Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production
Abstract
1. Introduction
2. Results
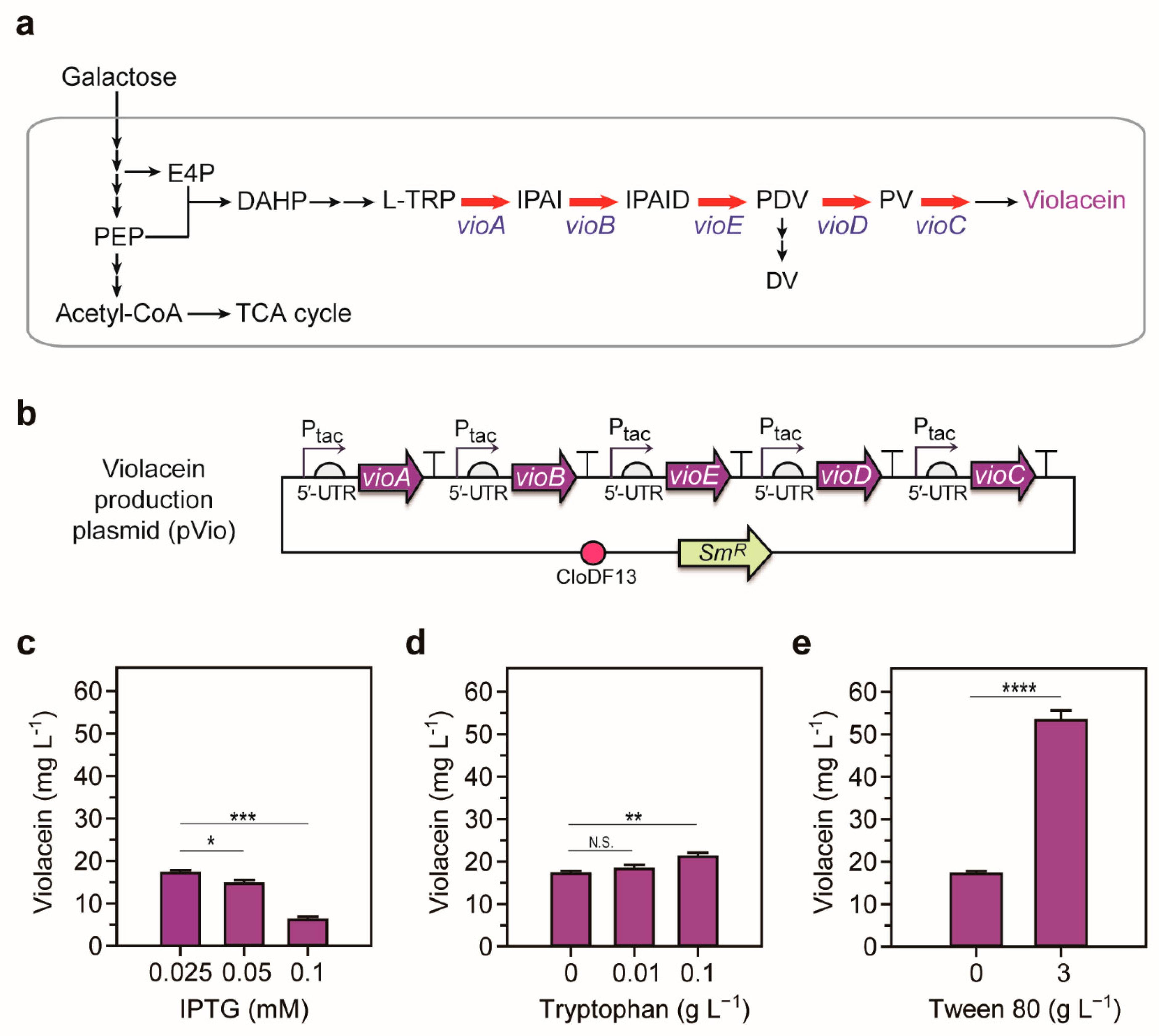
2.1. Optimization of the Violacein Production System
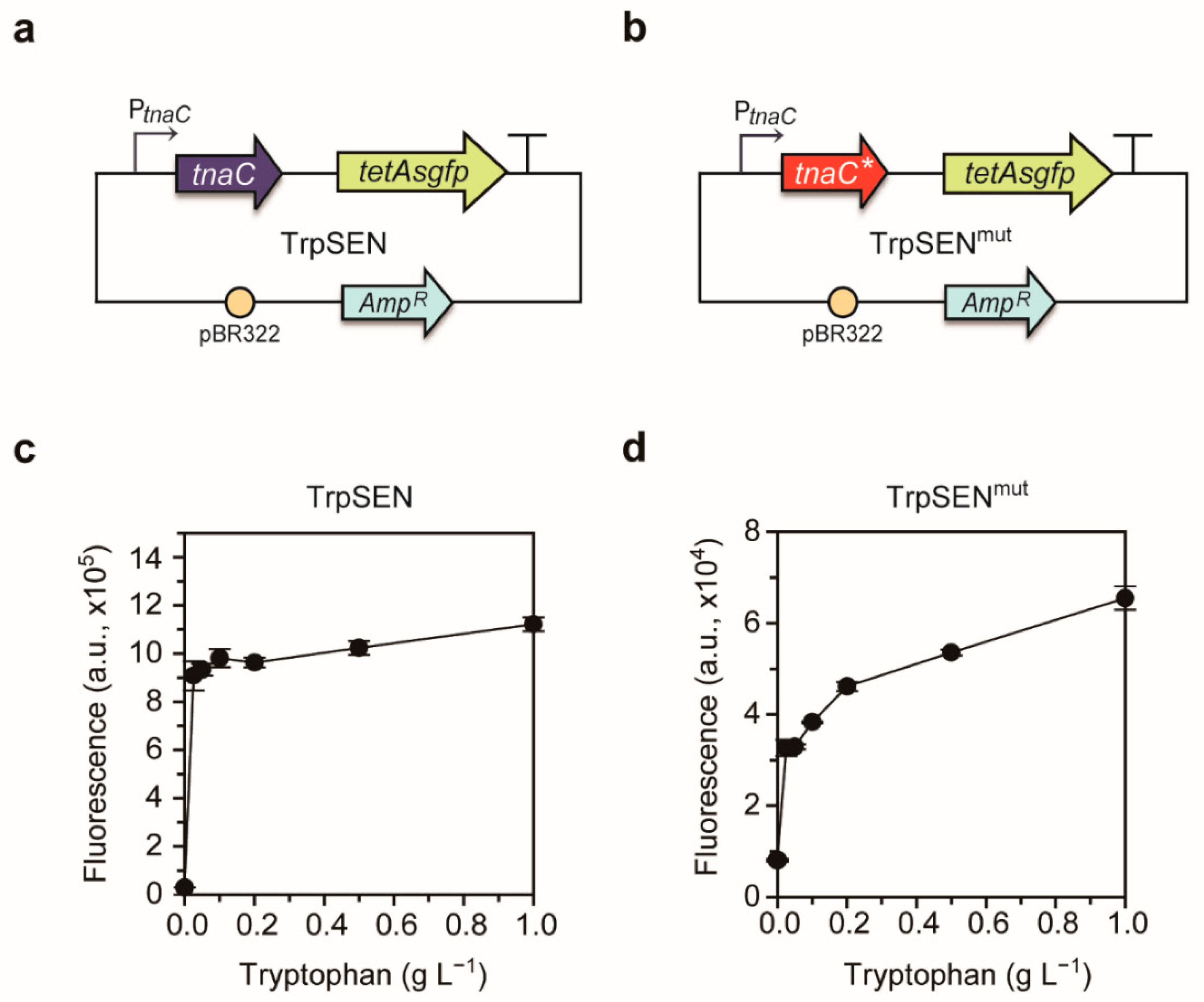
2.2. Development of Tryptophan-Responsive Sensors
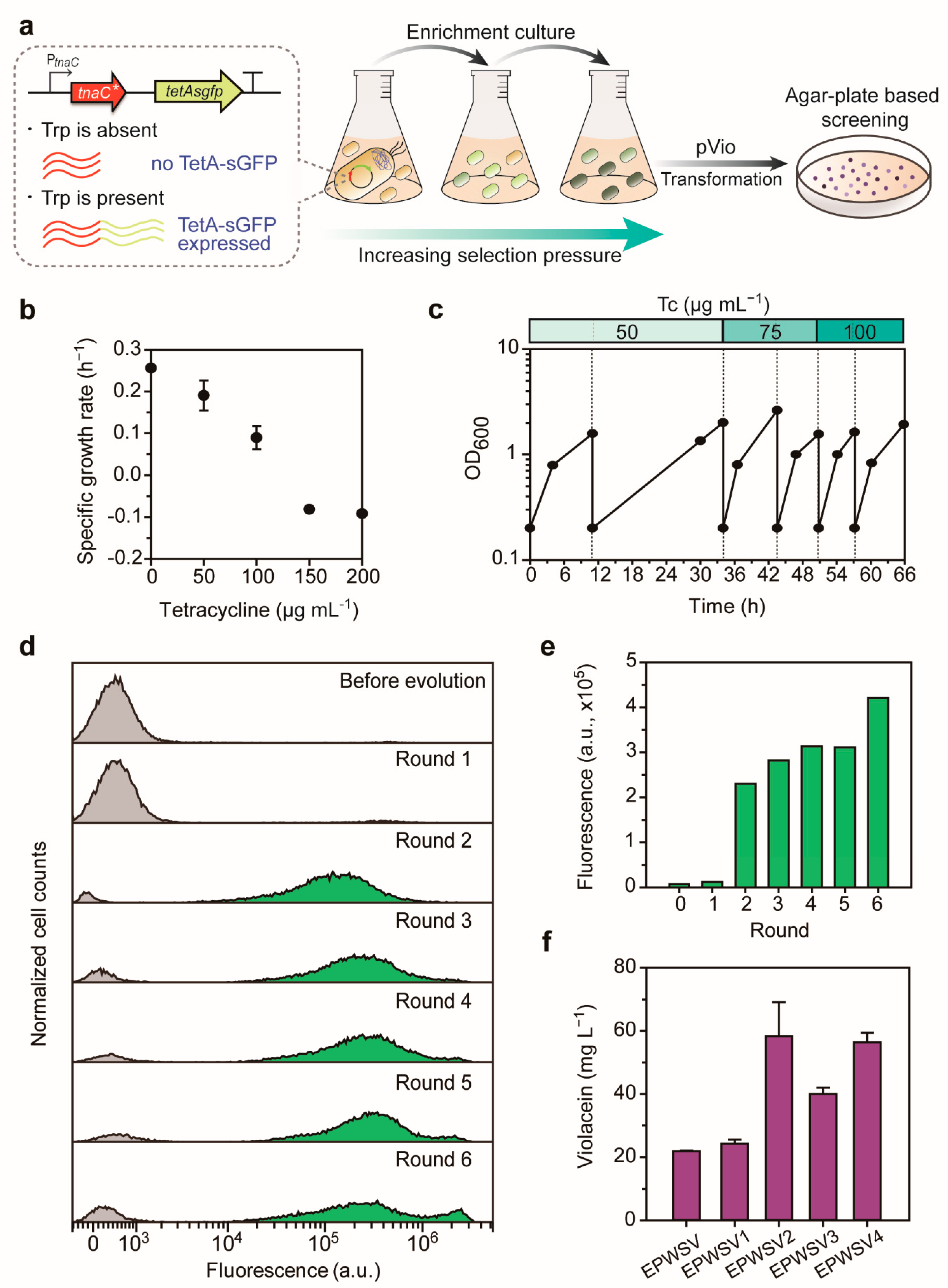
2.3. Tryptophan-Responsive Sensor-Driven ALE for Violacein Overproduction
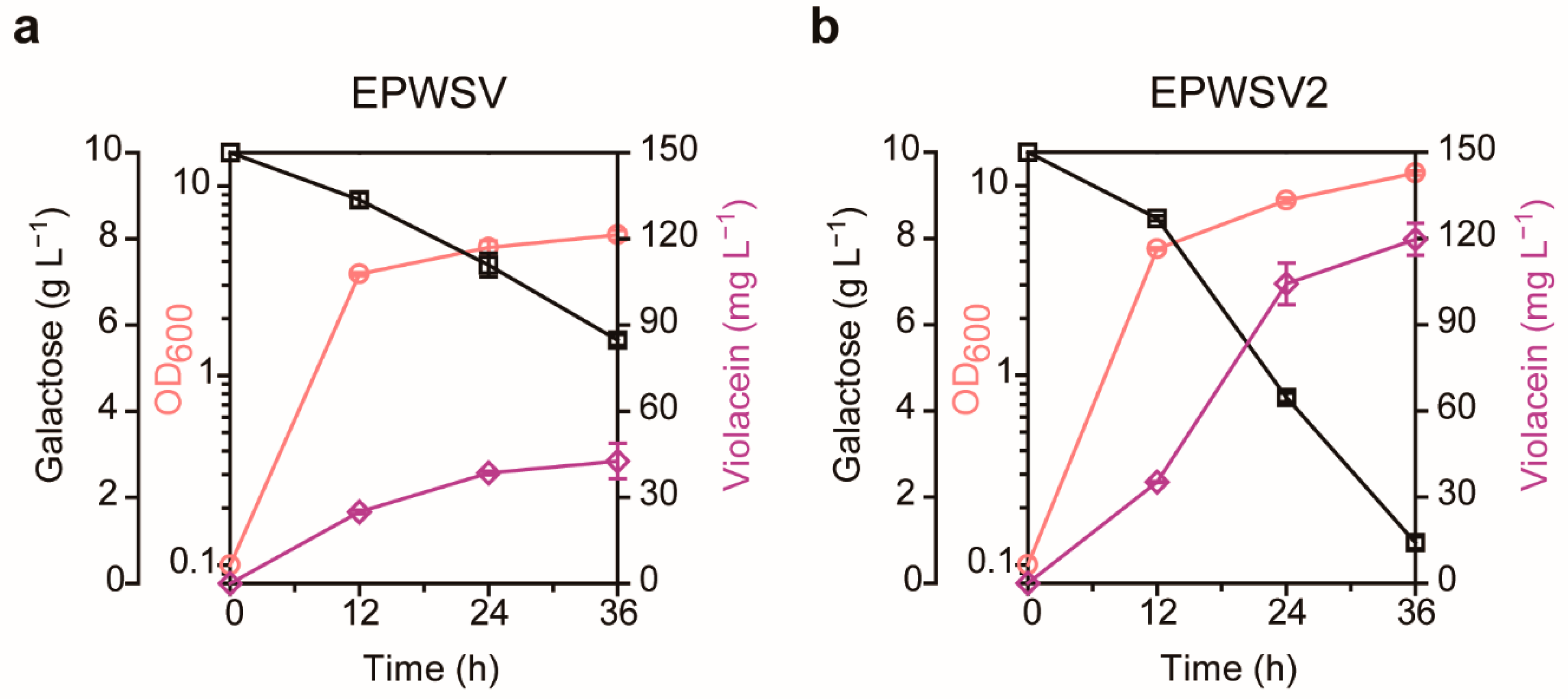
2.4. Examination of the Evolved Strain
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Reagents
4.2. Construction of Heterologous Violacein Pathway
4.3. Development of Tryptophan-Responsive Sensors
4.4. Validation of Tryptophan-Responsive Sensors
4.5. Adaptive Laboratory Evolution Procedure
4.6. Culture Conditions for Violacein Production
4.7. Analytical Methods
4.8. Whole-Genome Sequencing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALE | Adaptive Laboratory Evolution |
| E. coli | Escherichia coli |
| C. violaceum | Chromobacterium violaceum |
| E4P | Erythrose 4-phosphate |
| PEP | Phosphoenolpyruvate |
| DAHP | 3-deoxy-d-arabinoheptulosonate 7-phosphate |
| L-TRP | L-tryptophan |
| IPAI | indole-3-pyruvic acid imine dimer |
| PDV | prodeoxyviolacein |
| PV | proviolacein |
| DV | deoxyviolacein |
| 5′-UTR | 5′-untranslated region |
| SNP | single nucleotide polymorphism |
References
- Durán, N.; Justo, G.Z.; Durán, M.; Brocchi, M.; Cordi, L.; Tasic, L.; Castro, G.R.; Nakazato, G. Advances in Chromobacterium violaceum and properties of violacein-Its main secondary metabolite: A review. Biotechnol. Adv. 2016, 34, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Andrighetti-Fröhner, C.R.; Antonio, R.V.; Creczynski-Pasa, T.B.; Barardi, C.R.M.; Simões, C.M.O. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Memórias do Instituto Oswaldo Cruz 2003, 98, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Kanelli, M.; Mandić, M.; Kalakona, M.; Vasilakos, S.; Kekos, D.; Nikodinovic-Runic, J.; Topakas, E. Microbial production of violacein and process optimization for dyeing polyamide fabrics with acquired antimicrobial properties. Front. Microbiol. 