The mTORC2 Regulator Homer1 Modulates Protein Levels and Sub-Cellular Localization of the CaSR in Osteoblast-Lineage Cells
Abstract
1. Introduction
2. Results
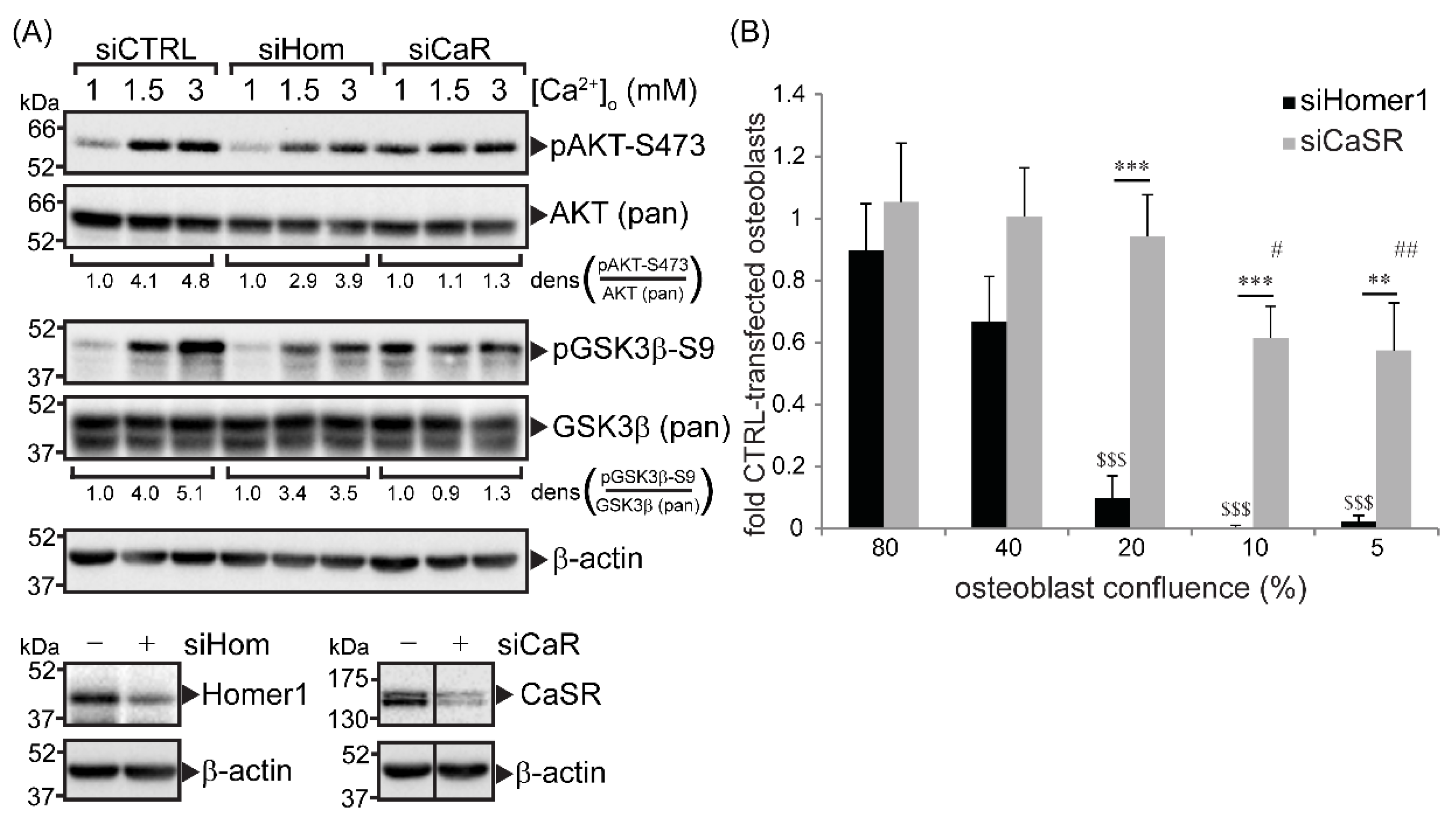
2.1. CaSR and Homer1 Were Required for Extracellular Ca2+-Dependent AKT and GSK3β Phosphorylation in Immortalized Primary Human Osteoblasts and Promoted Osteoblast Viability
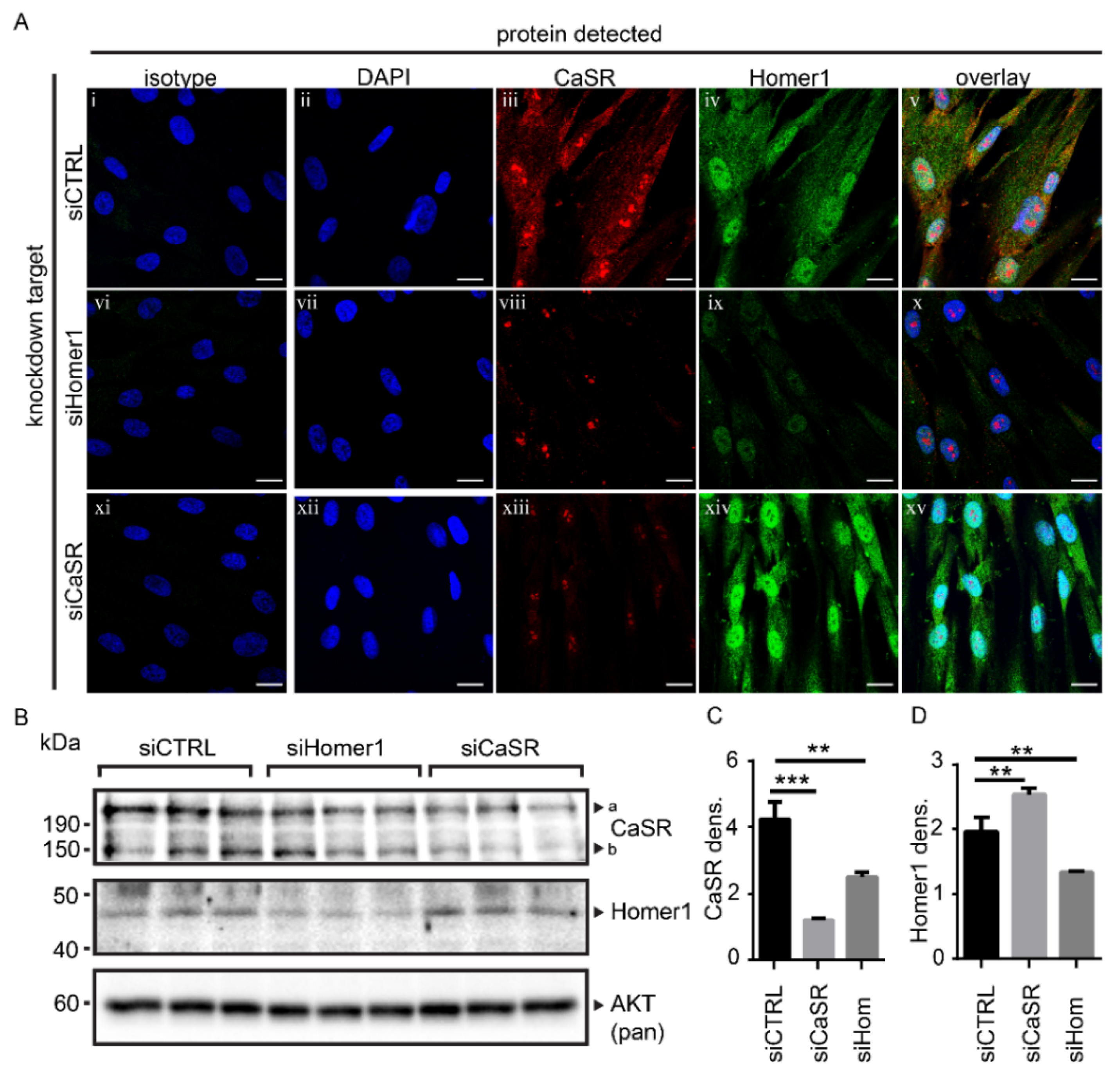
2.2. Protein Levels of Homer1 and CaSR Were Linked in Primary Human Osteoblasts
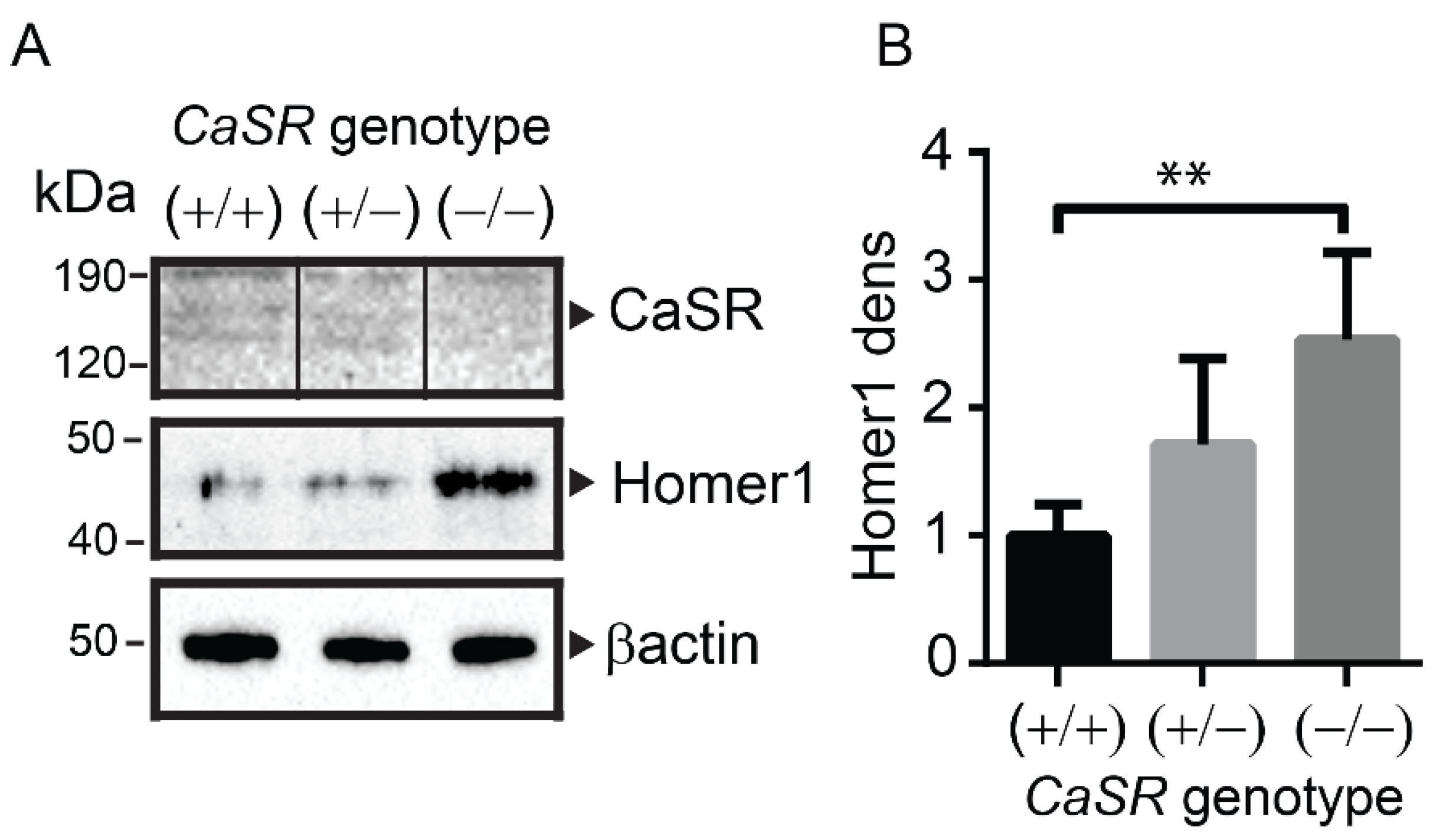
2.3. Homer1 Protein Expression Was Increased in the Long Bones of Osteoblast-Specific CaSR Knockout Mice
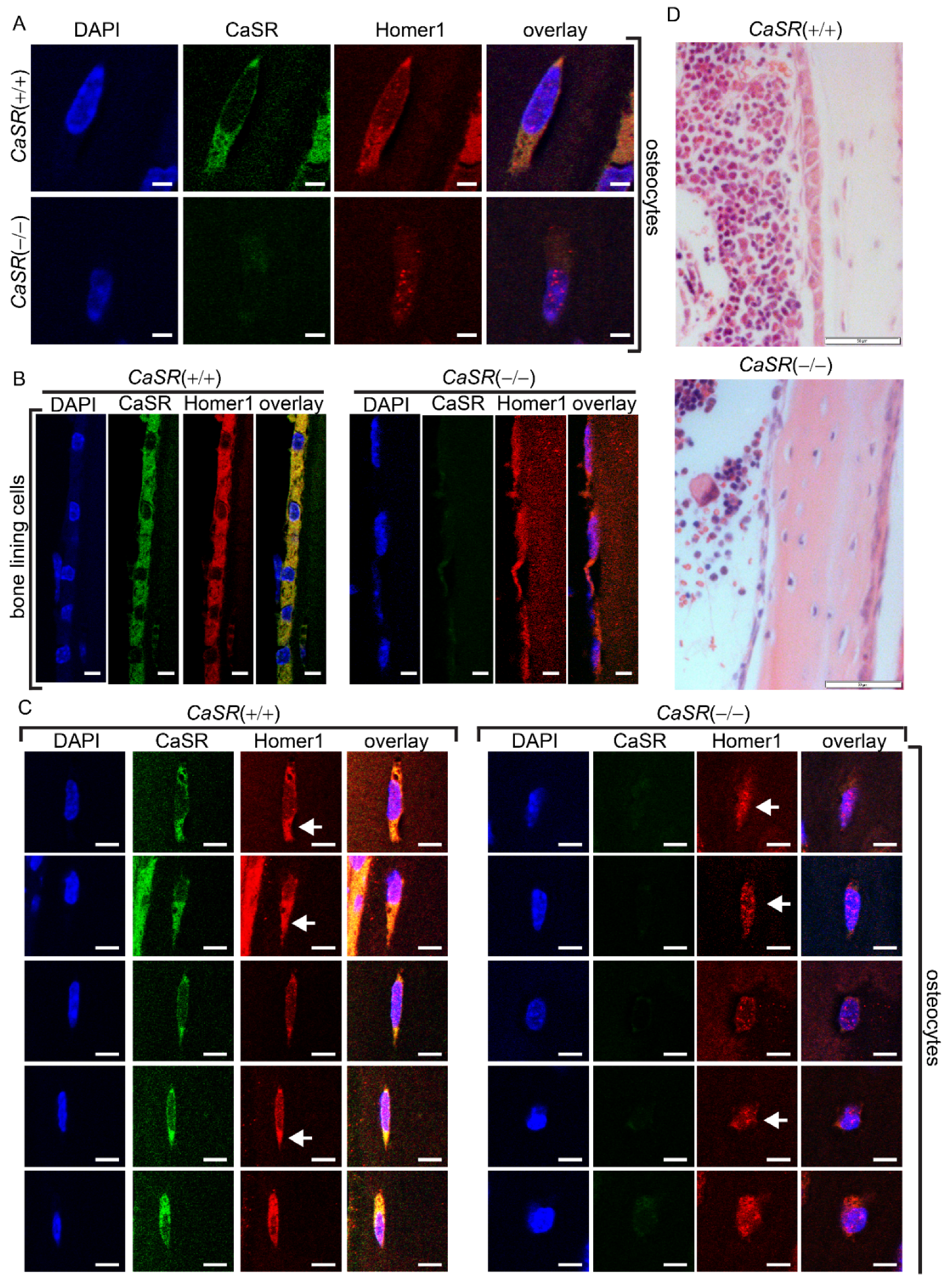
2.4. CaSR Modulated the Sub-Cellular Localization of Homer1 in Osteoblast-Lineage Cells In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CaSR Knockout Mice
4.3. Immunofluorescence (Mouse Bones)
4.4. Immunofluorescence (Human Osteoblasts)
4.5. siRNA Transfection
4.6. Chemical Denaturation of Whole Mouse Bones Followed by Protein Level Measurement by Western Blot
4.7. qPCR mRNA Quantificiation
4.8. Western Blot Analyses
4.9. Statistics and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin Mediates Bone Response to Mechanical Unloading via Antagonizing Wnt/beta-Catenin Signaling. J. Bone Miner. Res. 2009. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Slater, M.; Conigrave, A.D.; Mason, R.S. An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J. Biol. Chem. 2011, 286, 23771–23779. