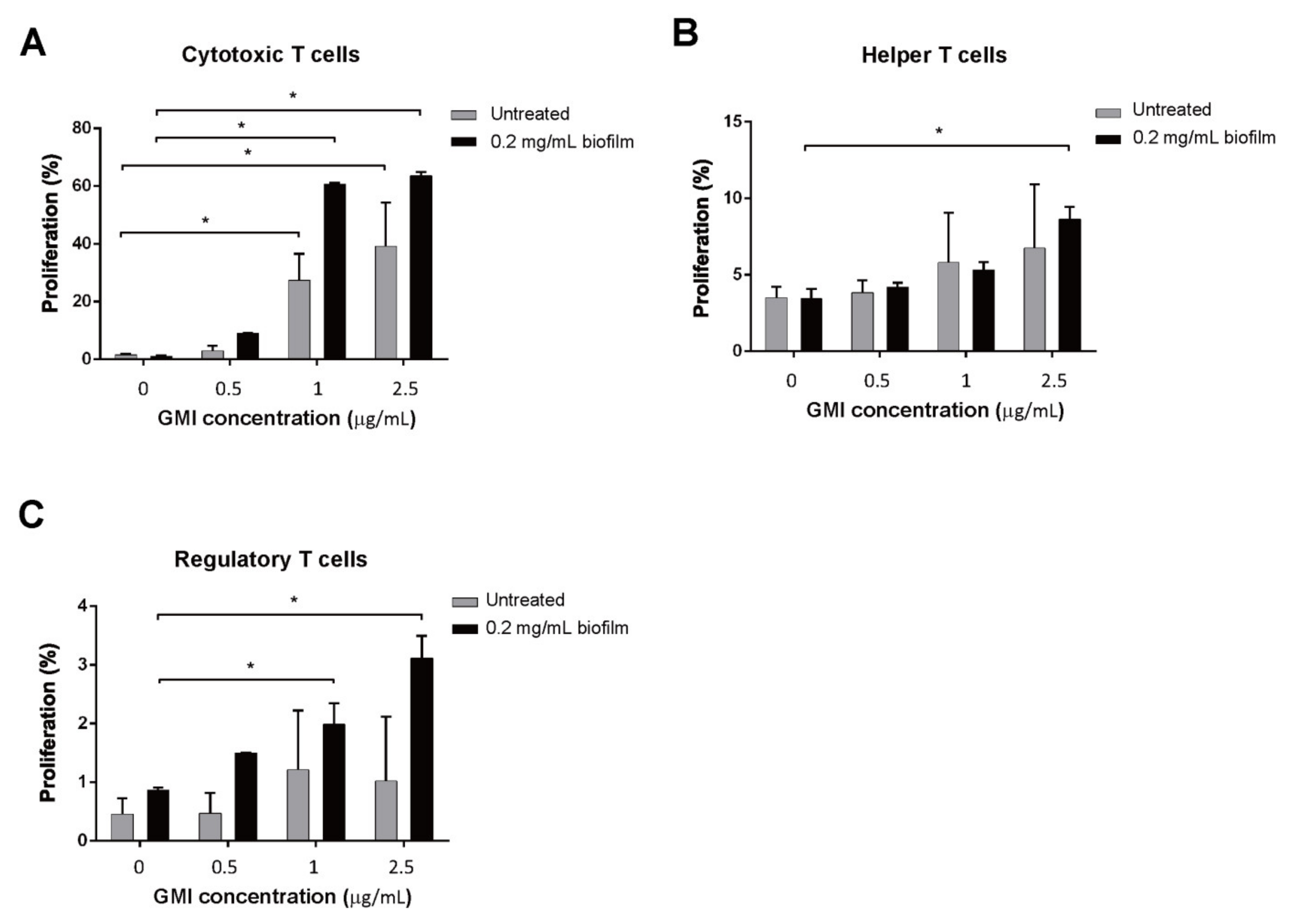
3.5. Promotion of T Cell Proliferation by GMI
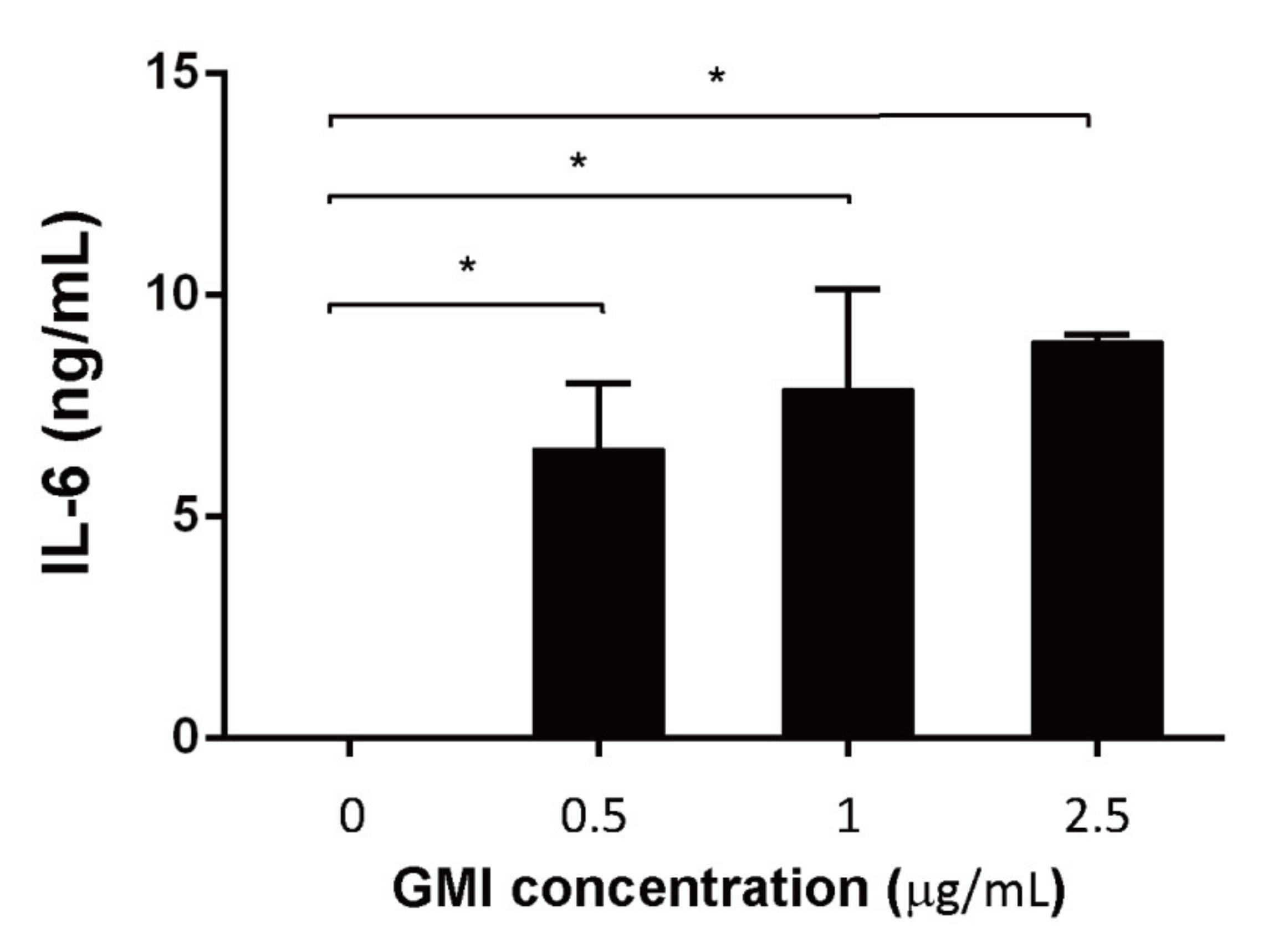
3.6. Effects of GMI on the Production of Cytokines
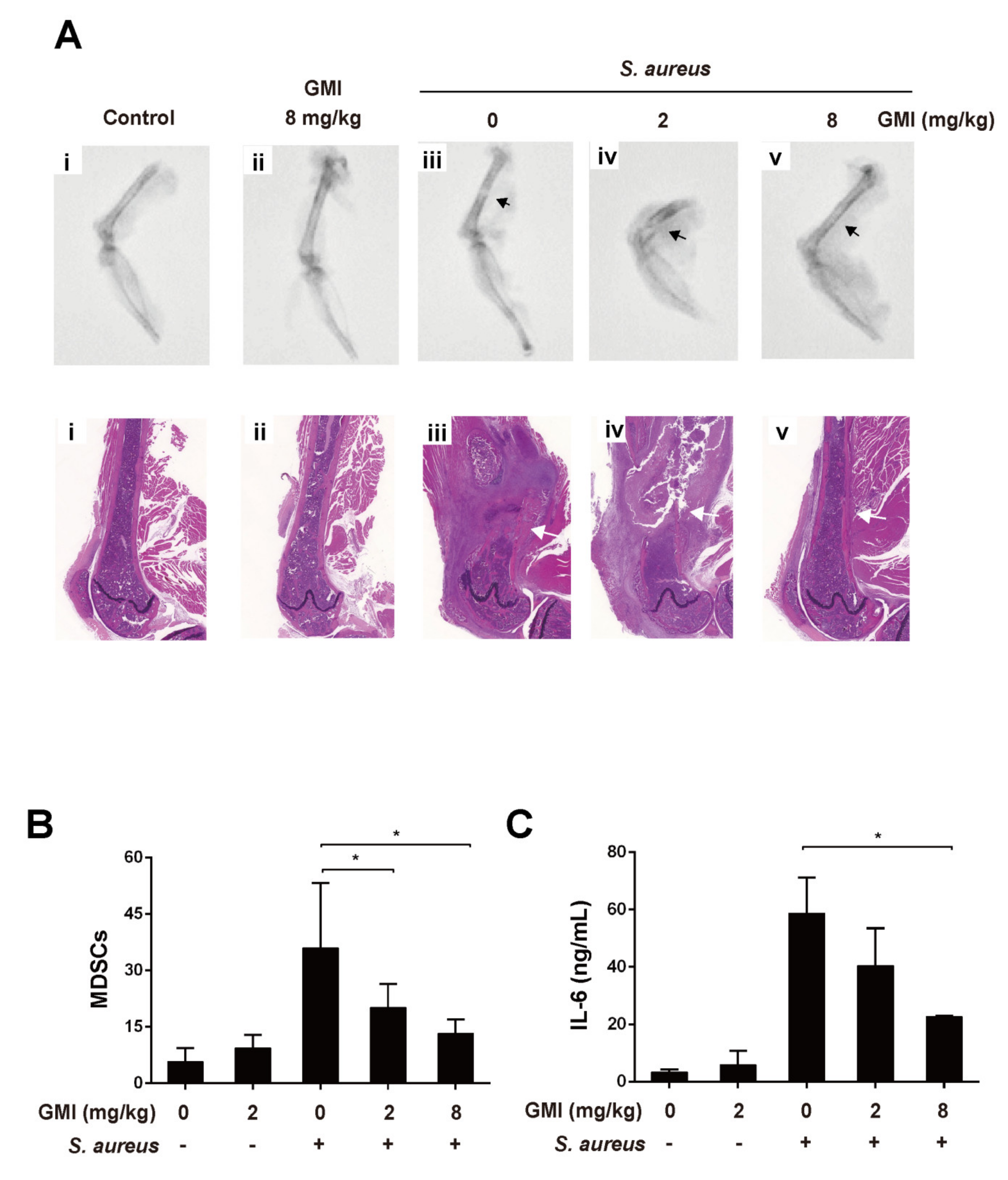
3.7. Effects of GMI on S. aureus-Induced Mouse PJI Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Gallo, J.; Kolar, M.; Novotny, R.; Rihakova, P.; Ticha, V. Pathogenesis of prosthesis-related infection. Biomed. Pap. 2003, 147, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.; Ziran, B.H.; Lipsky, B.A. Treating osteomyelitis: Antibiotics and surgery. Plast. Reconstr. Surg. 2011, 127 (Suppl. S1), S177–S187. [Google Scholar] [CrossRef]
- Fagotti, L.; Tatka, J.; Salles, M.J.C.; Queiroz, M.C. Risk Factors and Treatment Options for Failure of a Two-Stage Exchange. Curr. Rev. Musculoskelet. Med. 2018, 11, 420–427. [Google Scholar] [CrossRef]
- Vilchez, F.; Martinez-Pastor, J.C.; Garcia-Ramiro, S.; Bori, G.; Macule, F.; Sierra, J.; Font, L.; Mensa, J.; Soriano, A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin. Microbiol. Infect. 2011, 17, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, A.J.; Abdel, M.P.; Sanders, T.L.; Fitzgerald, S.F.; Hanssen, A.D.; Berry, D.J. Irrigation and Debridement with Component Retention for Acute Infection After Hip Arthroplasty: Improved Results with Contemporary Management. J. Bone Jt. Surg. Am. 2017, 99, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Nodzo, S.R.; Boyle, K.K.; Spiro, S.; Nocon, A.A.; Miller, A.O.; Westrich, G.H. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee 2017, 24, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27 (Suppl. S8), 61.e1–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Dowsey, M.M.; Buising, K.L.; Liew, D.; Choong, P.F. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin. Microbiol. Infect. 