Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives
Abstract
:1. Introduction
2. Inflammation in COVID-19
3. The Cytokine Storm in COVID-19
4. Inflammation and Pro-Inflammatory Cytokines in Atherosclerosis
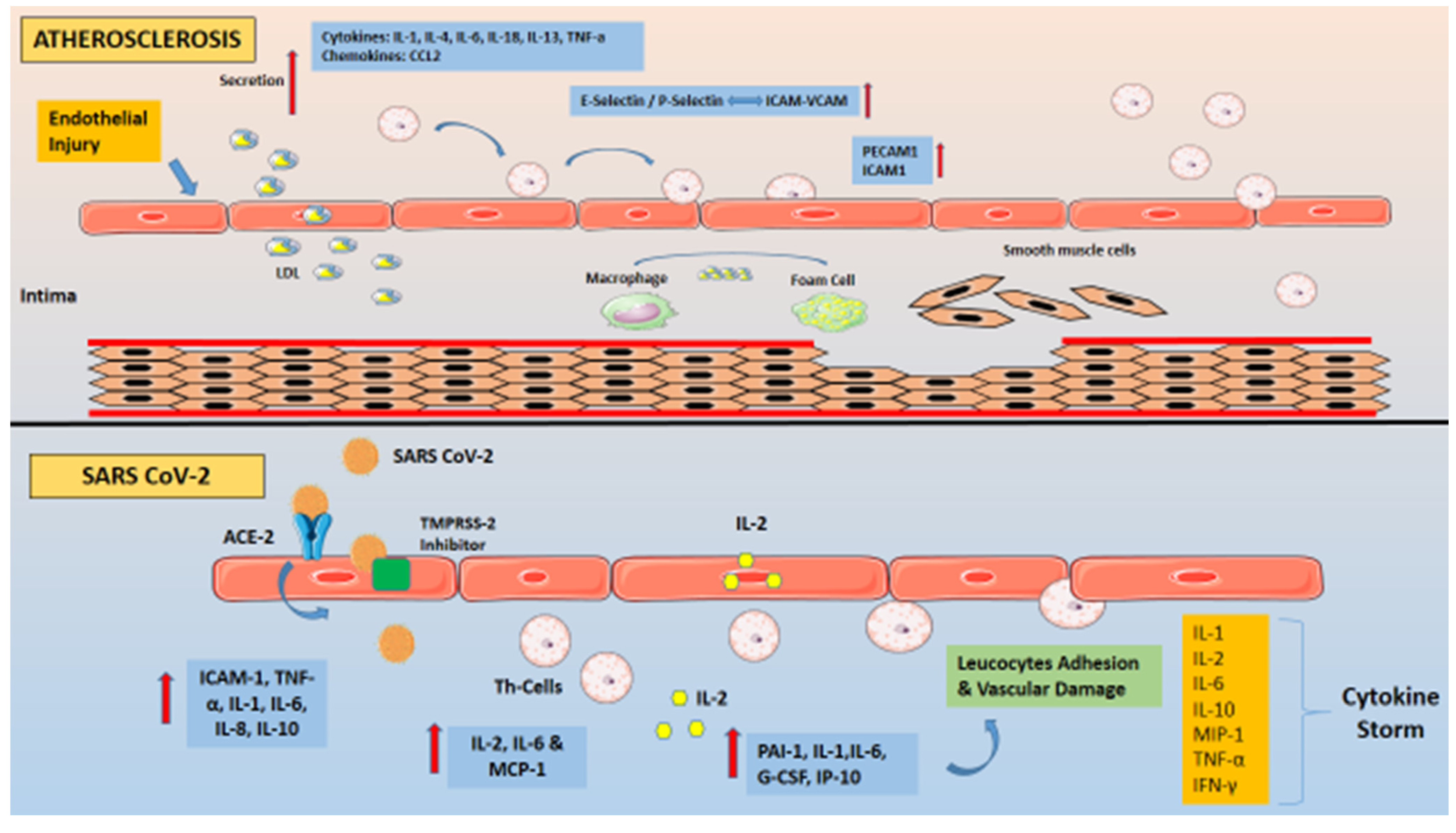
5. Similarities in the Inflammatory Processes Operating in COVID-19 and Atherosclerosis
6. Inflammatory Responses: Differences between COVID-19 and Atherosclerosis
7. Therapeutic Implications of Inflammation in COVID 19 and Atherosclerosis
7.1. Atherosclerosis
7.2. COVID-19
8. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| IFN | Interferon |
| NK | natural killers |
| CRP | C-reactive protein |
| MAS | macrophage activation syndrome |
| sHLH | secondary haemophagocytic lymphohistocytosis |
| TLRs | Toll-like receptors |
| IL | Interleukin |
| G-CSF | granulocyte colony-stimulating factor |
| MCP-1 | monocyte chemoattractant protein 1 |
| ICAM-1 | intercellular adhesion molecule-1 |
| VCAM-1 | vascular adhesion molecule-1 |
| MMPs | matrix metalloproteinases |
| TIMPs | tissue inhibitors of metalloproteinases |
| PGI2 | prostaglandins |
| NO | nitric oxide |
| ΡAΙ-1 | Plasminogen activator inhibitor-1 |
| TNF | Tumor Necrosis Factor |
| IP-10 | Interferon gamma-induced protein 10 |
| MIP-1 | Macrophage inflammatory protein-1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [PubMed]
- Shin, H.S. Empirical Treatment and Prevention of COVID-19. Infect. Chemother. 2020, 52, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Chang, D.; Lin, M.; Wei, L.; Xie, L.; Zhu, G.; Cruz, C.S.D.; Sharma, L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA 2020, 323, 1092–1093. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the Cytokine Storm in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Li, J.; Li, X.; Qi, X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020, 295, 18. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.W.; Yuan, S.; Kok, K.H.; To, K.K.W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.Y.; Poon, R.W.S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef]
- Brott, T.G.; Hobson, R.W.; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Rodríguez-Carrio, J. Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open 2020, 6, e001295. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Gu, X. A close-up on COVID-19 and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1057–1060. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.L.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 1. [Google Scholar]
- Cheung, C.Y.; Poon, L.L.M.; Ng, I.H.Y.; Luk, W.; Sia, S.-F.; Wu, M.H.S.; Chan, K.-H.; Yuen, K.-Y.; Gordon, S.; Guan, Y.; et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: Possible relevance to pathogenesis. J. Virol. 2005, 79, 7819–7826. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.K.P.; Lau, C.C.Y.; Chan, K.-H.; Li, C.P.Y.; Chen, H.; Jin, D.-Y.; Chan, J.F.W.; Woo, P.C.Y.; Yuen, K.-Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. J. Gen. Virol. 2013, 94, 2679–2690. [Google Scholar] [CrossRef]
- Law, H.K.-W.; Cheung, C.Y.; Ng, H.Y.; Sia, S.F.; Chan, Y.O.; Luk, W.; Nicholls, J.M.; Peiris, J.S.M.; Lau, Y.L. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 2005, 106, 2366–2374. [Google Scholar] [CrossRef] [Green Version]
- Hogner, K.; Wolff, T.; Pleschka, S.; Plog, S.; Gruber, A.D.; Kalinke, U.; Herold, S.; Walmrath, H.-D.; Bodner, J.; Gattenlohner, S.; et al. Correction: Macrophage-expressed IFN-beta Contributes to Apoptotic Alveolar Epithelial Cell Injury in Severe Influenza Virus Pneumonia. PLoS Pathog. 2016, 12, e1005716. [Google Scholar] [CrossRef] [Green Version]