2018, 9, 1495. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Fong, S.S. Challenges and advances for genetic engineering of non-model bacteria and uses in consolidated bioprocessing. Front. Microbiol. 2017, 8, 2060. [Google Scholar] [CrossRef]
- Patijanasoontorn, B.; Boonma, P.; Wilailackana, C.; Sitthikesorn, J.; Lumbiganon, P.; Chetchotisakd, P.; Noppawinyoowong, C.; Simajareuk, K. Hospital acquired Janthinobacterium lividum septicemia in Srinagarind Hospital. J. Med. Assoc. Thail. Chotmaihet Thangphaet 1992, 75, 6–10. [Google Scholar]
- Ti, T.-Y.; Tan, W.C.; Chong, A.P.Y.; Lee, E.H. Nonfatal and fatal infections caused by Chromobacterium violaceum. Clin. Infect. Dis. 1993, 17, 505–507. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Trachtmann, N.; Becker, J.; Lohanatha, A.F.; Blotenberg, J.; Bolten, C.J.; Korneli, C.; Lima, A.O.D.S.; Porto, L.M.; Sprenger, G.A.; et al. Systems metabolic engineering of Escherichia coli for production of the antitumor drugs violacein and deoxyviolacein. Metab. Eng. 2013, 20, 29–41. [Google Scholar] [CrossRef]
- Fang, M.-Y.; Zhang, C.; Yang, S.; Cui, J.-Y.; Jiang, P.-X.; Lou, K.; Wachi, M.; Xing, X.-H. High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microb. Cell Factories 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Kwak, D.H.; Lim, H.G.; Yang, J.; Seo, S.W.; Jung, G.Y. Synthetic redesign of Escherichia coli for cadaverine production from galactose. Biotechnol. Biofuels 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Lim, H.G.; Seo, S.W.; Jung, G.Y. Engineered Escherichia coli for simultaneous utilization of galactose and glucose. Bioresour. Technol. 2013, 135, 564–567. [Google Scholar] [CrossRef]