2013, 19, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Darbani, R.; Farshadfar, C.; Tavana, S.; Saljoughi, H.; Zonouri, S.S.; Branch, S.; Zonouri, S.S. Identification of DNA gyrase Subunit a Mutations Associated with Ciprofloxacin Resistance in Staphylococcus aureus Isolated from Nasal Infection in Kurdistan, Iran. J. Mol. Biol. Res. 2017, 7, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, E.S.; Aslantas, O. Antimicrobial resistance and underlying mechanisms in Staphylococcus aureus isolates. Asian Pac. J. Trop. Med. 2017, 10, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus: Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Nicola, L.; Gismondo, M.R. In vitro evaluation of antibiotics’ combinations for empirical therapy of suspected methicillin resistant Staphylococcus aureus severe respiratory infections. BMC Infect. Dis. 2007, 7, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, K.; Katayama, Y.; Yuzawa, H.; Ito, T. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2002, 292, 67–74. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macia, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Medina, E.; Hartl, D. Myeloid-Derived Suppressor Cells in Infection: A General Overview. J. Innate Immun. 2018, 10, 407–413. [Google Scholar] [CrossRef]
- Peng, K.T.; Hsieh, C.C.; Huang, T.Y.; Chen, P.C.; Shih, H.N.; Lee, M.S.; Chang, P.J. Staphylococcus aureus biofilm elicits the expansion, activation and polarization of myeloid-derived suppressor cells in vivo and in vitro. PLoS ONE 2017, 12, e0183271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrecque, J.; Charlebois, S. Functional foods: An empirical study on perceived health benefits in relation to pre-purchase intentions. Nutr. Food Sci. 2011, 41, 308–318. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Li, Q.Z.; Wang, X.F.; Zhou, X.W. Recent status and prospects of the fungal immunomodulatory protein family. Crit. Rev. Biotechnol. 2011, 31, 365–375. [Google Scholar] [CrossRef]
