Treatment with the Probiotic Product Aviguard® Alleviates Inflammatory Responses during Campylobacter jejuni-Induced Acute Enterocolitis in Mice
Abstract
1. Introduction
2. Results
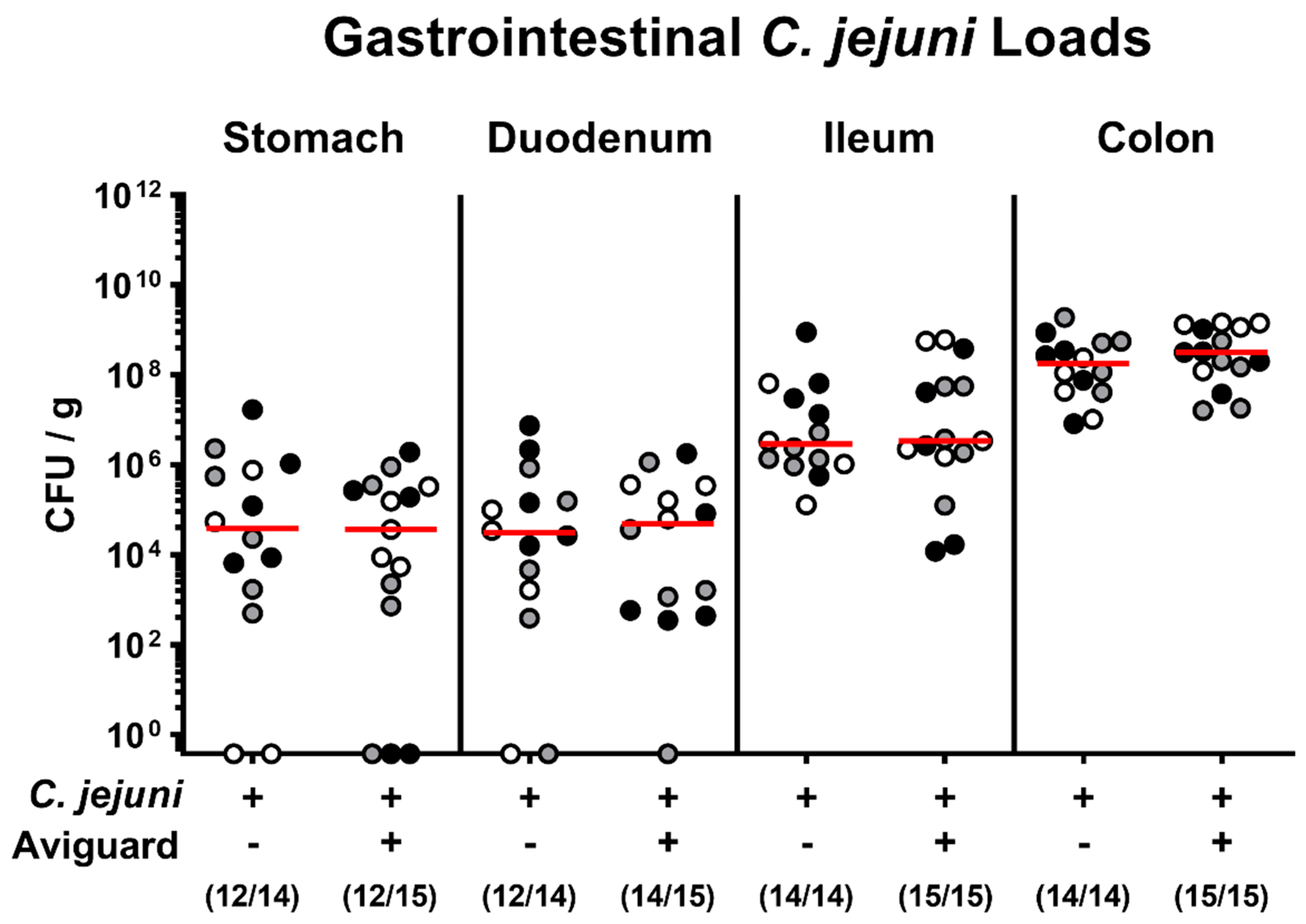
2.1. Aviguard® Treatment and Gastrointestinal C. jejuni Infection of Microbiota-Depleted IL-10−/− Mice
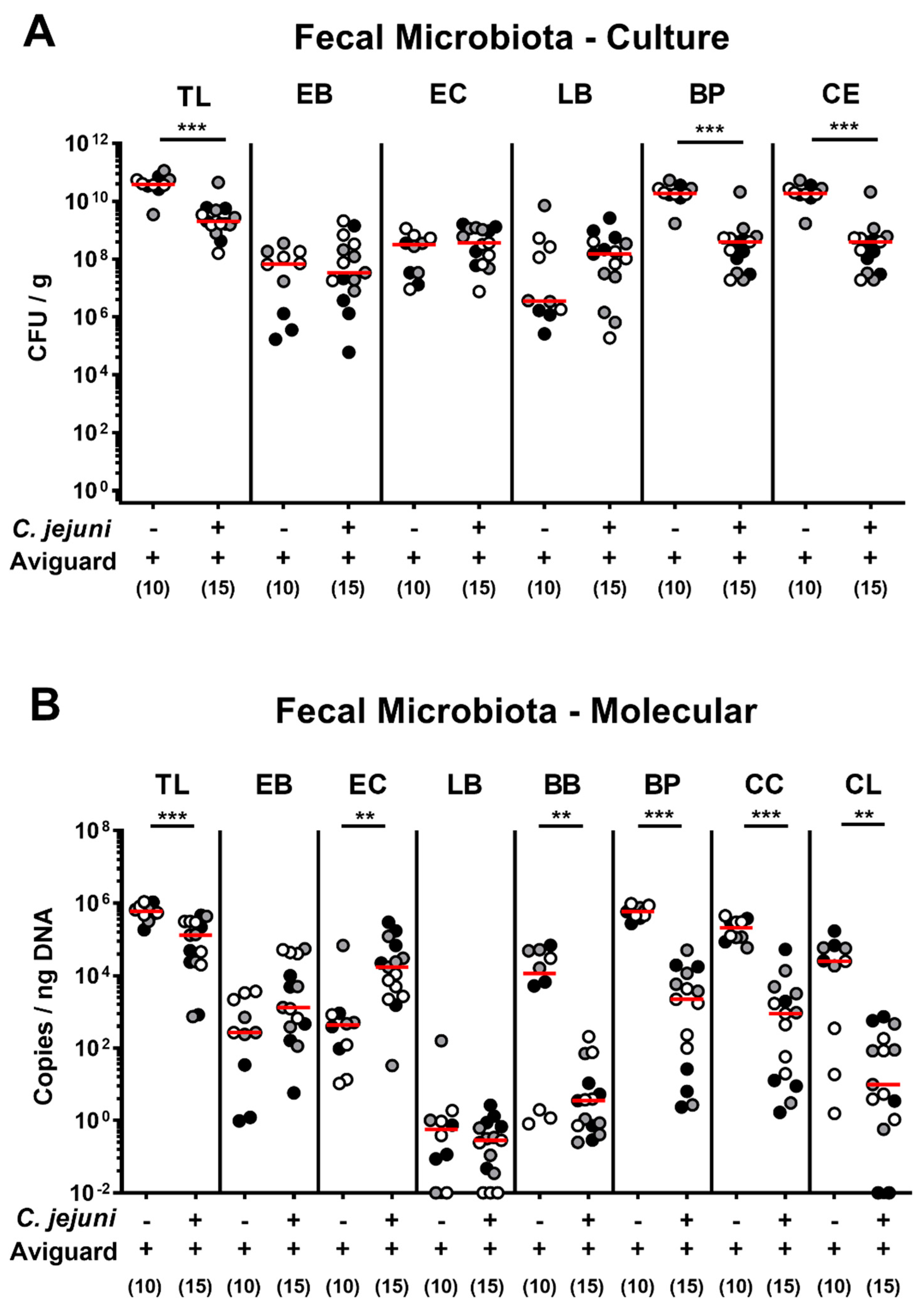
2.2. Aviguard® Treatment and Intestinal Microbiota Composition of C. jejuni-Infected Microbiota-Depleted IL-10−/− Mice
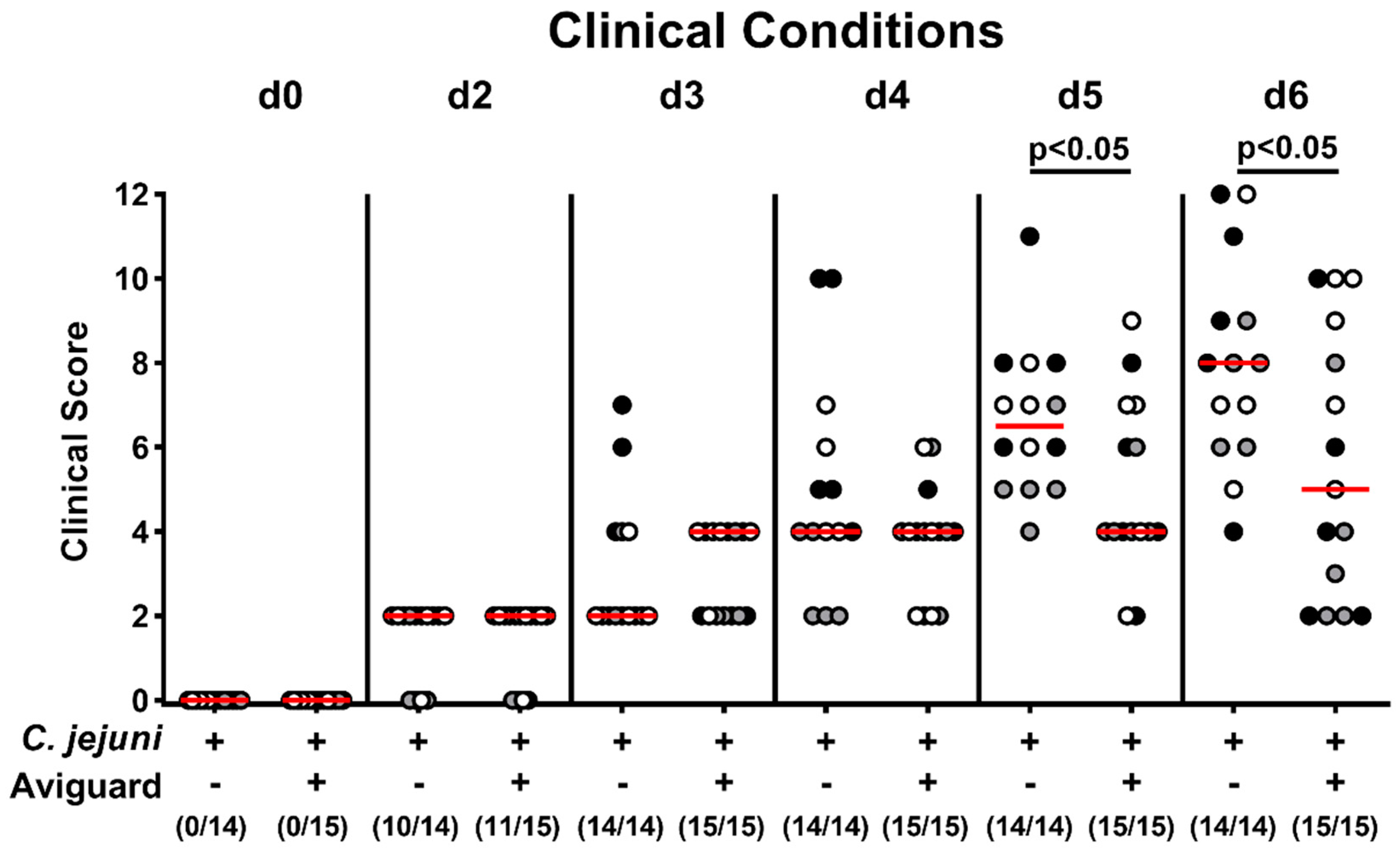
2.3. Aviguard® Treatment and Clinical Outcome in C. jejuni-Infected Microbiota-Depleted IL-10−/− Mice
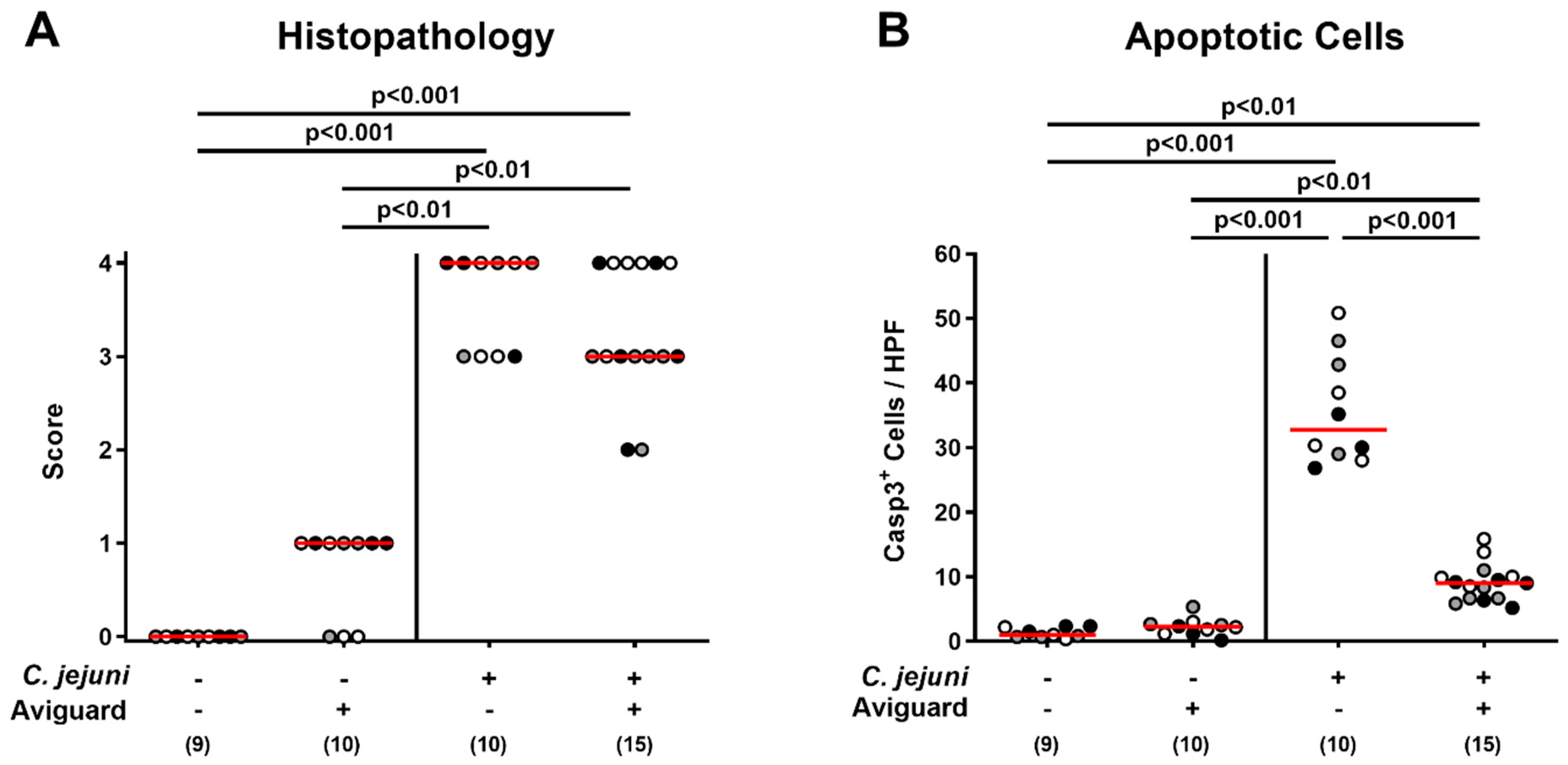
2.4. Aviguard® Treatment and Microscopic Inflammatory Changes in the Colon of C. jejuni-Infected Microbiota-Depleted IL-10−/− Mice
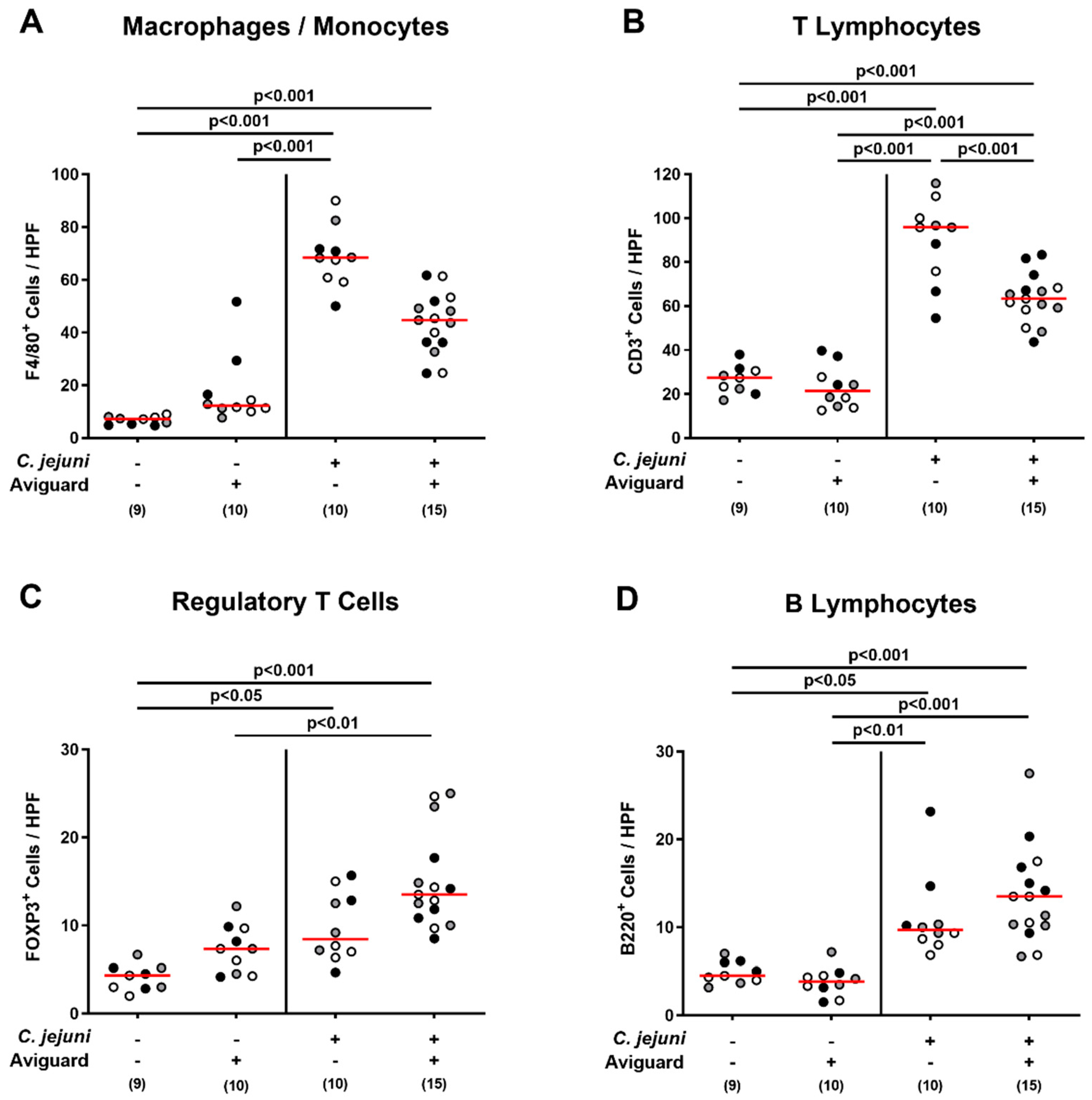
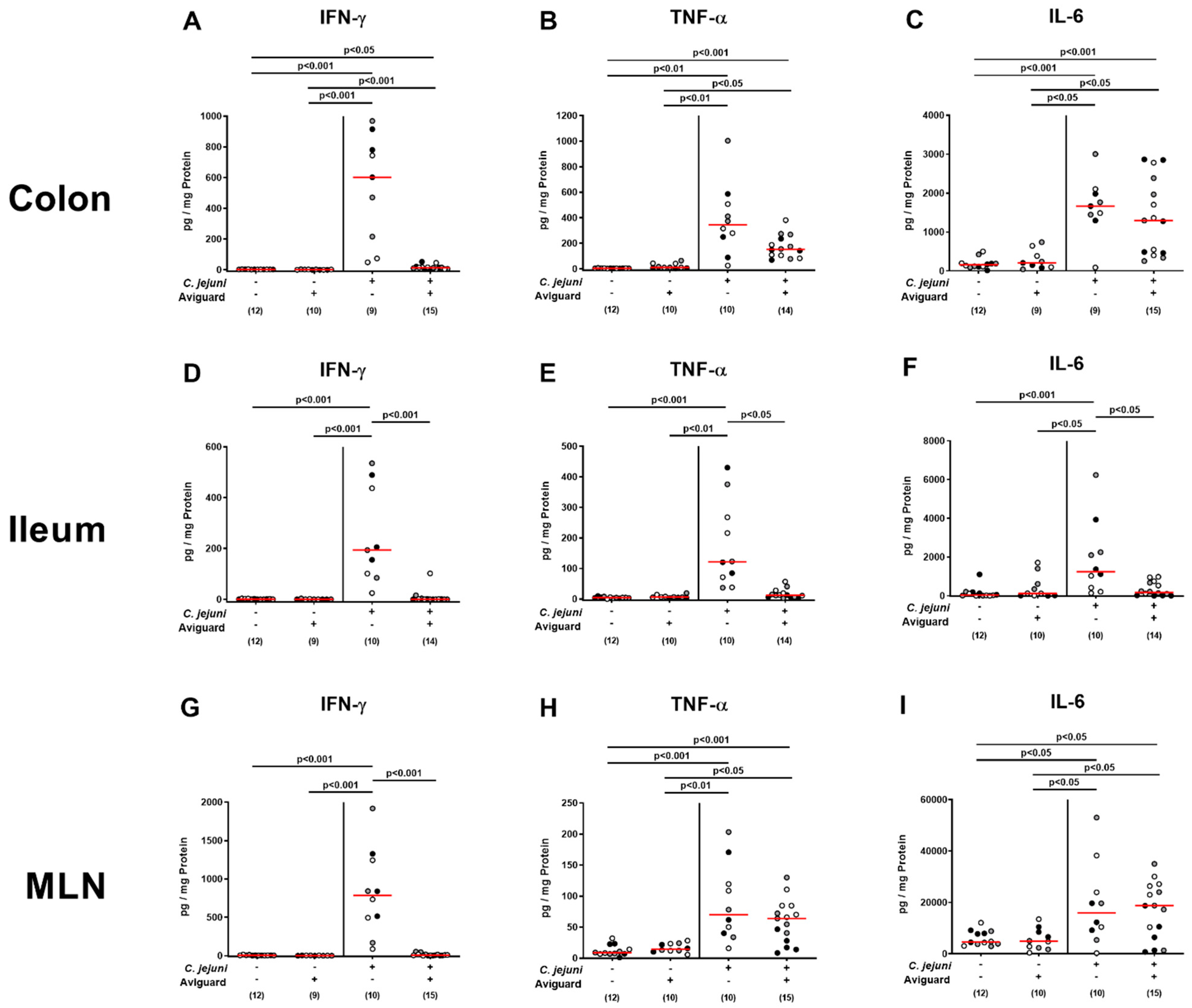
2.5. Aviguard® Treatment and Pro-Inflammatory Immune Responses in the Intestinal Tract of C. jejuni-Infected Microbiota-Depleted IL-10−/− Mice
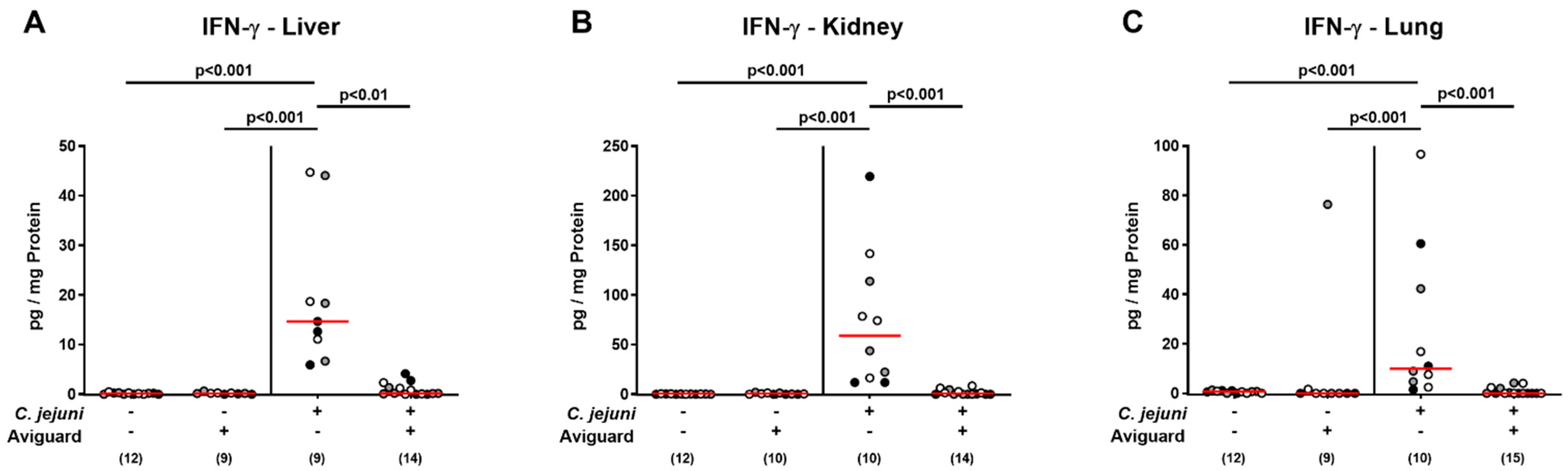
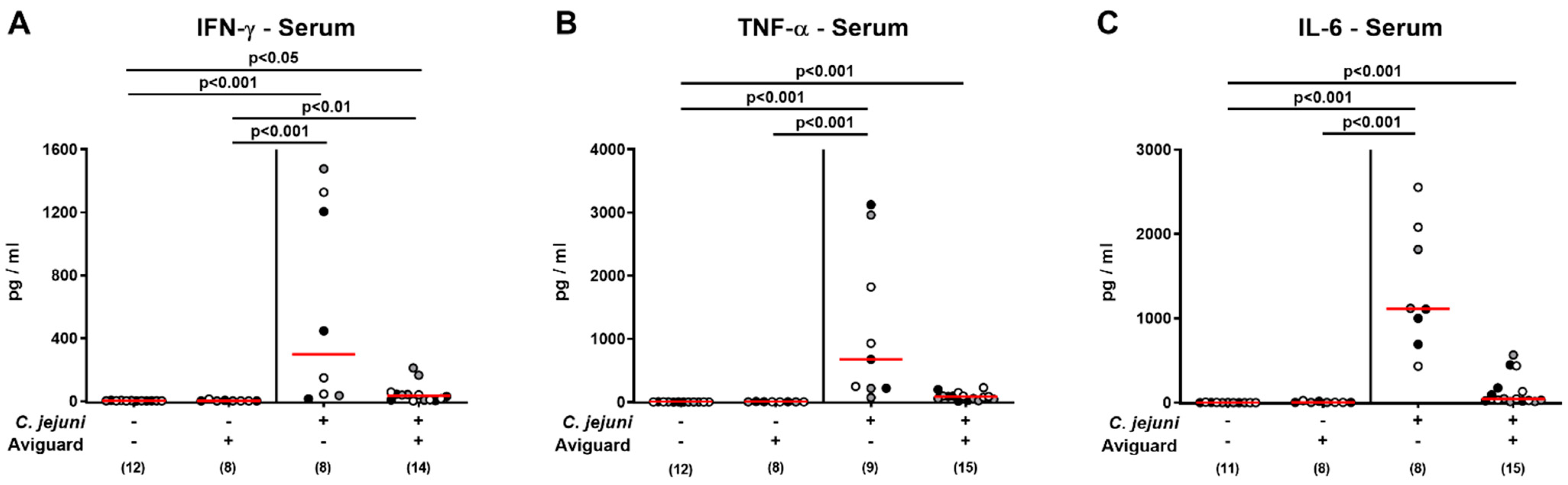
2.6. Aviguard® Treatment and Pro-Inflammatory Cytokine Secretion in Extra-Intestinal and Systemic Tissue Sites of C. jejuni-Infected Microbiota-Depleted IL-10−/− Mice
3. Discussion
4. Material and Methods
4.1. Microbiota-Depleted IL-10−/− Mice
4.2. Campylobacter jejuni Infection
4.3. Treatment of Mice with Commercial Exclusion Product Aviguard®
4.4. Gastrointestinal Colonization by C. jejuni
4.5. Cultural Intestinal Microbiota Analysis
4.6. Molecular Intestinal Microbiota Analysis
4.7. Clinical Outcome
4.8. Sampling Procedures
4.9. Histopathology
4.10. In Situ Immunohistochemistry
4.11. Pro-Inflammatory Cytokine Measurements