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Islam, K.S.; Brennan-Speranza, T.C.; Cheng, Z.; Brennan, S.C.; Chang, W.; Mason, R.S.; Conigrave, A.D. Homer1 mediates CaSR-dependent activation of mTOR complex 2 and initiates a novel pathway for AKT-dependent beta-catenin stabilization in osteoblasts. J. Biol. Chem. 2019, 294, 16337–16350. [Google Scholar] [CrossRef]
- Chang, W.; Tu, C.; Chen, T.H.; Bikle, D.; Shoback, D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal. 2008, 1, ra1. [Google Scholar] [CrossRef] [PubMed]
- Dvorak-Ewell, M.M.; Chen, T.H.; Liang, N.; Garvey, C.; Liu, B.; Tu, C.; Chang, W.; Bikle, D.D.; Shoback, D.M. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. JBMR 2011, 26, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D.; Hendy, G.N. The calcium-sensing receptor in bone--mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015, 11, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, M.M.; Siddiqua, A.; Ward, D.T.; Carter, D.H.; Dallas, S.L.; Nemeth, E.F.; Riccardi, D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. USA 2004, 101, 5140–5145. [Google Scholar] [CrossRef]
- Cianferotti, L.; Gomes, A.R.; Fabbri, S.; Tanini, A.; Brandi, M.L. The calcium-sensing receptor in bone metabolism: From bench to bedside and back. Osteoporos. Int. 2015, 26, 2055–2071. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.; Hannan, F.M.; Josephs, T.M.; Keller, A.N.; Møller, T.C.; Ward, D.T.; Kallay, E.; Mason, R.S.; Thakker, R.V.; Riccardi, D.; et al. International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2020, 72, 558–604. [Google Scholar] [CrossRef]