- Liu, Y.; Bastiaan-Net, S.; Wichers, H.J. Current Understanding of the Structure and Function of Fungal Immunomodulatory Proteins. Front. Nutr. 2020, 7, 132. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chow, Y.H.; Sun, H.L.; Liu, Y.F.; Lee, Y.T.; Lue, K.H.; Ko, J.L. Alleviation of respiratory syncytial virus replication and inflammation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. Antivir. Res. 2014, 110, 124–131. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chang, C.Y.; Li, J.R.; Wang, J.D.; Wu, C.C.; Kuan, Y.H.; Liao, S.L.; Wang, W.Y.; Chen, C.J. Anti-inflammatory and Neuroprotective Effects of Fungal Immunomodulatory Protein Involving Microglial Inhibition. Int. J. Mol. Sci. 2018, 19, 3678. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-y.; Hsu, M.-F.; Huang, C.-S.; Fu, H.-Y.; Wang, A.; Hseu, R.-S.; Huang, C.-T.; Yang, C.-S. A 2.0 Å Structure of the Fungal Immunomodulatory Protein GMI from Ganoderma microsporum. In Proceedings of the 2nd Asia-Oceania Forum for Synchrotron Radiation Research, Hsinchu, Taiwan, 2–3 November 2007. [Google Scholar]
- Hsin, I.L.; Sheu, G.T.; Jan, M.S.; Sun, H.L.; Wu, T.C.; Chiu, L.Y.; Lue, K.H.; Ko, J.L. Inhibition of lysosome degradation on autophagosome formation and responses to GMI, an immunomodulatory protein from Ganoderma microsporum. Br. J. Pharm. 2012, 167, 1287–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsin, I.L.; Ou, C.C.; Wu, T.C.; Jan, M.S.; Wu, M.F.; Chiu, L.Y.; Lue, K.H.; Ko, J.L. GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy 2011, 7, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Hsiao, Y.M.; Ou, C.C.; Lin, Y.W.; Chiu, Y.L.; Lue, K.H.; Chang, J.G.; Ko, J.L. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor alpha-induced expression of matrix metalloproteinase 9 via NF-κB pathway in human alveolar epithelial A549 cells. J. Agric. Food Chem. 2010, 58, 12014–12021. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Sheu, G.-T.; Lin, Y.-W.; Yeh, C.-S.; Huang, Y.-H.; Lai, Y.-C.; Chang, J.-G.; Ko, J.-L. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process. Biochem. 2010, 45, 1537–1542. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [PubMed]
- Tankersley, A.; Frank, M.B.; Bebak, M.; Brennan, R. Early effects of Staphylococcus aureus biofilm secreted products on inflammatory responses of human epithelial keratinocytes. J. Inflamm. 2014, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohl, K.; Tenbrock, K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front. Immunol. 2018, 9, 2499. [Google Scholar] [CrossRef] [Green Version]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.T.; Chiang, Y.C.; Huang, T.Y.; Chen, P.C.; Chang, P.J.; Lee, C.W. Curcumin nanoparticles are a promising anti-bacterial and anti-inflammatory agent for treating periprosthetic joint infections. Int. J. Nanomed. 2019, 14, 469–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skabytska, Y.; Wolbing, F.; Gunther, C.; Koberle, M.; Kaesler, S.; Chen, K.M.; Guenova, E.; Demircioglu, D.; Kempf, W.E.; Volz, T.; et al. Cutaneous innate immune sensing of Toll-like receptor 2–6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity 2014, 41, 762–775. [Google Scholar] [CrossRef] [Green Version]
- Heim, C.E.; Vidlak, D.; Kielian, T. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J. Leukoc. Biol. 2015, 98, 1003–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Hartman, C.W.; Garvin, K.L.; Kielian, T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 2015, 194, 3861–3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorhoi, A.; Du Plessis, N. Monocytic Myeloid-Derived Suppressor Cells in Chronic Infections. Front. Immunol. 