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. European Food Safety Authority-Campylobacter and Salmonella Cases Stable in EU. Available online: https://www.efsa.europa.eu/en/news/campylobacter-and-salmonella-cases-stable-eu (accessed on 3 May 2021).
- WHO. World Health Organisation. Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 4 June 2020).
- Mortensen, N.; Jonasson, S.A.; Lavesson, I.V.; Emberland, K.E.; Litleskare, S.; Wensaas, K.A.; Rortveit, G.; Langeland, N.; Hanevik, K. Characteristics of hospitalized patients during a large waterborne outbreak of Campylobacter jejuni in Norway. PLoS ONE 2021, 16, e0248464. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Chapter 1—Human campylobacteriosis. In Campylobacter; Klein, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–25. [Google Scholar] [CrossRef]
- Acheson, D.; Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Walter, E.J.S.; Crim, S.M.; Bruce, B.B.; Griffin, P.M. Postinfectious Irritable Bowel Syndrome After Campylobacter Infection. Am. J. Gastroenterol. 2019, 114, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Koga, M.; Yuki, N.; Hattori, T.; Kuwabara, S. Bickerstaff’s brainstem encephalitis after an outbreak of Campylobacter jejuni enteritis. J. Neuroimmunol. 2008, 196, 143–146. [Google Scholar] [CrossRef]
- Dingle, K.E.; Van Den Braak, N.; Colles, F.M.; Price, L.J.; Woodward, D.L.; Rodgers, F.G.; Endtz, H.P.; Van Belkum, A.; Maiden, M.C. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barré and Miller-Fisher syndromes are of diverse genetic lineage, serotype, and flagella type. J. Clin. Microbiol. 2001, 39, 3346–3349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godschalk, P.C.; Heikema, A.P.; Gilbert, M.; Komagamine, T.; Ang, C.W.; Glerum, J.; Brochu, D.; Li, J.; Yuki, N.; Jacobs, B.C.; et al. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J. Clin. Investig. 2004, 114, 1659–1665. [Google Scholar] [CrossRef]
