The “Angiogenic Switch” and Functional Resources in Cyclic Sports Athletes
Abstract
1. Introduction
2. Materials
3. Results
3.1. Vascular Endothelial Growth Factor (VEGF)
3.1.1. Role of VEGF in Angiogenesis in Athletes
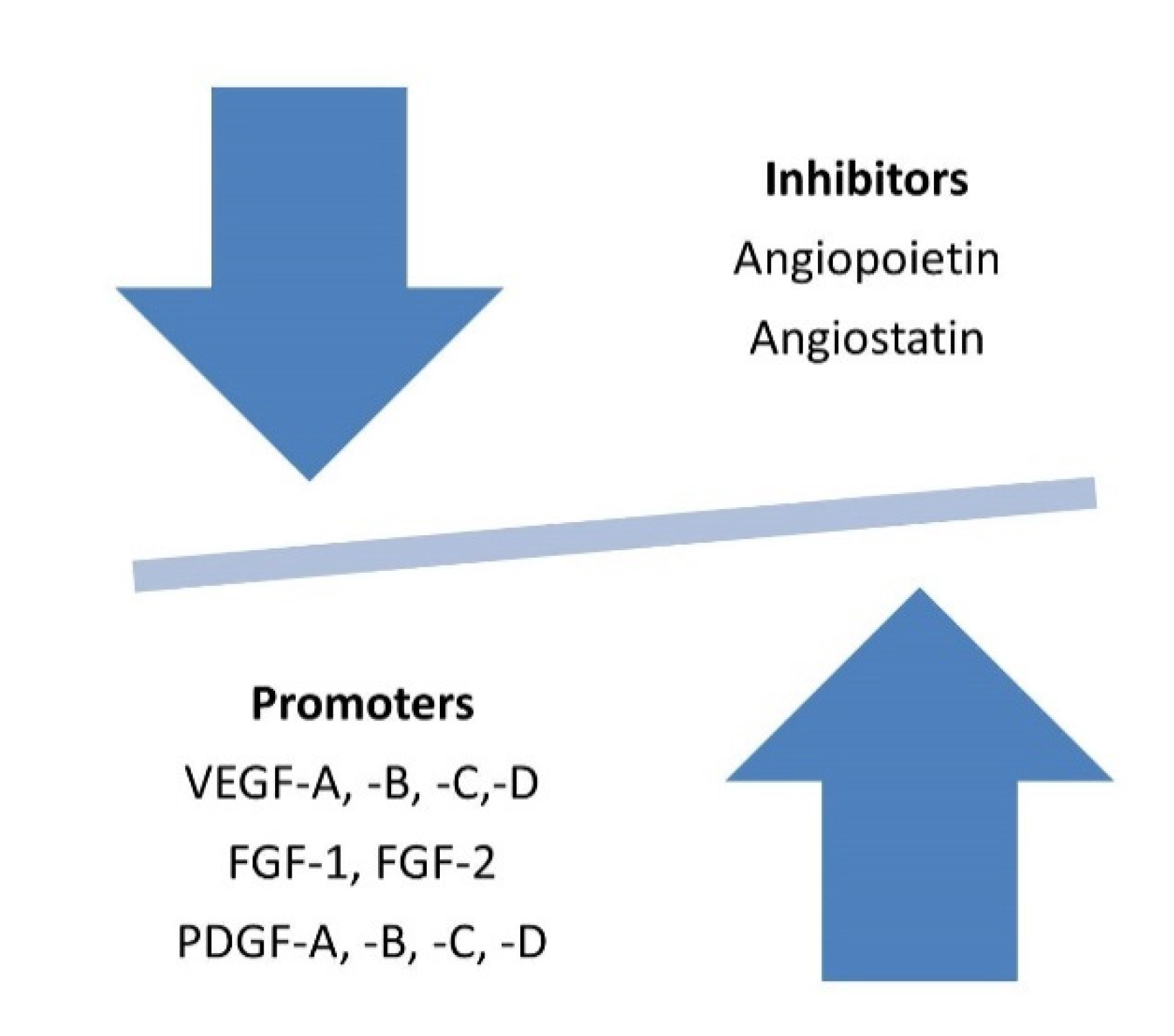
VEGF and the “Angiogenic Switch” in Skeletal Muscle
VEGF and “Angiogenic Switch” in the Myocardium
VEGF and “Angiogenic Switch” in Cerebrovascular and Cardiovascular Systems
VEGF and “Angiogenic Switch” in Lung Tissue
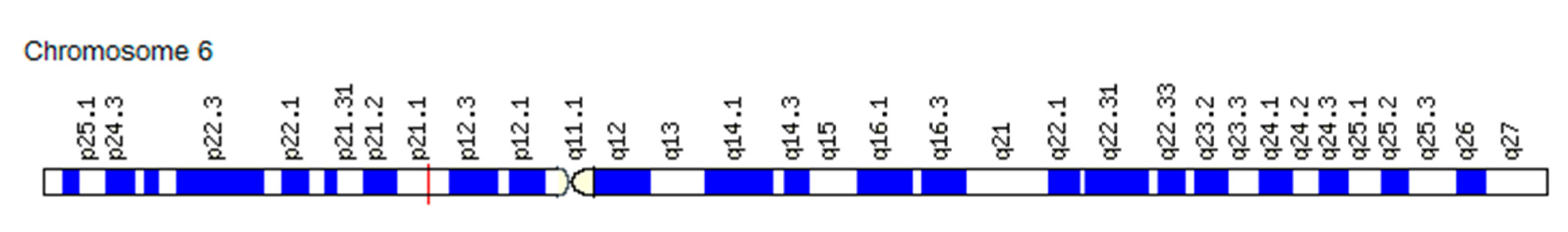
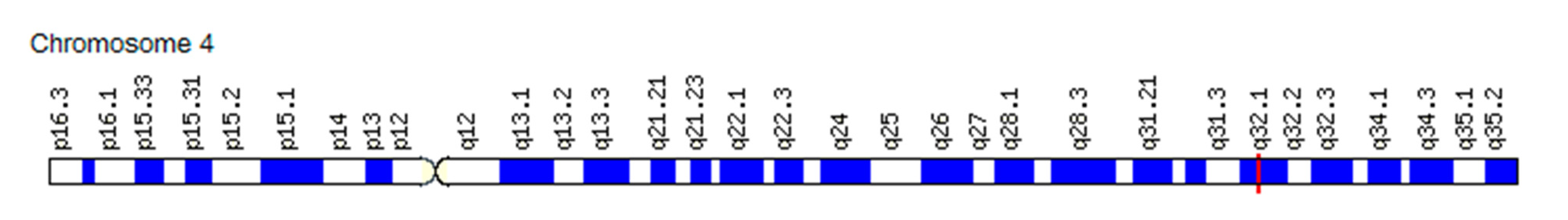
3.1.2. Association of the VEGF Gene SNVs with Changes in the “Angiogenic Switch” Stroke and Functional Resources in Athletes
3.2. Fibroblast Growth Factor (FGF)
3.2.1. The Role of FGF in Angiogenesis in Athletes
FGF and the “Angiogenic Switch” in Skeletal Muscle
FGF and “Angiogenic Switch” in the Myocardium
FGF and the “Angiogenic Switch” in the Cerebrovascular and Cardiovascular Systems
FGF and the “Angiogenic Switch” in Lung Tissue
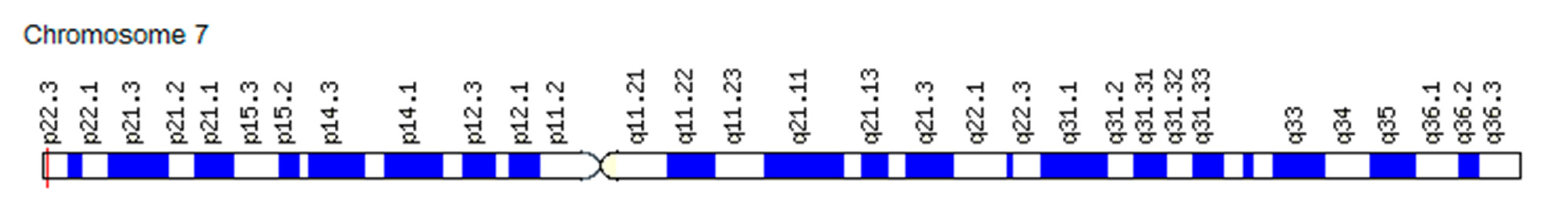
3.2.2. Association of FGF Gene SNVs with Changes in the “Angiogenic Switch” Stroke and Functional Resources in Athletes
3.3. Platelet Growth Factor (PDGF)
3.3.1. Role of PDGF in Angiogenesis in Athletes
PDGF and the “Angiogenic Switch” in Skeletal Muscle
PDGF and “Angiogenic Switch” in the Myocardium
PDGF and the “Angiogenic Switch” in the Cerebrovascular and Cardiovascular Systems
PDGF and the “Angiogenic Switch” in Lung Tissue
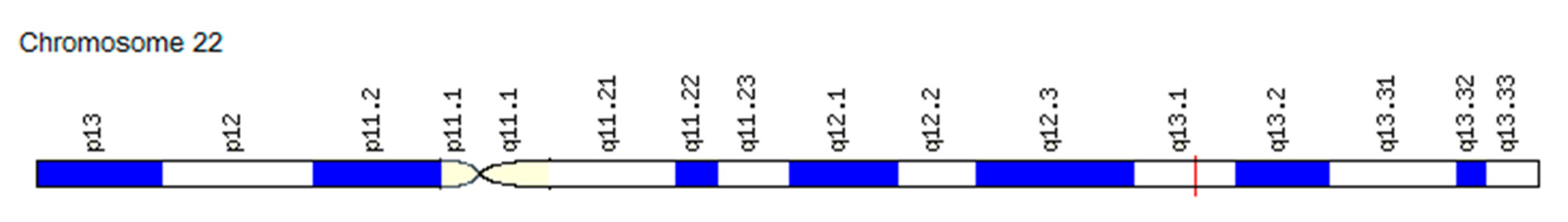
3.3.2. Association of the PDGF Gene SNVs with Changes in the “Angiogenic Switch” Stroke and Functional Resources in Athletes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Crivellato, E.; Roccaro, A.M.; Vacca, A. The history of the angiogenic switch concept. Leukemia 2007, 21, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Akhmetov, I.I.; Khakimullina, A.M.; Popov, D.V.; Missina, S.S.; Vinogradova, O.L.; Rogozkin, V.A. Polymorphism of the vascular endothelial growth factor gene (VEGF) and aerobic performance in athletes. Fiziol. Cheloveka 2008, 34, 97–101. [Google Scholar]
- Barroso, G.C.; Thiele, E.S. Muscle Injuries in Athletes. Rev. Bras. Ortop. 2015, 46, 354–358. [Google Scholar] [CrossRef]
- Yan, Z.; Choi, S.; Liu, X.; Zhang, M.; Schageman, J.J.; Lee, S.Y.; Hart, R.; Lin, L.; Thurmond, F.A.; Williams, R.S. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 2003, 278, 8826–8836. [Google Scholar] [CrossRef]
- Arsic, N.; Zacchigna, S.; Zentilin, L.; Ramirez-Correa, G.; Pattarini, L.; Salvi, A.; Sinagra, G.; Giacca, M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in Vivo. Mol. Ther. 2004, 5, 844–854. [Google Scholar] [CrossRef]
- Murry, C.; Soonpaa, M.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.S.; Virag, J.I.; Bartelmez, S.H.; Poppa, V.; et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef]