- Rodrigue-Gervais, I.G.; Labbé, K.; Dagenais, M.; Dupaul-Chicoine, J.; Champagne, C.; Morizot, A.; Skeldon, A.; Brincks, E.L.; Vidal, S.M.; Griffith, T.S.; et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 2014, 15, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis. Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Bot, A.; Holz, A.; Christen, U.; Wolfe, T.; Temann, A.; Flavell, R.; von Herrath, M. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology 2000, 269, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.L.; Chen, D.; Yan, J.; Yang, Q.; Han, Q.; Li, S.; Cheng, L. Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID-19 patients. FEBS J. 2020. [Google Scholar] [CrossRef]
- Shieh, J.M.; Tseng, H.-Y.; Jung, F.; Yang, S.-H.; Lin, J.-C. Elevation of IL-6 and IL-33 Levels in Serum Associated with Lung Fibrosis and Skeletal Muscle Wasting in a Bleomycin-Induced Lung Injury Mouse Model. Mediat. Inflamm. 2019, 2019, 7947596. [Google Scholar] [CrossRef] [PubMed]
- Mahallawi, W.H.; Khabour, O.F.; Zhang, Q.; Makhdoum, H.M.; Suliman, B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018, 104, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.-S.; et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020, 5, eabd1554. [Google Scholar] [CrossRef] [PubMed]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.F.; Siegel, M.R.; Lenardo, J.M. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity 2000, 13, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Pena, E.; De La Torre, R.; Arderiu, G.; Slevin, M.; Badimon, L. mCRP triggers angiogenesis by inducing F3 transcription and TF signalling in microvascular endothelial cells. Thromb. Haemost. 2017, 117, 357–370. [Google Scholar]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion during Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Swirski, F.K.; Nahrendorf, M.; Libby, P. Mechanisms of Myeloid Cell Modulation of Atherosclerosis. Microbiol. Spectr. 2016, 4, 813–824. [Google Scholar] [CrossRef] [Green Version]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, M.E.; Ross, R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 1990, 10, 680–687. [Google Scholar] [CrossRef] [Green Version]
- Kaartinen, M.; Penttila, A.; Kovanen, P.T. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arter. Thromb. 1994, 14, 966–972. [Google Scholar] [CrossRef] [Green Version]
- Henney, A.M.; Wakeley, P.R.; Davies, M.J.; Foster, K.; Hembry, R.; Murphy, G.; Humphries, S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc. Natl. Acad. Sci. USA 1991, 88, 8154–8158. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, H.; Zhang, Y.; Zhao, Y. TLRs are important inflammatory factors in atherosclerosis and may be a therapeutic target. Med. Hypotheses 2008, 70, 314–316. [Google Scholar] [CrossRef]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 ligand system: Linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Invest. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Lane, T.; Wassef, N.; Poole, S.; Mistry, Y.; Lachmann, H.J.; Gillmore, J.D.; Hawkins, P.N.; Pepys, M.B. Infusion of pharmaceutical-grade natural human C-reactive protein is not proinflammatory in healthy adult human volunteers. Circ. Res. 2014, 114, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, S.K.; Devaraj, S.; Jialal, I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation 2003, 108, 1676–1678. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Wang, C.H.; Li, S.H.; Dumont, A.S.; Fedak, P.W.; Badiwala, M.V.; Stewart, D.J.; Dhillon, B.; Weisel, R.D.; Li, R.-K.; et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002, 106, 913–919. [Google Scholar] [CrossRef]