- Verdu, E.F.; Mauro, M.; Bourgeois, J.; Armstrong, D. Clinical onset of celiac disease after an episode of Campylobacter jejuni enteritis. Can. J. Gastroenterol. 2007, 21, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.D.; Newell, D.G. Campylobacter in poultry: Filling an ecological niche. Avian Dis. 2006, 50, 1–9. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Rovira, J. Campylobacter in the Food Chain. Adv. Food Nutr. Res. 2018, 86, 215–252. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Ó Cróinín, T.; Backert, S. Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front. Cell. Infect. Microbiol. 2012, 2, 25. [Google Scholar] [CrossRef]
- Backert, S.; Boehm, M.; Wessler, S.; Tegtmeyer, N. Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: Paracellular, transcellular or both? Cell Commun. Signal. CCS 2013, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis. Microorganisms 2020, 8, 482. [Google Scholar] [CrossRef]
- John, D.A.; Williams, L.K.; Kanamarlapudi, V.; Humphrey, T.J.; Wilkinson, T.S. The Bacterial Species Campylobacter jejuni Induce Diverse Innate Immune Responses in Human and Avian Intestinal Epithelial Cells. Front. Microbiol. 2017, 8, 1840. [Google Scholar] [CrossRef]
- Nurmi, E.; Rantala, M. New aspects of Salmonella infection in broiler production. Nature 1973, 241, 210–211. [Google Scholar] [CrossRef]
- Rantala, M.; Nurmi, E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973, 14, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Hirn, J.; Nurmi, E.; Johansson, T.; Nuotio, L. Long-term experience with competitive exclusion and salmonellas in Finland. Int. J. Food Microbiol. 1992, 15, 281–285. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Ferreira, C.S.; Knobl, T.; Moreno, A.M.; Bacarro, M.R.; Chen, M.; Robach, M.; Mead, G.C. Comparison of three commercial competitive-exclusion products for controlling Salmonella colonization of broilers in Brazil. J. Food Prot. 2003, 66, 490–492. [Google Scholar] [CrossRef]