- Brakeman, P.R.; Lanahan, A.A.; O’Brien, R.; Roche, K.; Barnes, C.A.; Huganir, R.L.; Worley, P.F. Homer: A protein that selectively binds metabotropic glutamate receptors. Nature 1997, 386, 284–288. [Google Scholar] [CrossRef]
- Rong, R.; Ahn, J.Y.; Huang, H.; Nagata, E.; Kalman, D.; Kapp, J.A.; Tu, J.; Worley, P.F.; Snyder, S.H.; Ye, K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat. Neurosci. 2003, 6, 1153–1161. [Google Scholar] [CrossRef]
- Mao, L.; Yang, L.; Tang, Q.; Samdani, S.; Zhang, G.; Wang, J.Q. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J. Neurosci. 2005, 25, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Weir, M.D.; Xu, H.H. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement-fiber scaffold. Tissue Eng. Part A 2011, 17, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Tomasetti, C.; Cicale, M.; Yuan, P.X.; Manji, H.K. Chronic treatment with lithium or valproate modulates the expression of Homer1b/c and its related genes Shank and Inositol 1,4,5-trisphosphate receptor. Eur. Neuropsychopharmacol. 2012, 22, 527–535. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.B.; Permenter, L.K.; Lake, R.; Worley, P.F.; Kalivas, P.W. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur. J. Neurosci. 2003, 18, 1645–1651. [Google Scholar] [CrossRef]
- Cavanaugh, A.; McKenna, J.; Stepanchick, A.; Breitwieser, G.E. Calcium-sensing receptor biosynthesis includes a cotranslational conformational checkpoint and endoplasmic reticulum retention. J. Biol. Chem. 2010, 285, 19854–19864. [Google Scholar] [CrossRef]