- Balsam, L.B.; Wagers, A.J.; Christensen, J.L.; Kofidis, T.; Weissman, I.L.; Robbins, R.C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004, 428, 668–673. [Google Scholar] [CrossRef]
- Wu, G.; Rana, J.S.; Wykrzykowska, J.; Du, Z.; Ke, Q.; Kang, P.; Li, J.; Laham, R.J. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H389–H395. [Google Scholar] [CrossRef] [PubMed]
- Vona, M.; Rossi, A.; Capodaglio, P.; Rizzo, S.; Servi, P.; de Marchi, M.; Cobelli, F. Impact of physical training and detraining on endothelium-dependent vasodilation in patients with recent acute myocardial infarction. Am. Heart J. 2004, 147, 1039–1046. [Google Scholar] [CrossRef]
- Ardakanizade, M. The effects of mid- and long-term endurance exercise on heart angiogenesis and oxidative stress. Iran. J. Basic Med. Sci. 2018, 21, 800–805. [Google Scholar] [CrossRef]
- Gogiraju, R.; Bochenek, M.L.; Schäfer, K. Angiogenic Endothelial Cell Signaling in Cardiac Hypertrophy and Heart Failure. Front. Cardiovasc. Med. 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Stern, D.F.; Polverini, P.J.; Bender, J.R. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am. J. Physiol. 1999, 277, H2205–H2211. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, G.; Zhao, T.C. HDAC4: Mechanism of regulation and biological functions. Epigenomics 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Hyatt, H.W.; Smuder, A.J.; Sollanek, K.J.; Morton, A.B.; Roberts, M.D.; Kavazis, A.N. Comparative changes in antioxidant enzymes and oxidative stress in cardiac, fast twitch and slow twitch skeletal muscles following endurance exercise training. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 160–168. [Google Scholar]
- Powers, S.K.; DeRuisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Semenza, G.L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 2010, 86, 236–242. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Stavrou, N.A.; Kounalakis, S.N.; Eiken, O.; Mekjavic, I.B. Severe hypoxia during incremental exercise to exhaustion provokes negative post-exercise affects. Physiol. Behav. 2016, 156, 171–176. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Wu, H.; Naya, F.J.; McKinsey, T.A.; Mercer, B.; Shelton, J.M.; Chin, E.R.; Simard, A.R.; Michel, R.N.; Bassel-Duby, R.; Olson, E.N.; et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000, 19, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Xu, Z.; Duh, E.J. Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothelial cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3640–3648. [Google Scholar] [CrossRef]
- Zhu, Y.; Lee, C.; Shen, F.; Du, R.; Young, W.L.; Yang, G.Y. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke 2005, 36, 1533–1537. [Google Scholar] [CrossRef]