- Venugopal, S.K.; Devaraj, S.; Yuhanna, I.; Shaul, P.; Jialal, I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 2002, 106, 1439–1441. [Google Scholar] [CrossRef]
- Cermak, J.; Key, N.S.; Bach, R.R.; Balla, J.; Jacob, H.S.; Vercellotti, G.M. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood 1993, 82, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Amento, E.P.; Ehsani, N.; Palmer, H.; Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arter. Thromb. 1991, 11, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.K.; Hellstrand, M.; Rymo, L.; Rubbia, L.; Gabbiani, G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J. Exp. Med. 1989, 170, 1595–1608. [Google Scholar] [CrossRef] [Green Version]
- Ovchinnikova, O.; Robertson, A.K.L.; Wågsäter, D.; Folco, E.J.; Hyry, M.; Myllyharju, J.; Eriksson, P.; Libby, P.; Hansson, G.K. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe(-/-) mice. Am. J. Pathol. 2009, 174, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, K. Association between COVID-19 and cardiovascular disease. Int. J. Cardiol. Heart Vasc. 2020, 29, 100583. [Google Scholar]
- Min, C.K.; Cheon, S.; Ha, N.Y.; Sohn, K.M.; Kim, Y.; Aigerim, A.; Kim, Y.S.; Shin, H.-M.; Choi, J.-Y.; Inn, K.-S.; et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016, 6, 25359. [Google Scholar] [CrossRef]
- Mallat, Z.; Heymes, C.; Corbaz, A.; Logeart, D.; Alouani, S.; Cohen-Solal, A.; Seidler, T.; Hasenfuss, G.; Chvatchko, Y.; Shah, A.M.; et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004, 18, 1752–1754. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; Von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, T.; Petit, I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Weber, C.; Schober, A.; Zernecke, A. Chemokines: Key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arter. Thromb. Vasc. Biol. 2004, 24, 1997–2008. [Google Scholar] [CrossRef] [Green Version]
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef]
- Dhakal, B.P.; Sweitzer, N.K.; Indik, J.H.; Acharya, D.; William, P. SARS-CoV-2 Infection and Cardiovascular Disease: COVID-19 Heart. Heart Lung. Circ. 2020, 29, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.J.; Mohiddin, S.A.; DiMarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Cao, Z.; Wu, Y.; Faucon, E.; Mouhat, S.; Kovacic, H.; Sabatier, J.M. Counter-regulatory ’Renin-Angiotensin’ System-based Candidate Drugs to Treat COVID-19 Diseases in SARS-CoV-2-infected patients. Infect. Disord. Drug Targets 2020, 20, 407–408. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Sagris, M.; Kokkinidis, D.G.; Lempesis, I.G.; Giannopoulos, S.; Rallidis, L.; Mena-Hurtado, C.; Bakoyiannis, C. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev. Cardiovasc. Med. 2020, 21, 565–575. [Google Scholar]
- Pastrana, J.L.; Sha, X.; Virtue, A.; Mai, J.; Cueto, R.; Lee, I.A.; Yang, X.F.; Wang, H. Regulatory T cells and Atherosclerosis. J. Clin. Exp. Cardiol. 2012, 2012, 2. [Google Scholar] [CrossRef]
- Richards, C.D. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm. 2013, 2013, 512103. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Yuan, W.; Li, J.; Zhou, H.; Xu, L.; Weng, J.; Li, X.; Zhang, X.; Wang, Z.; Yan, J. Cyclophilin A mediates the ox-LDL-induced activation and apoptosis of macrophages via autophagy. Int. J. Cardiol. 2017, 230, 142–148. [Google Scholar] [CrossRef]