- Lim, H.G.; Lim, J.H.; Jung, G.Y. Modular design of metabolic network for robust production of n-butanol from galactose–glucose mixtures. Biotechnol. Biofuels 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Lee, J.W.; Na, D.; Park, J.M.; Lee, J.; Choi, S.; Lee, S.Y. Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat. Chem. Biol. 2012, 8, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem. Soc. Rev. 2020, 49, 4615–4636. [Google Scholar] [CrossRef]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dragosits, M.; Mattanovich, D. Adaptive laboratory evolution—Principles and applications for biotechnology. Microb. Cell Factories 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, V.A.; Bezdan, D.; Zengler, K. Adaptive laboratory evolution—Harnessing the power of biology for metabolic engineering. Curr. Opin. Biotechnol. 2011, 22, 590–594. [Google Scholar] [CrossRef]
- Mundhada, H.; Seoane, J.M.; Schneider, K.; Koza, A.; Christensen, H.B.; Klein, T.; Phaneuf, P.V.; Herrgard, M.; Feist, A.M.; Nielsen, A.T. Increased production of L-serine in Escherichia coli through adaptive laboratory evolution. Metab. Eng. 2017, 39, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.H.; Gomez, J.M.; Kao, K.C. Improving carotenoids production in yeast via adaptive laboratory evolution. Metab. Eng. 2014, 21, 26–33. [Google Scholar] [CrossRef]
- Pfeifer, E.; Gätgens, C.; Polen, T.; Frunzke, J. Adaptive laboratory evolution of Corynebacterium glutamicum towards higher growth rates on glucose minimal medium. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Sathesh-Prabu, C.; Shin, K.S.; Kwak, G.H.; Jung, S.-K.; Lee, S.K. Microbial production of fatty acid via metabolic engineering and synthetic biology. Biotechnol. Bioprocess. Eng. 2019, 24, 23–40. [Google Scholar] [CrossRef]
- Raman, S.; Rogers, J.K.; Taylor, N.D.; Church, G.M. Evolution-guided optimization of biosynthetic pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 17803–17808. [Google Scholar] [CrossRef]
