IPA1 Negatively Regulates Early Rice Seedling Development by Interfering with Starch Metabolism via the GA and WRKY Pathways
Abstract
:1. Introduction
2. Results
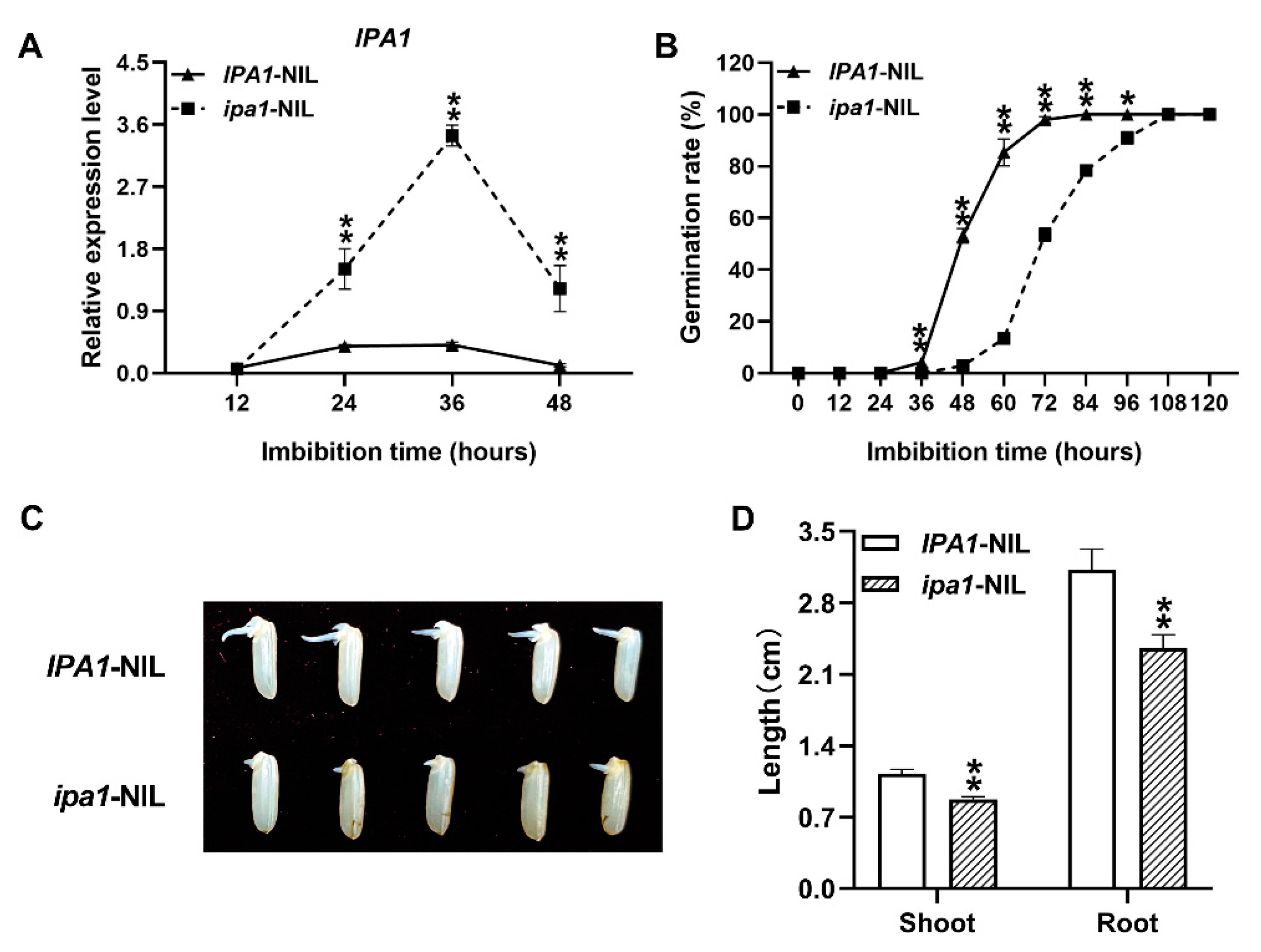
2.1. ipa1 Retards Seed Germination and Early Seedling Growth of Rice
2.2. IPA1 Negatively Regulates Starch Metabolism during Seed Germination and Early Seedling Growth
2.3. The Retarding Effect of Seed Germination and Early Seeding Growth in the ipa1-NIL Seeds Caused by GA Defect
2.4. Enhanced GA Deactivation in the ipa1-NIL
2.5. IPA1 Directly Binds to OsWRKY51 and OsWRKY71 and Promotes Their Expression
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Germination Analysis
4.3. Determination of α-Amylase Activity
4.4. Measurement of Soluble Sugar Content
4.5. Measurement of Endogenous Bioactive GA Content
4.6. RNA Extraction and qPCR
4.7. Dual-Luciferase Assay
4.8. Yeast One-Hybrid Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [Green Version]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukura, C.; Saitoh, T.; Hirose, T.; Ohsugi, R.; Perata, P.; Yamaguchi, J. Sugar uptake and transport in rice embryo. Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol. 2000, 124, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujadas, G.; Palau, J. Evolution of alpha-amylases: Architectural features and key residues in the stabilization of the (β/α)8 scaffold. Mol. Biol. Evol. 2001, 18, 38–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.-M.; Lo, S.-F.; Ho, T.-H.D. Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Chen, P.-W.; Chiang, C.-M.; Tseng, T.-H.; Yu, S.-M. Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of α-Amylase genes. Plant Cell 2006, 18, 2326–2340. [Google Scholar] [CrossRef] [Green Version]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.-F.; Ho, T.-H.; Wu, C.-F.; Ho, S.-L.; Yeh, R.-H.; Lu, C.-A.; Chen, P.-W.; Yu, L.-C.; Chao, A.; Yu, S.-M. Convergent starvation signals and hormone crosstalk in regulating nutrient mobilization upon germination in cereals. Plant Cell 2012, 24, 2857–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, M.; Inukai, Y.; Ueguchi-Tanaka, M.; Itoh, H.; Izawa, T.; Kobayashi, Y.; Hattori, T.; Miyao, A.; Hirochika, H.; Ashikari, M.; et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell 2004, 16, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.R.; Rodriguez, R.L. Metabolite signals regulate gene expression and source/sink relations in cereal seedlings. Plant Physiol. 1994, 106, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Guo, X.-L.; Hou, X.-M.; Fang, J.; Wei, P.-W.; Xu, B.; Chen, M.-L.; Feng, Y.-Q.; Chu, C.-C. The rice GERMINATION DEFECTIVE 1, encoding a B3 domain transcriptional repressor, regulates seed germination and seedling development by integrating GA and carbohydrate metabolism. Plant J. 2013, 75, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Feng, J.-H.; Zhang, L.-H.; Zhang, J.-F.; Mispan, M.S.; Cao, Z.-Q.; Beighley, D.H.; Yang, J.-C.; Gu, X.-Y. Map-based cloning of seed dormancy1-2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiol. 2015, 169, 2152–2165. [Google Scholar] [PubMed] [Green Version]