- O’Brien, E.R.; Garvin, M.R.; Stewart, D.K.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M.; Giachelli, C.M. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arter. Thromb. 1994, 14, 1648–1656. [Google Scholar] [CrossRef] [Green Version]
- Schoppet, M.; Preissner, K.T.; Hofbauer, L.C. RANK ligand and osteoprotegerin: Paracrine regulators of bone metabolism and vascular function. Arter. Thromb. Vasc. Biol. 2002, 22, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef]
- Sakellarios, A.I.; Fotiadis, D.I. Editorial commentary: The pleiotropic effect of statins on the atherosclerotic plaque and coronary heart disease. Trends Cardiovasc. Med. 2019, 29, 456–457. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Cyrus, T.; Sung, S.; Zhao, L.; Funk, C.D.; Tang, S.; Praticò, D. Effect of low-dose aspirin on vascular inflammation, plaque stability, and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation 2002, 106, 1282–1287. [Google Scholar] [CrossRef] [Green Version]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, M.; Thompson, P.L. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am. J. Cardiol. 2007, 99, 805–807. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Tardif, J.-C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef]
- Monteagudo, L.A.; Boothby, A.; Gertner, E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020, 2, 276–282. [Google Scholar] [CrossRef]
- Cacciapaglia, F.; Anelli, M.G.; Rinaldi, A.; Fornaro, M.; Lopalco, G.; Scioscia, C.; Lapadula, G.; Iannone, F. Lipids and Atherogenic Indices Fluctuation in Rheumatoid Arthritis Patients on Long-Term Tocilizumab Treatment. Mediators Inflamm. 2018, 2018, 2453265. [Google Scholar] [CrossRef]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.-A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef] [PubMed]
- van Kraaij, T.D.; Mostard, R.L.; Ramiro, S.; Checa, C.M.; van Dongen, C.M.; van Haren, E.H.; Bujis, J.; Landewé, R.B. Tocilizumab in Severe COVID-19 Pneumonia and Concomitant Cytokine Release Syndrome. Eur. J. Case Rep. Intern. Med. 2020, 7, 001675. [Google Scholar] [CrossRef] [PubMed]
- Strang, A.C.; Bisoendial, R.J.; Kootte, R.S.; Schulte, D.M.; Dallinga-Thie, G.M.; Levels, J.H.; Kok, M.; Vos, K.; Tas, S.W.; Tietge, U.J.; et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 2013, 229, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, U.; Jovinge, S.; Persson, J.; Jacobsson, L.T.; Turesson, C. Effects of Treatment with Adalimumab on Blood Lipid Levels and Atherosclerosis in Patients with Rheumatoid Arthritis. Curr. Ther. Res. Clin. Exp. 2018, 89, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Yan, B.X.; Man, X.Y. TNFalpha inhibitor may be effective for severe COVID-19: Learning from toxic epidermal necrolysis. Ther. Adv. Respir. Dis. 2020, 14, 1753466620926800. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Gniadecki, R.; Iversen, L.; Bryld, L.; Lasthein, S.; Lindhardsen, J.; Kristensen, S.L.; Torp-Pedersen, C.; et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J. Eur. Acad. Derm. Venereol. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Jacobsson, L.T.; Turesson, C.; Gülfe, A.; Kapetanovic, M.C.; Petersson, I.F.; Saxne, T.; Geborek, P. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1213–1218. [Google Scholar]