- Kajstura, J.; Rota, M.; Cappetta, D.; Ogórek, B.; Arranto, C.; Bai, Y.; Ferreira-Martins, J.; Signore, S.; Sanada, F.; Matsuda, A.; et al. Cardiomyogenesis in the aging and failing human heart. Circulation 2012, 126, 1869–1881. [Google Scholar] [CrossRef]
- Dorn, G.W. The fuzzy logic of physiological cardiac hypertrophy. Hypertension 2007, 49, 962–970. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef] [PubMed]
- Jaipersad, A.S.; Lip, G.Y.; Silverman, S.; Shantsila, E. The role of monocytes in angiogenesis and atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Rissanen, T.T.; Vajanto, I.; Hartikainen, J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J. Am. Coll. Cardiol. 2007, 49, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.M.; Deng, X.T.; Qi, R.M.; Xiao, L.Y.; Yang, C.Q.; Gong, T. Mechanism of Chronic Stress-induced Reduced Atherosclerotic Medial Area and Increased Plaque Instability in Rabbit Models of Chronic Stress. Chin. Med. J. Engl. 2018, 131, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cialoni, D.; Sponsiello, N.; Marabotti, C.; Marroni, A.; Pieri, M.; Maggiorelli, F.; Tonerini, M.; Frammartino, B. Prevalence of acute respiratory symptoms in breath-hold divers. Undersea Hyperb. Med. 2012, 39, 837–844. [Google Scholar]
- Bove, A.A. Pulmonary Aspects of Exercise and Sports. Methodist. Debakey. Cardiovasc. J. 2016, 12, 93–97. [Google Scholar] [CrossRef]
- Tuder, R.M.; Yun, J.H. Vascular endothelial growth factor of the lung: Friend or foe. Curr. Opin. Pharmacol. 2008, 8, 255–260, Epub 28 May 2008. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Flower, V.A.; Pauling, J.D.; Millar, A.B. VEGF (Vascular Endothelial Growth Factor) and Fibrotic Lung Disease. Int. J. Mol. Sci. 2018, 19, 1269. [Google Scholar] [CrossRef]
- Mura, M.; dos Santos, C.C.; Stewart, D.; Liu, M. Vascular endothelial growth factor and related molecules in acute lung injury. J. Appl. Physiol. 2004, 97, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Hillan, K.J.; Ryan, A.M.; Kowalski, J.; Keller, G.A.; Rangell, L.; Wright, B.D.; Radtke, F.; Aguet, M.; Ferrara, N. VEGF is required for growth and survival in neonatal mice. Development 1999, 126, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Compernolle, V.; Brusselmans, K.; Acker, T.; Hoet, P.; Tjwa, M.; Beck, H.; Plaisance, S.; Dor, Y.; Keshet, E.; Lupu, F.; et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 2002, 8, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Blythe, T.; Jarrett, C.; Ourradi, K.; Shelley-Fraser, G.; Day, M.J.; Qiu, Y.; Harper, S.; Maher, T.M.; Oltean, S.; et al. Differential Expression of VEGF-Axxx Isoforms Is Critical for Development of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 479–493. [Google Scholar] [CrossRef]
- Henson, P.M.; Bratton, D.L.; Fadok, V.A. The phosphatidylserine receptor: A crucial molecular switch? Nat. Rev. Mol. Cell Biol. 2001, 2, 627–633. [Google Scholar] [CrossRef]