- Huo, H.-Q.; Wei, S.-H.; Bradford, K.J. DELAY OF GERMINATION1 (DOG1) regulates both seed dormancy and flowering time through microRNA pathways. Proc. Natl. Acad. Sci. USA 2016, 113, E2199–E2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, C.-B.; Wang, Z.; Zhang, L.; Yao, J.-J.; Hua, K.; Liu, X.; Shi, H.-Z.; Zhu, J.-K. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 2019, 10, 3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.-Q.; Wang, Y.-H.; Xue, D.-W.; Wang, J.; Yan, M.-X.; Liu, G.-F.; Dong, G.-J.; Zeng, D.-L.; Lu, Z.-F.; Zhu, X.-D.; et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef]
- Miura, K.; Ikeda, M.; Matsubara, A.; Song, X.-J.; Ito, M.; Asano, K.; Matsuoka, M.; Kitano, H.; Ashikari, M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010, 42, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, T.-W.; Song, Q.-X.; Ye, W.-X.; Song, X.-G.; Chu, J.-F.; Li, J.-Y.; Chen, Z.J. Rice circadian clock regulates tiller growth and panicle development through strigolactone signaling and sugar sensing. Plant Cell 2020, 32, 3124–3138. [Google Scholar] [CrossRef]
- Liu, M.-M.; Shi, Z.-Y.; Zhang, X.-H.; Wang, M.-X.; Zhang, L.; Zheng, K.-Z.; Liu, J.-Y.; Hu, X.-M.; Di, C.-R.; Qian, Q.; et al. Inducible overexpression of Ideal Plant Architecture1 improves both yield and disease resistance in rice. Nat. Plants 2019, 5, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Shi, H.; Chern, M.; Yu, H.; Yi, H.; He, M.; Yin, J.-J.; Zhu, X.-B.; Li, Y.; et al. A single transcription factor promotes both yield and immunity in rice. Science 2018, 361, 1026–1028. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.S.; Thomas, B.R.; Rodriguez, R.L. Differential expression of rice α-amylase genes during seedling development under anoxia. Plant Mol. Biol. 1999, 40, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Cen, W.; Li, R.-B.; Wang, S.-K.; Luo, J.-J. The molecular regulatory pathways and metabolic adaptation in the seed germination and early seedling growth of rice in response to low O2 stress. Plants 2020, 9, 1363. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Z.-L.; Zou, X.-L.; Yang, G.-X.; Komatsu, S.; Shen, Q.J. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J. 2006, 46, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-F.; Yu, H.; Xiong, G.-S.; Wang, J.; Jiao, Y.-Q.; Liu, G.-F.; Jing, Y.-H.; Meng, X.-B.; Hu, X.-M.; Qian, Q.; et al. Genome-wide binding analysis of the transcription activator IDEAL PLANT ARCHITECTURE1 reveals a complex network regulating rice plant architecture. Plant Cell 2013, 25, 3743–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, E.-C.; Wang, Y.-H.; Li, X.-H.; Lin, Q.-B.; Zhang, T.; Wang, Y.-P.; Zhou, C.-L.; Zhang, H.; Jiang, L.; Wang, J.-L.; et al. OsSHI1 regulates plant architecture through modulating the transcriptional activity of IPA1 in rice. Plant Cell 2019, 31, 1026–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Yang, P.-F. Studies on the molecular mechanisms of seed germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef]
- Mishra, P.; Dubey, R.S. Effect of aluminium on metabolism of starch and sugars in growing rice seedlings. Acta Physiol. Plant 2007, 30, 265–275. [Google Scholar] [CrossRef]
- Lo, S.-F.; Yang, S.-Y.; Chen, K.-T.; Hsing, Y.-I.; Zeevaart, J.A.D.; Chen, L.-J.; Yu, S.-M. A novel class of Gibberellin 2-Oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [Green Version]