- Johnson, R.M.; Vinetz, J.M. Dexamethasone in the management of covid -19. Br. Med. J. 2020, 370, m2648. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020, 84, 106560. [Google Scholar] [CrossRef]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Din, C.T.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325-e331. [Google Scholar] [CrossRef]
- Bozzi, G.; Mangioni, D.; Minoia, F.; Aliberti, S.; Grasselli, G.; Barbetta, L.; Bandera, A.; Castelli, V.; Palomba, E.; Alagna, L.; et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: An observational cohort study. J. Allergy Clin. Immunol. 2020, 147, 561–566. [Google Scholar] [CrossRef]
- Rossotti, R.; Travi, G.; Ughi, N.; Corradin, M.; Baiguera, C.; Fumagalli, R.; Bottiroli, M.; Mondino, M.; Merli, M.; Bellone, A.; et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis. J. Infect. 2020, 81, e11–e17. [Google Scholar] [CrossRef]
- Hossen, M.S.; Barek, M.A.; Jahan, N.; Islam, M.S. A Review on Current Repurposing Drugs for the Treatment of COVID-19: Reality and Challenges. SN Compr. Clin. Med. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Thompson, P.L. Why Colchicine Should Be Considered for Secondary Prevention of Atherosclerosis: An Overview. Clin. Ther. 2019, 41, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Deftereos, S.G.; Siasos, G.; Giannopoulos, G.; Vrachatis, D.A.; Angelidis, C.; Giotaki, S.G.; Gargalianos, P.; Giamarellou, H.; Gogos, C.; Daikos, G.; et al. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J. Cardiol. 2020, 61, 42–45. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Holte, E.; Kleveland, O.; Ueland, T.; Kunszt, G.; Bratlie, M.; Broch, K.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Aakhus, S.; et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction. Heart 2017, 103, 1521–1527. [Google Scholar] [CrossRef]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular Safety of Tocilizumab Versus Etanercept in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 2020, 72, 31–40. [Google Scholar] [CrossRef]
| IL-/TNF- and IFN-Family Cytokines | |
|---|---|
| Factor | Prognostic Value |
| IL-1β | Elevated levels IL-1β have been associated with hypercoagulation, disseminated intravascular coagulation, and severe symptoms [28]. |
| IL-2 | Increases in IL-2 or its receptor IL-2R are directly proportional to the severity of the disease [13]. |
| IL-4 | IL-4 has negative effects on CD8+ memory T cells; elevated IL-4 levels are associated with cytokine storm and severe respiratory symptoms [29]. |
| IL-6 | Higher levels of IL-6 accelerates the inflammatory process, contributing to the cytokine storm and worsening the prognosis [18]. |
| IL-12 | NA |
| IL-17 | Elevated IL-17 levels have been reported in patients with SARS-CoV-2 as part of the cytokine storm, and they are associated with viral load and disease severity [30]. |
| IL-18 | NA |
| IL-21 | NA |
| IL-33 | Higher IL-33 levels have been associated with lung fibrosis and skeletal muscle wasting [31]. |
| TNF-alpha | TNF-alpha was one of the cytokines whose overproduction was related to a poor prognosis in patients with SARS-CoV-2, finding an inverse relationship between TNF-alpha levels and T cell counts [32]. |
| TGF-β | NA |
| IFN-α | NA |
| IFN-γ | IFN-γ levels are associated with greater viral load and lung damage [33]. |
| Chemokines | |
| CCL2/MCP-1 | CCL2 levels were higher in patients with COVID-19 and even higher among those admitted to the Intensive Care Unit [9]. |
| CCL3/MIP-1A | NA |
| CCL5 | NA |
| CXCL9 | NA |