- Golpon, H.A.; Fadok, V.A.; Taraseviciene-Stewart, L.; Scerbavicius, R.; Sauer, C.; Welte, T.; Henson, P.M.; Voelkel, N.F. Life after corpse engulfment: Phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004, 18, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.K.; Huang, T.H.; Yang, C.T.; Shi, C.S. Roles of lung-recruited monocytes and pulmonary Vascular Endothelial Growth Factor (VEGF) in resolving Ventilator-Induced Lung Injury (VILI). PLoS ONE 2021, 16, e0248959. [Google Scholar] [CrossRef]
- Crosby, L.M.; Waters, C.M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.J.; Tatsunami, R.; Yamamura, H.; Fukai, T.; Ushio-Fukai, M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017, 312, C749–C764. [Google Scholar] [CrossRef]
- Medford, A.R.; Douglas, S.K.; Godinho, S.I.; Uppington, K.M.; Armstrong, L.; Gillespie, K.M.; van Zyl, B.; Tetley, T.D.; Ibrahim, N.B.; Millar, A.B. Vascular Endothelial Growth Factor (VEGF) isoform expression and activity in human and murine lung injury. Respir. Res. 2009, 10, 27. [Google Scholar] [CrossRef]
- Herold, S.; Mayer, K.; Lohmeyer, J. Acute lung injury: How macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2011, 2, 65. [Google Scholar] [CrossRef] [PubMed]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.; Moss, M.; Downey, G.P. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef]
- Loftus, T.J.; Thomson, A.J.; Kannan, K.B.; Alamo, I.G.; Millar, J.K.; Plazas, J.M.; Whitley, E.E.; Efron, P.A.; Mohr, A.M. Clonidine restores vascular endothelial growth factor expression and improves tissue repair following severe trauma. Am. J. Surg. 2017, 214, 610–615. [Google Scholar] [CrossRef]
- Koh, H.; Tasaka, S.; Hasegawa, N.; Yamada, W.; Shimizu, M.; Nakamura, M.; Yonemaru, M.; Ikeda, E.; Adachi, Y.; Fujishima, S.; et al. Protective role of vascular endothelial growth factor in endotoxin-induced acute lung injury in mice. Respir. Res. 2007, 8, 60. [Google Scholar] [CrossRef]
- Medford, A.R.; Ibrahim, N.B.; Millar, A.B. Vascular endothelial growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. J. Crit. Care 2009, 24, 236–242. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Varet, J.; Douglas, S.K.; Gilmartin, L.; Medford, A.R.; Bates, D.O.; Harper, S.J.; Millar, A.B. VEGF in the lung: A role for novel isoforms. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L768–L774. [Google Scholar] [CrossRef]