- Gubler, F.; Kalla, R.; Roberts, J.K.; Jacobsen, J.V. Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI α-amylase gene promoter. Plant Cell 1995, 7, 1879–1891. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-L.; Shin, M.; Zou, X.-L.; Huang, J.-Z.; Ho, T.-H.D.; Shen, Q.-X.J. A negative regulator encoded by a rice WRKY gene represses both abscisic acid and gibberellins signaling in aleurone cells. Plant Mol. Biol. 2009, 70, 139–151. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hou, Y.-X.; Wang, S.; Tong, X.-H.; Tang, L.-Q.; Abolore Adijat, A.; Zhang, J.; Wang, Y.-F. WRKY72 negatively regulates seed germination through interfering gibberellin pathway in rice. Rice Sci. 2021, 28, 1–5. [Google Scholar]
- Zhang, Z.-L.; Xie, Z.; Zou, X.-L.; Casaretto, J.; Ho, T.-H.D.; Shen, Q.-X.J. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004, 134, 1500–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yang, A.; Zhang, W.-H. Higher endogenous bioactive gibberellins and α-amylase activity confer greater tolerance of rice seed germination to saline-alkaline stress. Environ. Exp. Bot. 2019, 162, 357–363. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Cui, Y.-T.; Hu, G.-H.; Wang, X.-D.; Chen, H.-Z.; Shi, Q.-H.; Xiang, J.; Zhang, Y.-K.; Zhu, D.-F.; Zhang, Y.-P. Reduced bioactive gibberellin content in rice seeds under low temperature leads to decreased sugar consumption and low seed germination rates. Plant Physiol. Biochem. 2018, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Moller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Chen, M.-L.; Fu, X.-M.; Liu, J.-Q.; Ye, T.-T.; Hou, S.-Y.; Huang, Y.-Q.; Yuan, B.-F.; Wu, Y.; Feng, Y.-Q. Highly sensitive and quantitative profiling of acidic phytohormones using derivatization approach coupled with nano-LC–ESI-Q-TOF-MS analysis. J. Chromatogr. B 2012, 905, 67–74. [Google Scholar] [CrossRef]
- He, F.; Zhang, F.; Sun, W.-X.; Ning, Y.-S.; Wang, G.-L. A versatile vector toolkit for functional analysis of rice genes. Rice 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, J.-B.; Duan, S.; Ao, Y.; Dai, J.-R.; Liu, J.; Wang, P.; Li, Y.-G.; Liu, B.; Feng, D.-R.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhu, M.; Li, Z.; Jiang, S.; He, Z.; Xu, S.; Chen, X.; Hu, Z.; Zhang, Z. IPA1 Negatively Regulates Early Rice Seedling Development by Interfering with Starch Metabolism via the GA and WRKY Pathways. Int. J. Mol. Sci. 2021, 22, 6605. https://doi.org/10.3390/ijms22126605
He Y, Zhu M, Li Z, Jiang S, He Z, Xu S, Chen X, Hu Z, Zhang Z. IPA1 Negatively Regulates Early Rice Seedling Development by Interfering with Starch Metabolism via the GA and WRKY Pathways. International Journal of Molecular Sciences. 2021; 22(12):6605. https://doi.org/10.3390/ijms22126605
Chicago/Turabian StyleHe, Yonggang, Menghao Zhu, Zhihui Li, Shan Jiang, Zijun He, Shuang Xu, Xiangsong Chen, Zhongli Hu, and Zhihong Zhang. 2021. "IPA1 Negatively Regulates Early Rice Seedling Development by Interfering with Starch Metabolism via the GA and WRKY Pathways" International Journal of Molecular Sciences 22, no. 12: 6605. https://doi.org/10.3390/ijms22126605
APA StyleHe, Y., Zhu, M., Li, Z., Jiang, S., He, Z., Xu, S., Chen, X., Hu, Z., & Zhang, Z. (2021). IPA1 Negatively Regulates Early Rice Seedling Development by Interfering with Starch Metabolism via the GA and WRKY Pathways. International Journal of Molecular Sciences, 22(12), 6605. https://doi.org/10.3390/ijms22126605