- Breitwieser, G.E. Minireview: The intimate link between calcium sensing receptor trafficking and signaling: Implications for disorders of calcium homeostasis. Mol. Endocrinol. 2012, 26, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Pidasheva, S.; Grant, M.; Canaff, L.; Ercan, O.; Kumar, U.; Hendy, G.N. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: Biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum. Mol. Genet. 2006, 15, 2200–2209. [Google Scholar] [CrossRef]
- Gama, L.; Wilt, S.G.; Breitwieser, G.E. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J. Biol. Chem. 2001, 276, 39053–39059. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Tu, J.; Yang, T.; Vernon, P.S.; Allen, P.D.; Worley, P.F.; Pessah, I.N. Homer regulates gain of ryanodine receptor type 1 channel complex. J. Biol. Chem. 2002, 277, 44722–44730. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wei, F.; Zou, S.; Robbins, M.T.; Sugiyo, S.; Ikeda, T.; Tu, J.C.; Worley, P.F.; Dubner, R.; Ren, K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J. Neurosci. 2004, 24, 9161–9173. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; López, J.J.; Berna-Erro, A.; Salido, G.M.; Rosado, J.A. Homer proteins in Ca²⁺ entry. IUBMB Life 2013, 65, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.C.; Xiao, B.; Yuan, J.P.; Lanahan, A.A.; Leoffert, K.; Li, M.; Linden, D.J.; Worley, P.F. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 1998, 21, 717–726. [Google Scholar] [CrossRef]