2017, 8, 1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.Y.; Hua, W.J.; Yeh, H.; Tseng, A.J. Functional proteomic analysis reveals that fungal immunomodulatory protein reduced expressions of heat shock proteins correlates to apoptosis in lung cancer cells. Phytomedicine 2021, 80, 153384. [Google Scholar] [CrossRef] [PubMed]
- Haak-Frendscho, M.; Kino, K.; Sone, T.; Jardieu, P. Ling Zhi-8: A novel T cell mitogen induces cytokine production and upregulation of ICAM-1 expression. Cell Immunol. 1993, 150, 101–113. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hua, K.F.; Wu, W.C.; Hsu, J.; Weng, S.T.; Lin, T.L.; Liu, C.Y.; Hseu, R.S.; Huang, C.T. Reishi immuno-modulation protein induces interleukin-2 expression via protein kinase-dependent signaling pathways within human T cells. J. Cell Physiol. 2008, 215, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Li, J.; Yu, X.; Zhang, D.; Ren, G.; Shi, B.; Wang, C.; Kosinska, A.D.; Wang, S.; Zhou, X.; et al. Polarization of Monocytic Myeloid-Derived Suppressor Cells by Hepatitis B Surface Antigen Is Mediated via ERK/IL-6/STAT3 Signaling Feedback and Restrains the Activation of T Cells in Chronic Hepatitis B Virus Infection. J. Immunol. 2015, 195, 4873–4883. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Spector, S.A. HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J. Infect. Dis. 2014, 209, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Rochman, I.; Paul, W.E.; Ben-Sasson, S.Z. IL-6 increases primed cell expansion and survival. J. Immunol 2005, 174, 4761–4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissenbach, M.; Clahsen, T.; Weber, C.; Spitzer, D.; Wirth, D.; Vestweber, D.; Heinrich, P.C.; Schaper, F. Interleukin-6 is a direct mediator of T cell migration. Eur. J. Immunol. 2004, 34, 2895–2906. [Google Scholar] [CrossRef]