- Mahr, R.; Gätgens, C.; Gätgens, J.; Polen, T.; Kalinowski, J.; Frunzke, J. Biosensor-driven adaptive laboratory evolution of l-valine production in Corynebacterium glutamicum. Metab. Eng. 2015, 32, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-D.; Wu, Y.-N.; Wang, T.-M.; Zhang, C.; Xing, X.-H. Maltose utilization as a novel selection strategy for continuous evolution of microbes with enhanced metabolite production. ACS Synth. Biol. 2017, 6, 2326–2338. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Kim, J.-R.; Park, S. Development of biosensor for 3-hydroxypropionic acid. Biotechnol. Bioprocess. Eng. 2019, 24, 109–118. [Google Scholar] [CrossRef]
- Ganesh, I.; Gwon, D.; Lee, J.W. Gas-sensing transcriptional regulators. Biotechnol. J. 2020, 15, e1900345. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.W. Genetic biocontainment systems for the safe use of engineered microorganisms. Biotechnol. Bioprocess. Eng. 2020, 25, 974–984. [Google Scholar] [CrossRef]
- Dietrich, J.A.; Shis, D.L.; Alikhani, A.; Keasling, J.D. Transcription factor-based screens and synthetic selections for microbial small-molecule biosynthesis. ACS Synth. Biol. 2013, 2, 47–58. [Google Scholar] [CrossRef]
- Seok, J.Y.; Yang, J.; Choi, S.J.; Lim, H.G.; Choi, U.J.; Kim, K.-J.; Park, S.; Yoo, T.H.; Jung, G.Y. Directed evolution of the 3-hydroxypropionic acid production pathway by engineering aldehyde dehydrogenase using a synthetic selection device. Metab. Eng. 2018, 47, 113–120. [Google Scholar] [CrossRef]
- Dietrich, J.A.; McKee, A.E.; Keasling, J.D. High-throughput metabolic engineering: Advances in small-molecule screening and selection. Annu. Rev. Biochem. 2010, 79, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Yang, J.-S.; Kim, I.; Yang, J.; Min, B.E.; Kim, S.; Jung, G.Y. Predictive design of mRNA translation initiation region to control prokaryotic translation efficiency. Metab. Eng. 2013, 15, 67–74. [Google Scholar] [CrossRef]
- Balibar, C.J.; Walsh, C.T. In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum. Biochemistry 2006, 45, 15444–15457. [Google Scholar] [CrossRef] [PubMed]