- Richard, C.; Huo, R.; Samadfam, R.; Bolivar, I.; Miao, D.; Brown, E.M.; Hendy, G.N.; Goltzman, D. The calcium-sensing receptor and 25-hydroxyvitamin D-1alpha-hydroxylase interact to modulate skeletal growth and bone turnover. J. Bone Miner. Res. 2010, 25, 1627–1636. [Google Scholar] [CrossRef]
- Breitwieser, G.E. The calcium sensing receptor life cycle: Trafficking, cell surface expression, and degradation. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 303–313. [Google Scholar] [CrossRef]
- Serge, A.; Fourgeaud, L.; Hemar, A.; Choquet, D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J. Neurosci. 2002, 22, 3910–3920. [Google Scholar] [CrossRef] [PubMed]
- Ango, F.; Robbe, D.; Tu, J.C.; Xiao, B.; Worley, P.F.; Pin, J.P.; Bockaert, J.; Fagni, L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol. Cell. Neurosci. 2002, 20, 323–329. [Google Scholar] [CrossRef]
- Jaubert, P.J.; Golub, M.S.; Lo, Y.Y.; Germann, S.L.; Dehoff, M.H.; Worley, P.F.; Kang, S.H.; Schwarz, M.K.; Seeburg, P.H.; Berman, R.F. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007, 6, 141–154. [Google Scholar] [CrossRef]
- Stiber, J.A.; Zhang, Z.S.; Burch, J.; Eu, J.P.; Zhang, S.; Truskey, G.A.; Seth, M.; Yamaguchi, N.; Meissner, G.; Shah, R.; et al. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol. Cell Biol. 2008, 28, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Worley, P.F.; Zeng, W.; Huang, G.; Kim, J.Y.; Shin, D.M.; Kim, M.S.; Yuan, J.P.; Kiselyov, K.; Muallem, S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 2007, 42, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Guo, Y.F.; He, H.; Lin, X.; Wang, X.F.; Zhou, R.; Li, W.T.; Pan, D.Y.; Shen, J.; Deng, H.W. Integrative Analysis of Genomics and Transcriptome Data to Identify Potential Functional Genes of BMDs in Females. J. Bone Miner. Res. 2016, 31, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.W.; Tu, J.C.; Petralia, R.S.; Xiao, B.; Wenthold, R.J.; Worley, P.F. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J. Biol. Chem. 1999, 274, 25953–25957. [Google Scholar] [CrossRef]
- Slater, M.; Patava, J.; Kingham, K.; Mason, R.S. Modulation of growth factor incorporation into ECM of human osteoblast-like cells in vitro by 17 beta-estradiol. Am. J. Physiol. 1994, 267, E990–E1001. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.; Patava, J.; Mason, R.S. Role of chondroitin sulfate glycosaminoglycans in mineralizing osteoblast-like cells: Effects of hormonal manipulation. J. Bone Miner. Res. 1994, 9, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.C.; Rybchyn, M.S.; Green, W.; Atwa, S.; Conigrave, A.D.; Mason, R.S. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol. 2009, 157, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, S. Influence of Glucocorticoids on Osteoclast Generation, Activity and Life Span. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2005. [Google Scholar]