- Stern, N.J.; Cox, N.A.; Bailey, J.S.; Berrang, M.E.; Musgrove, M.T. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 2001, 80, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ota, Y.; Mizukami, A.; Ito, T.; Ngwai, Y.B.; Adachi, Y. Evaluation of aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella. Poult. Sci. 2002, 81, 1653–1660. [Google Scholar] [CrossRef]
- Abudabos, A.M. Use of a Competitive Exclusion Product (Aviguard®) to Prevent Clostridium perfringens Colonization in Broiler Chicken under Induced Challenge. Pak. J. Zool. 2013, 45. [Google Scholar]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10−/− mice via Toll-like-receptor-2 and -4 signaling. PLoS ONE 2012, 7, e40761. [Google Scholar] [CrossRef] [PubMed]
- Bücker, R.; Krug, S.M.; Moos, V.; Bojarski, C.; Schweiger, M.R.; Kerick, M.; Fromm, A.; Janssen, S.; Fromm, M.; Hering, N.A.; et al. Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol. 2018, 11, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Escher, U.; Thunhorst, E.; Kittler, S.; Kehrenberg, C.; Bereswill, S.; Heimesaat, M.M. Vitamin C alleviates acute enterocolitis in Campylobacter jejuni infected mice. Sci. Rep. 2020, 10, 2921. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; de Sa, F.D.L.; Schulzke, J.D.; Bucker, R.; Bereswill, S.; Heimesaat, M.M. Vitamin D in Acute Campylobacteriosis-Results from an Intervention Study Applying a Clinical Campylobacter jejuni Induced Enterocolitis Model. Front. Immunol. 2019, 10, 2094. [Google Scholar] [CrossRef]
- Mousavi, S.; Schmidt, A.-M.; Escher, U.; Kittler, S.; Kehrenberg, C.; Thunhorst, E.; Bereswill, S.; Heimesaat, M.M. Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut Pathog. 2020, 12, 2. [Google Scholar] [CrossRef]
- Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Preclinical Evaluation of Oral Urolithin-A for the Treatment of Acute Campylobacteriosis in Campylobacter jejuni Infected Microbiota-Depleted IL-10−/− Mice. Pathogens 2021, 10, 7. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mousavi, S.; Escher, U.; de Sá, F.D.L.; Peh, E.; Schulzke, J.-D.; Kittler, S.; Bücker, R.; Bereswill, S. Resveratrol Alleviates Acute Campylobacter jejuni Induced Enterocolitis in a Preclinical Murine Intervention Study. Microorganisms 2020, 8, 1858. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mousavi, S.; Weschka, D.; Bereswill, S. Anti-Pathogenic and Immune-Modulatory Effects of Peroral Treatment with Cardamom Essential Oil in Acute Murine Campylobacteriosis. Microorganisms 2021, 9, 169. [Google Scholar] [CrossRef]
- Bereswill, S.; Mousavi, S.; Weschka, D.; Buczkowski, A.; Schmidt, S.; Heimesaat, M.M. Peroral Clove Essential Oil Treatment Ameliorates Acute Campylobacteriosis-Results from a Preclinical Murine Intervention Study. Microorganisms 2021, 9, 735. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mousavi, S.; Kløve, S.; Genger, C.; Weschka, D.; Tamas, A.; Reglodi, D.; Bereswill, S. Pituitary Adenylate Cyclase-Activating Polypeptide Alleviates Intestinal, Extra-Intestinal and Systemic Inflammatory Responses during Acute Campylobacter jejuni-induced Enterocolitis in Mice. Pathogens 2020, 9, 805. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mousavi, S.; Kløve, S.; Genger, C.; Weschka, D.; Giladi, E.; Bereswill, S.; Gozes, I. Immune-modulatory Properties of the Octapeptide NAP in Campylobacter jejuni Infected Mice Suffering from Acute Enterocolitis. Microorganisms 2020, 8, 802. [Google Scholar] [CrossRef]