- Watson, C.J.; Webb, N.J.; Bottomley, M.J.; Brenchley, P.E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: Correlation with variation in VEGF protein production. Cytokine 2000, 12, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.J.; Hagberg, J.M.; Paton, C.M.; Douglass, L.W.; Brown, M.D.; McLenithan, J.C.; Roth, S.M. DNA sequence variation in the promoter region of the VEGF gene impacts VEGF gene expression and maximal oxygen consumption. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1848–H1855. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Tureckova, J.; Wilson, E.M.; Cappalonga, J.L.; Rotwein, P. Insulin-like growth factor-mediated muscle differentiation: Collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J. Biol. Chem. 2001, 276, 39264–39270. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef]
- Floss, T.; Arnold, H.H.; Braun, T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997, 11, 2040–2051. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z.; Seger, R.; Rivera, A.J. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J. Histochem Cytochem. 1999, 47, 23–42. [Google Scholar] [CrossRef]
- Doukas, J.; Blease, K.; Craig, D.; Ma, C.; Chandler, L.A.; Sosnowski, B.A.; Pierce, G.F. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol. Ther. 2002, 5 Pt 1, 517–527. [Google Scholar] [CrossRef]
- Fiore, F.; Sébille, A.; Birnbaum, D. Skeletal muscle regeneration is not impaired in Fgf6 -/- mutant mice. Biochem Biophys Res Commun. 2000, 272, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S. Complementary studies of exercised-induced angiogenic growth factors in human skeletal muscle. Am. J. Physiol Heart Circ. Physiol. 2000, 279, H3146–H3147. [Google Scholar] [CrossRef]
- Lazarous, D.F.; Shou, M.; Stiber, J.A.; Dadhania, D.M.; Thirumurti, V.; Hodge, E.; Unger, E.F. Pharmacodynamics of basic fibroblast growth factor: Route of administration determines myocardial and systemic distribution. Cardiovasc. Res. 1997, 36, 78–85. [Google Scholar] [CrossRef]
- Rajanayagam, M.A.; Shou, M.; Thirumurti, V.; Lazarous, D.F.; Quyyumi, A.A.; Goncalves, L.; Stiber, J.; Epstein, S.E.; Unger, E.F. Intracoronary basic fibroblast growth factor enhances myocardial collateral perfusion in dogs. J. Am. Coll Cardiol. 2000, 35, 519–526. [Google Scholar] [CrossRef]
- Tomanek, R.J.; Doty, M.K.; Sandra, A. Early coronary angiogenesis in response to thyroxine: Growth characteristics and upregulation of basic fibroblast growth factor. Circ. Res. 1998, 82, 587–593. [Google Scholar] [CrossRef]
- Santiago, J.J.; McNaughton, L.J.; Koleini, N.; Ma, X.; Bestvater, B.; Nickel, B.E.; Fandrich, R.R.; Wigle, J.T.; Freed, D.H.; Arora, R.C.; et al. High molecular weight fibroblast growth factor-2 in the human heart is a potential target for prevention of cardiac remodeling. PLoS ONE 2014, 9, e97281. [Google Scholar] [CrossRef] [PubMed]
- Yajima, S.; Ishikawa, M.; Kubota, T.; Moroi, M.; Sugi, K.; Namiki, A. Intramyocardial injection of fibroblast growth factor-2 plus heparin suppresses cardiac failure progression in rats with hypertensive heart disease. Int. Heart J. 2005, 46, 289–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.Y.; Wang, F.; Yan, X.Y.; Zhou, Q.; Ling, Q.; Ling, J.X.; Rong, Y.Z.; Li, Y.G. Autologous transplantation of EPCs encoding FGF1 gene promotes neovascularization in a porcine model of chronic myocardial ischemia. Int. J. Cardiol. 2009, 135, 223–232. [Google Scholar] [CrossRef]