- Huccetogullari, D.; Luo, Z.W.; Lee, S.Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Factories 2019, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Katsumata, R. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl. Environ. Microbiol. 1999, 65, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Tribe, D.E.; Pittard, J. Hyperproduction of tryptophan by Escherichia coli: Genetic manipulation of the pathways leading to tryptophan formation. Appl. Environ. Microbiol. 1979, 38, 181–190. [Google Scholar] [CrossRef]
- Maiti, T.K.; Chatterjee, S.P. Microbial production of L-tryptophan: A review. Hindustan Antibiot. Bull. 1991, 33, 26–61. [Google Scholar]
- Aiba, S.; Tsunekawa, H.; Imanaka, T. New approach to tryptophan production by Escherichia coli: Genetic manipulation of composite plasmids in vitro. Appl. Environ. Microbiol. 1982, 43, 289–297. [Google Scholar] [CrossRef]
- Pardee, K.; Slomovic, S.; Nguyen, P.Q.; Lee, J.W.; Donghia, N.; Burrill, D.; Ferrante, T.; McSorley, F.R.; Furuta, Y.; Vernet, A.; et al. Portable, on-demand biomolecular manufacturing. Cell 2016, 167, 248–259.e12. [Google Scholar] [CrossRef]
- Luo, W.; Huang, J.; Zhu, X.C.; Huang, L.; Cai, J.; Xu, Z.N. Enhanced production of l-tryptophan with glucose feeding and surfactant addition and related metabolic flux redistribution in the recombinant Escherichia coli. Food Sci. Biotechnol. 2013, 22, 207–214. [Google Scholar] [CrossRef]
- Leavitt, J.M.; Wagner, J.M.; Tu, C.C.; Tong, A.; Liu, Y.; Alper, H.S. Biosensor-enabled directed evolution to improve muconic acid production in Saccharomyces cerevisiae. Biotechnol. J. 2017, 12, 10. [Google Scholar] [CrossRef]
- Wang, X.; Policarpio, L.; Prajapati, D.; Li, Z.; Zhang, H. Developing E. coli-E. coli co-cultures to overcome barriers of heterologous tryptamine biosynthesis. Metab. Eng. Commun. 2020, 10, e00110. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, X.; Ji, H.; Wang, T.-L.; Xing, X.-H.; Zhang, C. Dynamics of transcription–translation coordination tune bacterial indole signaling. Nat. Chem. Biol. 2019, 16, 440–449. [Google Scholar] [CrossRef]
- Weickert, M.J.; Adhya, S. The galactose regulon of Escherichia coli. Mol. Microbiol. 1993, 10, 245–251. [Google Scholar] [CrossRef]