- Ekmekciu, I.; Fiebiger, U.; Stingl, K.; Bereswill, S.; Heimesaat, M.M. Amelioration of intestinal and systemic sequelae of murine Campylobacter jejuni infection by probiotic VSL#3 treatment. Gut Pathog. 2017, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Kløve, S.; Genger, C.; Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathogens 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Genger, C.; Biesemeier, N.; Klove, S.; Weschka, D.; Mousavi, S.; Bereswill, S. Inflammatory Immune Responses and Gut Microbiota Changes Following Campylobacter coli Infection of IL-10−/− Mice with Chronic Colitis. Pathogens 2020, 9, 560. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Bohm, M.; Kuhl, A.A.; Gobel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell. Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Dasti, J.I.; Zautner, A.E.; Munoz, M.; Loddenkemper, C.; et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Stern, N.J. Mucosal competitive exclusion to diminish colonization of chickens by Campylobacter jejuni. Poult. Sci. 1994, 73, 402–407. [Google Scholar] [CrossRef]
- Stern, N.J.; Bailey, J.S.; Blankenship, L.; Cox, N.; McHan, F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988, 330–334. [Google Scholar] [CrossRef]
- Howarth, G.S.; Wang, H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients 2013, 5, 58–81. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef]
- Bereswill, S.; Ekmekciu, I.; Escher, U.; Fiebiger, U.; Stingl, K.; Heimesaat, M.M. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci. Rep. 2017, 7, 2138. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.H.; Whang, K.Y.; Kim, Y.J.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS ONE 2012, 7, e43197. [Google Scholar] [CrossRef] [PubMed]