- Parsons, B.D.; Schindler, A.; Evans, D.H.; Foley, E. A direct phenotypic comparison of siRNA pools and multiple individual duplexes in a functional assay. PLoS ONE 2009, 4, e8471. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybchyn, M.S.; Brennan-Speranza, T.C.; Mor, D.; Cheng, Z.; Chang, W.; Conigrave, A.D.; Mason, R.S. The mTORC2 Regulator Homer1 Modulates Protein Levels and Sub-Cellular Localization of the CaSR in Osteoblast-Lineage Cells. Int. J. Mol. Sci. 2021, 22, 6509. https://doi.org/10.3390/ijms22126509
Rybchyn MS, Brennan-Speranza TC, Mor D, Cheng Z, Chang W, Conigrave AD, Mason RS. The mTORC2 Regulator Homer1 Modulates Protein Levels and Sub-Cellular Localization of the CaSR in Osteoblast-Lineage Cells. International Journal of Molecular Sciences. 2021; 22(12):6509. https://doi.org/10.3390/ijms22126509
Chicago/Turabian StyleRybchyn, Mark S., Tara Clare Brennan-Speranza, David Mor, Zhiqiang Cheng, Wenhan Chang, Arthur D. Conigrave, and Rebecca S. Mason. 2021. "The mTORC2 Regulator Homer1 Modulates Protein Levels and Sub-Cellular Localization of the CaSR in Osteoblast-Lineage Cells" International Journal of Molecular Sciences 22, no. 12: 6509. https://doi.org/10.3390/ijms22126509
APA StyleRybchyn, M. S., Brennan-Speranza, T. C., Mor, D., Cheng, Z., Chang, W., Conigrave, A. D., & Mason, R. S. (2021). The mTORC2 Regulator Homer1 Modulates Protein Levels and Sub-Cellular Localization of the CaSR in Osteoblast-Lineage Cells. International Journal of Molecular Sciences, 22(12), 6509. https://doi.org/10.3390/ijms22126509