- Diehl, S.; Rincon, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Neumann, J.; Prezzemolo, T.; Vanderbeke, L.; Roca, C.P.; Gerbaux, M.; Janssens, S.; Willemsen, M.; Burton, O.; van Mol, P.; van Herck, Y.; et al. Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clin. Transl. Immunol. 2020, 9, e1204. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.-T.; Chen, J.-L.; Kuo, L.-T.; Yu, P.-A.; Hsu, W.-H.; Lee, C.-W.; Chang, P.-J.; Huang, T.-Y. GMI, an Immunomodulatory Peptide from Ganoderma microsporum, Restrains Periprosthetic Joint Infections via Modulating the Functions of Myeloid-Derived Suppressor Cells and Effector T Cells. Int. J. Mol. Sci. 2021, 22, 6854. https://doi.org/10.3390/ijms22136854
Peng K-T, Chen J-L, Kuo L-T, Yu P-A, Hsu W-H, Lee C-W, Chang P-J, Huang T-Y. GMI, an Immunomodulatory Peptide from Ganoderma microsporum, Restrains Periprosthetic Joint Infections via Modulating the Functions of Myeloid-Derived Suppressor Cells and Effector T Cells. International Journal of Molecular Sciences. 2021; 22(13):6854. https://doi.org/10.3390/ijms22136854
Chicago/Turabian StylePeng, Kuo-Ti, Jiun-Liang Chen, Liang-Tseng Kuo, Pei-An Yu, Wei-Hsiu Hsu, Chiang-Wen Lee, Pey-Jium Chang, and Tsung-Yu Huang. 2021. "GMI, an Immunomodulatory Peptide from Ganoderma microsporum, Restrains Periprosthetic Joint Infections via Modulating the Functions of Myeloid-Derived Suppressor Cells and Effector T Cells" International Journal of Molecular Sciences 22, no. 13: 6854. https://doi.org/10.3390/ijms22136854
APA StylePeng, K.-T., Chen, J.-L., Kuo, L.-T., Yu, P.-A., Hsu, W.-H., Lee, C.-W., Chang, P.-J., & Huang, T.-Y. (2021). GMI, an Immunomodulatory Peptide from Ganoderma microsporum, Restrains Periprosthetic Joint Infections via Modulating the Functions of Myeloid-Derived Suppressor Cells and Effector T Cells. International Journal of Molecular Sciences, 22(13), 6854. https://doi.org/10.3390/ijms22136854