- Hudcovic, T.; Kolinska, J.; Klepetar, J.; Stepankova, R.; Rezanka, T.; Srutkova, D.; Schwarzer, M.; Erban, V.; Du, Z.; Wells, J.M.; et al. Protective effect of Clostridium tyrobutyricum in acute dextran sodium sulphate-induced colitis: Differential regulation of tumour necrosis factor-α and interleukin-18 in BALB/c and severe combined immunodeficiency mice. Clin. Exp. Immunol. 2012, 167, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Mrazek, K.; Bereswill, S. Murine Fecal Microbiota Transplantation Alleviates Intestinal and Systemic Immune Responses in Campylobacter jejuni Infected Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019, 10, 2272. [Google Scholar] [CrossRef]
- Lee, S.K.; Yang, K.M.; Cheon, J.H.; Kim, T.I.; Kim, W.H. [Anti-inflammatory mechanism of Lactobacillus rhamnosus GG in lipopolysaccharide- stimulated HT-29 cell]. Korean J. Gastroenterol. 2012, 60, 86–93. [Google Scholar] [CrossRef]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G608–G617. [Google Scholar] [CrossRef]
- Benoit, S.L.; Maier, R.J.; Sawers, R.G.; Greening, C. Molecular Hydrogen Metabolism: A Widespread Trait of Pathogenic Bacteria and Protists. Microbiol. Mol. Biol. Rev. MMBR 2020, 84. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef]
- Lallemand. Lallemand Animal Nutrition—Product Details AVIGUARD. Available online: https://lallemandanimalnutrition.com/en/europe/our-products/product-details/aviguard/ (accessed on 3 December 2020).
- Heimesaat, M.M.; Fischer, A.; Jahn, H.K.; Niebergall, J.; Freudenberg, M.; Blaut, M.; Liesenfeld, O.; Schumann, R.R.; Gobel, U.B.; Bereswill, S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 2007, 56, 941–948. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Nogai, A.; Bereswill, S.; Plickert, R.; Fischer, A.; Loddenkemper, C.; Steinhoff, U.; Tchaptchet, S.; Thiel, E.; Freudenberg, M.A.; et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 2010, 59, 1079–1087. [Google Scholar] [CrossRef]