- Baneyx, F.; Georgiou, G. Construction and characterization of Escherichia coli strains deficient in multiple secreted proteases: Protease III degrades high-molecular-weight substrates in vivo. J. Bacteriol. 1991, 173, 2696–2703. [Google Scholar] [CrossRef]
- Awano, N.; Rajagopal, V.; Arbing, M.; Patel, S.; Hunt, J.; Inouye, M.; Phadtare, S. Escherichia coli RNase R has dual activities, helicase and RNase. J. Bacteriol. 2010, 192, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.M.; Burgess, R.R. Localization of a sigma70 binding site on the N terminus of the Escherichia coli RNA polymerase beta’ subunit. J. Biol. Chem. 1998, 273, 31381–31387. [Google Scholar] [CrossRef]
- Imlay, K.R.C.; Korshunov, S.; Imlay, J.A. Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J. Bacteriol. 2015, 197, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence (5′-3′) |
|---|---|
| Primers used for the construction of violacein production plasmid | |
| homology_pCDF_F2 | acaagcttgcggccgcataatgcttaagtc |
| homology_pCDF_R | attatacgagccgatgattaattgtcaaggatccccatggtatatctccttattaaagttaaacaaaattatttc |
| UTR_vioA_F | gtgagcggataacaattgaaacggacataaggaggaaattctatgaaacattcttccgatatctgcattg |
| term_vioA_R | gctgttgcagcgtatcgccgcgtaacgcaaaaaaccccgcttcggcggggttttttcgcgaattcttgac |
| UTR_vioB_F | tgtgtggaattgtgagcggataacaattaatcgagttcgaaggaggaaaagtcatgagcattctggatttcccgc |
| term_vioB_R | gcaaactgagccgcgaggcctaacgcaaaaaaccccgcttcggcggggttttttcgcgcggccgcata |
| homology_vioA_F2 | gggatccttgacaattaatcatcggctcgtataatgtgtggaattgtgagcggataacaattgaaacgga |
| term_vioA_R | gctgttgcagcgtatcgccgcgtaacgcaaaaaaccccgcttcggcggggttttttcgcgaattcttgac |
| homology_vioB_F2 | ttcggcggggttttttcgcgaattcttgacaattaatcatcggctcgtataatgtgtggaattgtgagcggataaca |
| term_vioB_R2 | gcaaactgagccgcgaggcctaacgcaaaaaaccccgcttcggcggggttttttcgcgagctcggcgcgcctgca |
| homology_vec_F | cctaggctgctgccaccgctgagcaataactagc |
| homology_vec_R | cttgcggccgcataatgcttaagtcgaaca |
| UTR_vioC_F | tgtgtggaattgtgagcggataacaattaaggacaacaaaaggaggagaactaatgaaacgtgcgattatcgttggtg |