- Tomita, Y.; Kusama, Y.; Seino, Y.; Munakata, K.; Kishida, H.; Hayakawa, H. Increased accumulation of acidic fibroblast growth factor in left ventricular myocytes of patients with idiopathic cardiomyopathy. Am. Heart J. 1997, 134, 779–786. [Google Scholar] [CrossRef]
- Murakami, M. Signaling required for blood vessel maintenance: Molecular basis and pathological manifestations. Int. J. Vasc. Med. 2012, 2012, 293641. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef]
- Murakami, M.; Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 2008, 15, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; White, A.C.; Park, C.; Smith, C.S.; Choi, K.; Long, F.; Hui, C.C.; Ornitz, D.M. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006, 20, 1651–1666. [Google Scholar] [CrossRef]
- Miller, D.L.; Ortega, S.; Bashayan, O.; Basch, R.; Basilico, C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol. Cell Biol. 2000, 20, 2260–2268, Erratum in 2000, 20, 3752. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nguyen, L.T.; Zhuang, Z.W.; Moodie, K.L.; Carmeliet, P.; Stan, R.V.; Simons, M. The FGF system has a key role in regulating vascular integrity. J. Clin. Investig. 2008, 118, 3355–3366, Erratum in 2009, 119, 2113. [Google Scholar] [CrossRef]
- Murakami, M.; Nguyen, L.T.; Hatanaka, K.; Schachterle, W.; Chen, P.Y.; Zhuang, Z.W.; Black, B.L.; Simons, M. FGF-dependent regulation of VEGF receptor 2 expression in mice. J. Clin. Investig. 2011, 121, 2668–2678. [Google Scholar] [CrossRef]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H.; et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Rabata, A.; Fedr, R.; Soucek, K.; Hampl, A.; Koledova, Z. 3D Cell Culture Models Demonstrate a Role for FGF and WNT Signaling in Regulation of Lung Epithelial Cell Fate and Morphogenesis. Front. Cell Dev. Biol. 2020, 8, 574. [Google Scholar] [CrossRef]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef]
- Karolak, J.A.; Gambin, T.; Honey, E.M.; Slavik, T.; Popek, E.; Stankiewicz, P. A de novo 2.2Mb recurrent 17q23.1q23.2 deletion unmasks novel putative regulatory non-coding SNVs associated with lethal lung hypoplasia and pulmonary hypertension: A case report. BMC Med. Genomics 2020, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Raica, M.; Cimpean, A.M. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Ranjbar, K.; Fayazi, B. Vascularisation of skeletal muscle. In Muscle Cells-Recent Advances and Future Perspectives; Valarmathi, M.T., Ed.; 2019; Available online: https://www.intechopen.com/books/muscle-cells-recent-advances-and-future-perspectives/vascularisation-of-s (accessed on 26 May 2021).