- Rausch, S.; Held, J.; Fischer, A.; Heimesaat, M.M.; Kühl, A.A.; Bereswill, S.; Hartmann, S. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS ONE 2013, 8, e74026. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Heimesaat, M.M.; Schmidt, A.-M.; Mousavi, S.; Escher, U.; Tegtmeyer, N.; Wessler, S.; Gadermaier, G.; Briza, P.; Hofreuter, D.; Bereswill, S. Peptidase PepP is a novel virulence factor of Campylobacter jejuni contributing to murine campylobacteriosis. Gut Microbes 2020, 12, 1770017. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Giladi, E.; Kuhl, A.A.; Bereswill, S.; Gozes, I. The octapetide NAP alleviates intestinal and extra-intestinal anti-inflammatory sequelae of acute experimental colitis. Peptides 2018, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimesaat, M.M.; Weschka, D.; Mousavi, S.; Bereswill, S. Treatment with the Probiotic Product Aviguard® Alleviates Inflammatory Responses during Campylobacter jejuni-Induced Acute Enterocolitis in Mice. Int. J. Mol. Sci. 2021, 22, 6683. https://doi.org/10.3390/ijms22136683
Heimesaat MM, Weschka D, Mousavi S, Bereswill S. Treatment with the Probiotic Product Aviguard® Alleviates Inflammatory Responses during Campylobacter jejuni-Induced Acute Enterocolitis in Mice. International Journal of Molecular Sciences. 2021; 22(13):6683. https://doi.org/10.3390/ijms22136683
Chicago/Turabian StyleHeimesaat, Markus M., Dennis Weschka, Soraya Mousavi, and Stefan Bereswill. 2021. "Treatment with the Probiotic Product Aviguard® Alleviates Inflammatory Responses during Campylobacter jejuni-Induced Acute Enterocolitis in Mice" International Journal of Molecular Sciences 22, no. 13: 6683. https://doi.org/10.3390/ijms22136683
APA StyleHeimesaat, M. M., Weschka, D., Mousavi, S., & Bereswill, S. (2021). Treatment with the Probiotic Product Aviguard® Alleviates Inflammatory Responses during Campylobacter jejuni-Induced Acute Enterocolitis in Mice. International Journal of Molecular Sciences, 22(13), 6683. https://doi.org/10.3390/ijms22136683