| term_vioC_R | ggtacaagattggtcgcgtgaattaacgcaaaaaaccccgcttcggcggggttttttcgc |
| UTR_vioD_F | tgtgtggaattgtgagcggataacaattccattggagagaaggagggaaattcatgaagattctggtcattggtgctg |
| term_vioD_R | tgcgttatgctttgcagcgctaacgcaaaaaaccccgcttcggcggggttttttcgcctcgagttg |
| UTR_vioE_F | aatgtgtggaattgtgagcggataacaattaaaacgaaaataaggaggaaattctatggagaaccgtgagccacc |
| term_vioE_R | cggttttcgcggccaagcgctaacgcaaaaaaccccgcttcggcggggttttttcgcggtaccttgacaattaatcat cggctcg |
| homology_vioC_F2 | cgcttcggcggggttttttcgcctcgagttgacaattaatcatcggctcgtataatgtgtggaattgtgagcggataac |
| term_vioC_R2 | cttcggcggggttttttcgccctaggctgctgccaccgctgagcaataactagc |
| homology_vioD_F2 | gcggtaccttgacaattaatcatcggctcgtataatgtgtggaattgtgagcggataac |
| term_vioD_R | tgcgttatgctttgcagcgctaacgcaaaaaaccccgcttcggcggggttttttcgcctcgagttg |
| homology_vioE_F2 | cttgcggccgcataatgcttaagtcgaacattgacaattaatcatcggctcgtataatgtgtggaattgtgagcggataac |
| term_vioE_R | cggttttcgcggccaagcgctaacgcaaaaaaccccgcttcggcggggttttttcgcggtaccttgacaattaatcatcg gctcg |
| Primers used for the construction of tryptophan-responsive sensor | |
| T7_term_F | ctagcataaccccttggggc |
| pET23b_vec_R | ccgagatctcgatcccgcgaaat |
| tnaC_homo_F | caacgctgcccgagatctcgatcccgcgaaatgcatgcccgcgcttacgaagccgcattc |
| tnaA_homo_w_tetA | ccgaggatgacgatgagcgcattgttagatttcatcggttcagggagatgtttaaagttttccattac |
| tetA_F | atgaaatctaacaatgcgctcatcgtcatc |
| sfgfp_homo_R | gcatggacgagctgtacaagtaaaccgctgagcaataactagcataaccccttggggc |
| D21T_blunt_F | acacaccgcccttgatttgccc |
| D21T_blunt_R | gacaattttgttgtcaatattgaacc |
| Gene | Function | Position | Mutation | Amino Acid Change | |
|---|---|---|---|---|---|
| EPWSV | EPWSV2 | ||||
| galR | DNA-binding transcriptional dual regulator [42] | 2975313 | G | C | R20P |
| ptrA | Protease 3 [43] | 2956475 | A | T | S356T |
| rnr | RNase R [44] | 4413454 | A | C | E707D |
| rpoC | RNA polymerase subunit β’ [45] | 3450328 | T | A | Q335L |
| tcyP | Cystine/sulfocystein:cation symporter [46] | 1812711 | A | C | T22P |
| Name | Relevant Characteristics 1 | Source |