| CXCL10/IP-10 | IP-10 levels were found to be elevated in patients with COVID-19 and even higher in those who required Intensive Care Unit admission, suggesting their relationship with lung damage and disease severity [9]. |
| Similarities | ||
|---|---|---|
| Factor | Atherosclerosis | COVID-19 |
| NO−1 | Decreased | Decreased |
| Coagulation Factors | Increased | Increased |
| IL-1β | Increased | Increased |
| IL-6 | Increased | Increased |
| IL-12 | Increased | Increased |
| IL-18 | Increased | Increased |
| IFN-α | Increased | Increased |
| IFN-γ | Increased | Increased |
| TGF-β | Increased | Increased |
| TNF-alpha | Increased | Increased |
| CCL2 | Increased | Increased |
| CCL3 | Increased | Increased |
| CXCL9 | Increased | Increased |
| CXCL10 | Increased | Increased |
| C-Reactive Protein | Increased | Increased |
| Differences | ||
| Factor | Atherosclerosis | COVID-19 |
| Angiotensin II | NA | Increased |
| IL-3 | Increased | NA |
| IL-8 | Increased | NA |
| IL-15 | Increased | NA |
| IL-17 | NA | Increased |
| IL-21 | NA | Increased |
| IL-33 | NA | Increased |
| M-CSF | Increased | NA |
| CXCL8 | NA | Increased |
| CXCL11 | Increased | NA |
| CXCL16 | Increased | NA |
| Oncostatin M | Increased | NA |
| Cyclophilin A | Increased | NA |
| Osteopontin | Increased | NA |
| Osteoprotogerin | Increased | NA |
| Ferritin | NA | Increased |
| Drugs | Action | COVID-19 Ongoing Trials | Atherosclerotic Disease Trials |
|---|---|---|---|
| Corticosteroids | Immunosuppression | NCT04273321 | - |
| Anakinra | Monoclonal antibody against IL-1 Receptor | NCT04339712, Phase 2 | Ikonomidis et al. [84] |
| Emapalumab | Monoclonal antibody against IL-1 Receptor | NCT04324021, Phase 2/3 | - |
| Canakinumab | monoclonal antibody against IL-1-beta | NCT04362813, Phase 2 | CANTOS Trial [102] |
| Tocilizumab | IL-6 Receptor Inhibitor | NCT04317092 | Holte et al. [103] |
| Sarilumab | IL-6 Receptor Inhibitor | NCT04280588, Phase2 | - |
| Heparin | Anticoagulant, anti-inflammatory, antiviral | NCT04345848, Phase 3 | - |
| Colchicine | Inhibition of NLRP3 inflammasome | NCT04326790 | COLCOT Trial [80] |
| Adalimumab | Anti-TNF-alpha antagonists | Case Series | ENTRACTE Trial [103] |
| Etanercept | Anti-TNF-alpha antagonists | Case Series | ENTRACTE Trial [103] |
| Aspirin | Inhibitor of the enzyme cyclooxygenase (COX) | NCT04363840 | Coronary Microvascular Angina Trial (CorMicA) [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, C.; Oikonomou, E.; Antoniades, C.; Crea, F.; Kaski, J.C.; Tousoulis, D. Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. Int. J. Mol. Sci. 2021, 22, 6607. https://doi.org/10.3390/ijms22126607
Sagris M, Theofilis P, Antonopoulos AS, Tsioufis C, Oikonomou E, Antoniades C, Crea F, Kaski JC, Tousoulis D. Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. International Journal of Molecular Sciences. 2021; 22(12):6607. https://doi.org/10.3390/ijms22126607
Chicago/Turabian StyleSagris, Marios, Panagiotis Theofilis, Alexios S. Antonopoulos, Costas Tsioufis, Evangelos Oikonomou, Charalambos Antoniades, Filippo Crea, Juan Carlos Kaski, and Dimitris Tousoulis. 2021. "Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives" International Journal of Molecular Sciences 22, no. 12: 6607. https://doi.org/10.3390/ijms22126607
APA StyleSagris, M., Theofilis, P., Antonopoulos, A. S., Tsioufis, C., Oikonomou, E., Antoniades, C., Crea, F., Kaski, J. C., & Tousoulis, D. (2021). Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. International Journal of Molecular Sciences, 22(12), 6607. https://doi.org/10.3390/ijms22126607