- Boström, P.; Mann, N.; Wu, J.; Quintero, P.A.; Plovie, E.R.; Panáková, D.; Gupta, R.K.; Xiao, C.; MacRae, C.A.; Rosenzweig, A.; et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 2010, 143, 1072–1083. [Google Scholar] [CrossRef]
- Leite, C.F.; Lopes, C.S.; Alves, A.C.; Fuzaro, C.S.; Silva, M.V.; Oliveira, L.F.; Garcia, L.P.; Farnesi, T.S.; Cuba, M.B.; Rocha, L.B.; et al. Endogenous resident c-Kit cardiac stem cells increase in mice with an exercise-induced, physiologically hypertrophied heart. Stem Cell Res. 2015, 15, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, P.; Qu, Y.; Yu, P.; Yao, J.; Wang, H.; Fu, S.; Bei, Y.; Chen, Y.; Che, L.; et al. Telocytes in exercise-induced cardiac growth. J. Cell Mol. Med. 2016, 20, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997, 277, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Gerhardt, H.; Kalén, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001, 153, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Zanini, A.; Chetta, A.; Imperatori, A.S.; Spanevello, A.; Olivieri, D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res. 2010, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Warshamana, G.S.; Corti, M.; Brody, A.R. TNF-alpha, PDGF, and TGF-beta(1) expression by primary mouse bronchiolar-alveolar epithelial and mesenchymal cells: Tnf-alpha induces TGF-beta(1). Exp. Mol. Pathol. 2001, 71, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Gabazza, E.C.; Hayashi, T.; Ido, M.; Adachi, Y.; Suzuki, K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.C.; Chu, H.W.; Westcott, J.Y.; Tucker, A.; Langmack, E.L.; Sutherland, E.R.; Kraft, M. Airway fibroblasts exhibit a synthetic phenotype in severe asthma. J. Allergy Clin. Immunol. 2005, 115, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.L.; Rice, A.; Geisenhoffer, K.; Madtes, D.K.; Bonner, J.C. Interleukin-13 stimulates the proliferation of lung myofibroblasts via a signal transducer and activator of transcription-6-dependent mechanism: A possible mechanism for the development of airway fibrosis in asthma. Chest 2003, 123 (Suppl. 3), 422S. [Google Scholar] [CrossRef] [PubMed]
- Kardas, G.; Daszyńska-Kardas, A.; Marynowski, M.; Brząkalska, O.; Kuna, P.; Panek, M. Role of Platelet-Derived Growth Factor (PDGF) in Asthma as an Immunoregulatory Factor Mediating Airway Remodeling and Possible Pharmacological Target. Front. Pharmacol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Hirst, S.J.; Barnes, P.J.; Twort, C.H. PDGF isoform-induced proliferation and receptor expression in human cultured airway smooth muscle cells. Am. J. Physiol. 1996, 270, 415–428. [Google Scholar] [CrossRef]