|---|---|---|
| Strains | ||
| Mach1-T1R | F− φ80(lacZ)ΔM15 ΔlacX74 hsdR(rK−mK+) ΔrecA1398 endA1 tonA | Invitrogen |
| E. coli W3110 | F− λ− rph-1 INV(rrnD, rrnE) | ATCC39936 |
| ATCC31743 | E. coli W3110-ΔtrpABCDE-ΔtrpR-ΔtnaA/pSC101-trpABCDE | ATCC31743 |
| EPW | ATCC31743, KanR | This study |
| EPWS | EPW/TrpSENmut | This study |
| EPWSV | EPWS/pVio | This study |
| EPWSV1 | Evolved strain derived from EPWSV | This study |
| EPWSV2 | Evolved strain derived from EPWSV | This study |
| EPWSV3 | Evolved strain derived from EPWSV | This study |
| EPWSV4 | Evolved strain derived from EPWSV | This study |
| Plasmids | ||
| pCDFDuet-1 | Expression vector, SmR, CloDF13 ori | Novagen |
| pET-23b | Expression vector, AmpR, pBR322 ori | Novagen |
| pCPA | pCDF-Ptac::synUTRvioA-vioA::Ter- Ptac::synUTRvioB-vioB::Ter | This study |
| pVio | pCDF-Ptac::synUTRvioA-vioA::Ter- Ptac::synUTRvioB-vioB::Ter-Ptac::synUTRvioE-vioE::T-Ptac::synUTRvioD-vioD::Ter-Ptac::synUTRvioC-vioC::Ter | This study |
| TrpSEN | pET-23b-PtnaC::tnaC-tetA-sfgfp::Ter | This study |
| TrpSENmut | pET-23b-PtnaC::tnaC(D21T)-tetA-sfgfp::Ter | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, D.-a.; Seok, J.Y.; Jung, G.Y.; Lee, J.W. Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production. Int. J. Mol. Sci. 2021, 22, 6594. https://doi.org/10.3390/ijms22126594
Gwon D-a, Seok JY, Jung GY, Lee JW. Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production. International Journal of Molecular Sciences. 2021; 22(12):6594. https://doi.org/10.3390/ijms22126594
Chicago/Turabian StyleGwon, Da-ae, Joo Yeon Seok, Gyoo Yeol Jung, and Jeong Wook Lee. 2021. "Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production" International Journal of Molecular Sciences 22, no. 12: 6594. https://doi.org/10.3390/ijms22126594
APA StyleGwon, D.-a., Seok, J. Y., Jung, G. Y., & Lee, J. W. (2021). Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production. International Journal of Molecular Sciences, 22(12), 6594. https://doi.org/10.3390/ijms22126594