- Bergsten, E.; Uutela, M.; Li, X.; Pietras, K.; Ostman, A.; Heldin, C.H.; Alitalo, K.; Eriksson, U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001, 3, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, L.J.; Varhaug, J.E.; Lillehaug, J.R. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005, 272, 5723–5741. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, X.; He, X. Platelet-derived growth factors and their receptors: Structural and functional perspectives. Biochim. Biophys. Acta 2013, 1834, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Lennartsson, J. Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009100. [Google Scholar] [CrossRef]
- Mamer, S.B.; Chen, S.; Weddell, J.C.; Palasz, A.; Wittenkeller, A.; Kumar, M.; Imoukhuede, P.I. Discovery of High-Affinity PDGF-VEGFR Interactions: Redefining RTK Dynamics. Sci Rep. 2017, 7, 16439. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, F.K.; Stivers, D.N.; Arnett, F.C. Microsatellites and intragenic polymorphisms of transforming growth factor beta and platelet-derived growth factor and their receptor genes in Native Americans with systemic sclerosis (scleroderma): A preliminary analysis showing no genetic association. Arthritis Rheum. 2000, 43, 1068–1073. [Google Scholar] [CrossRef]
- Joosten, P.H.; Toepoel, M.; Mariman, E.C.; Van Zoelen, E.J. Promoter haplotype combinations of the platelet-derived growth factor alpha-receptor gene predispose to human neural tube defects. Nat. Genet. 2001, 27, 215–217. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balberova, O.V.; Bykov, E.V.; Shnayder, N.A.; Petrova, M.M.; Gavrilyuk, O.A.; Kaskaeva, D.S.; Soloveva, I.A.; Petrov, K.V.; Mozheyko, E.Y.; Medvedev, G.V.; et al. The “Angiogenic Switch” and Functional Resources in Cyclic Sports Athletes. Int. J. Mol. Sci. 2021, 22, 6496. https://doi.org/10.3390/ijms22126496
Balberova OV, Bykov EV, Shnayder NA, Petrova MM, Gavrilyuk OA, Kaskaeva DS, Soloveva IA, Petrov KV, Mozheyko EY, Medvedev GV, et al. The “Angiogenic Switch” and Functional Resources in Cyclic Sports Athletes. International Journal of Molecular Sciences. 2021; 22(12):6496. https://doi.org/10.3390/ijms22126496
Chicago/Turabian StyleBalberova, Olga V., Evgeny V. Bykov, Natalia A. Shnayder, Marina M. Petrova, Oksana A. Gavrilyuk, Daria S. Kaskaeva, Irina A. Soloveva, Kirill V. Petrov, Elena Y. Mozheyko, German V. Medvedev, and et al. 2021. "The “Angiogenic Switch” and Functional Resources in Cyclic Sports Athletes" International Journal of Molecular Sciences 22, no. 12: 6496. https://doi.org/10.3390/ijms22126496
APA StyleBalberova, O. V., Bykov, E. V., Shnayder, N. A., Petrova, M. M., Gavrilyuk, O. A., Kaskaeva, D. S., Soloveva, I. A., Petrov, K. V., Mozheyko, E. Y., Medvedev, G. V., & Nasyrova, R. F. (2021). The “Angiogenic Switch” and Functional Resources in Cyclic Sports Athletes. International Journal of Molecular Sciences, 22(12), 6496. https://doi.org/10.3390/ijms22126496







