The PI-3-Kinase P110α Catalytic Subunit of T Lymphocytes Modulates Collagen-Induced Arthritis
Abstract
1. Introduction
2. Results
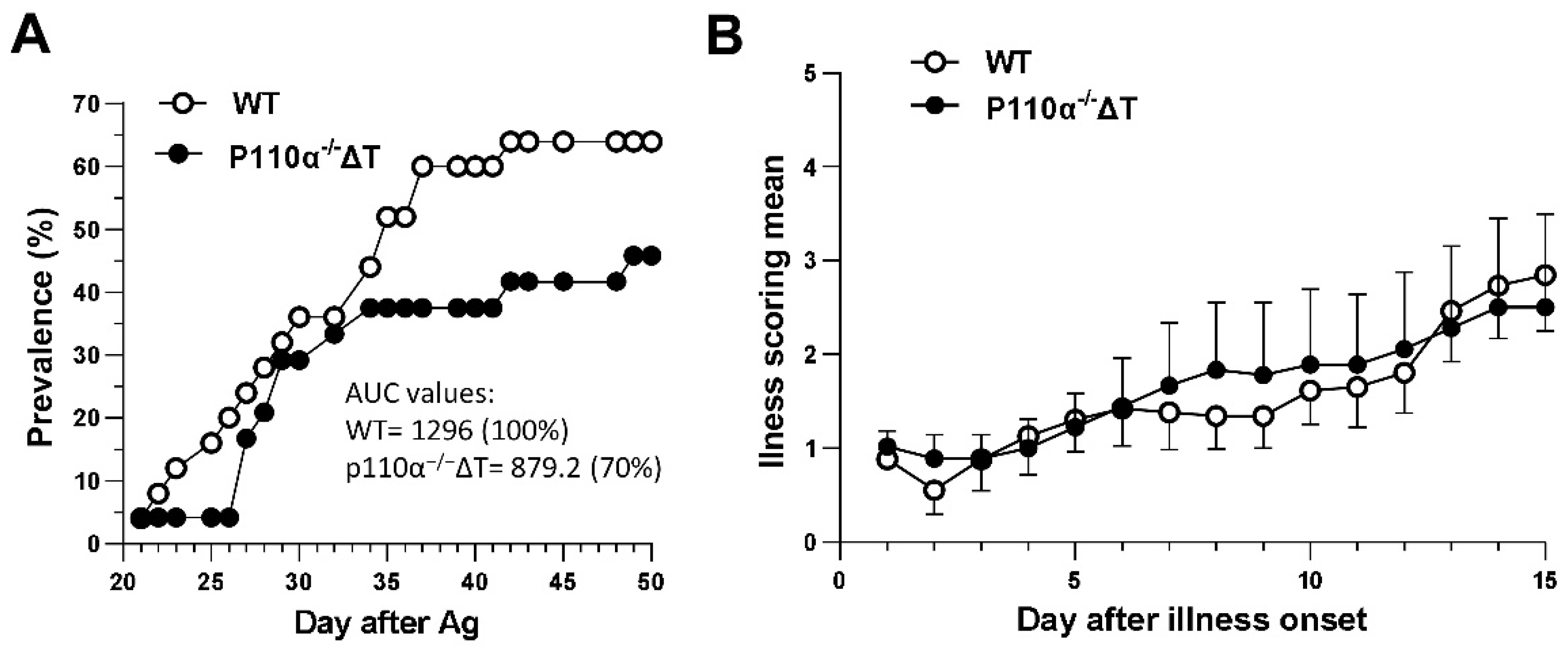
2.1. P110α−/−ΔT Mice Show a Lower Prevalence of CIA
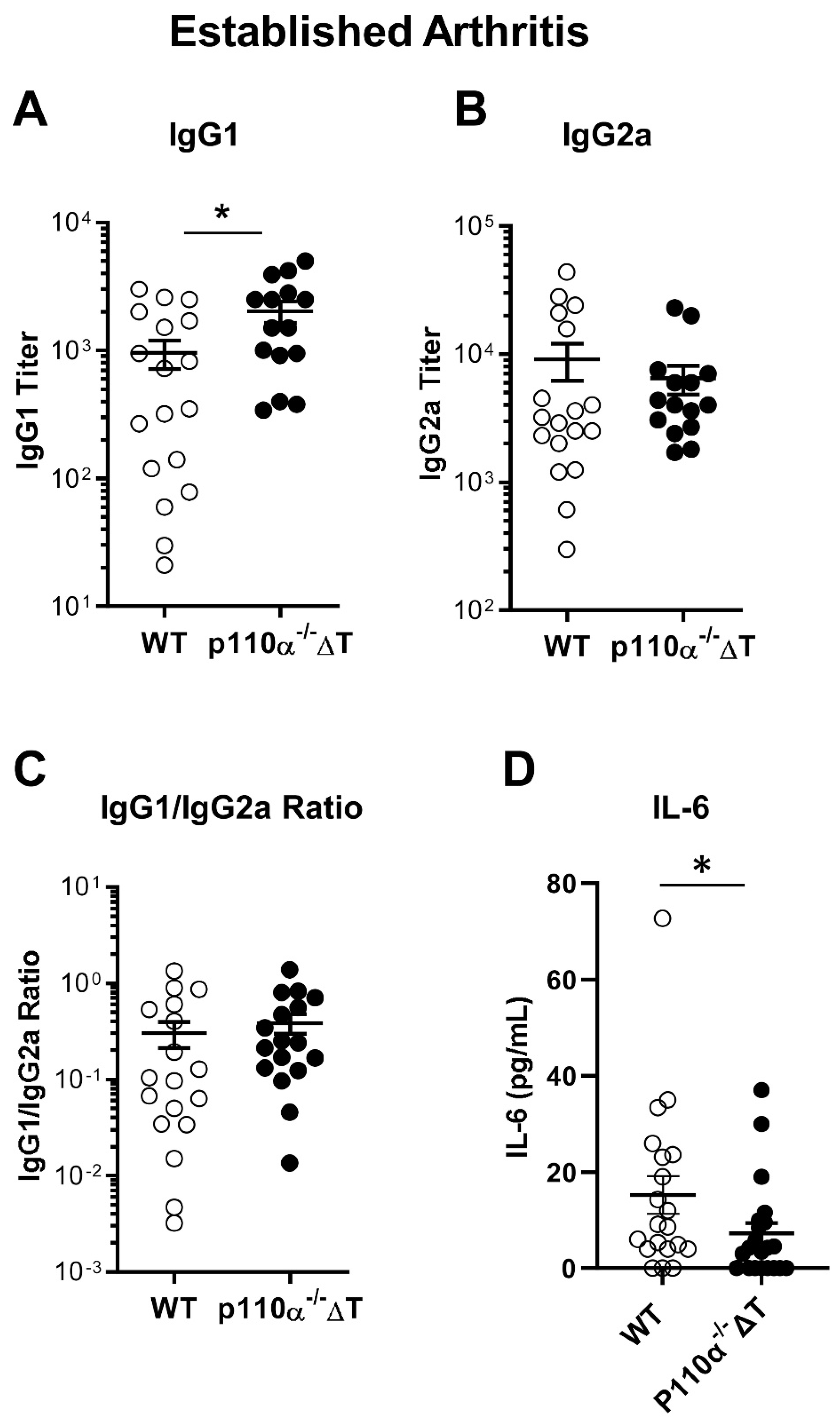
2.2. Impact of T-Cell-Specific Loss of p110α in Collagen-Specific Abs and Serum IL-6
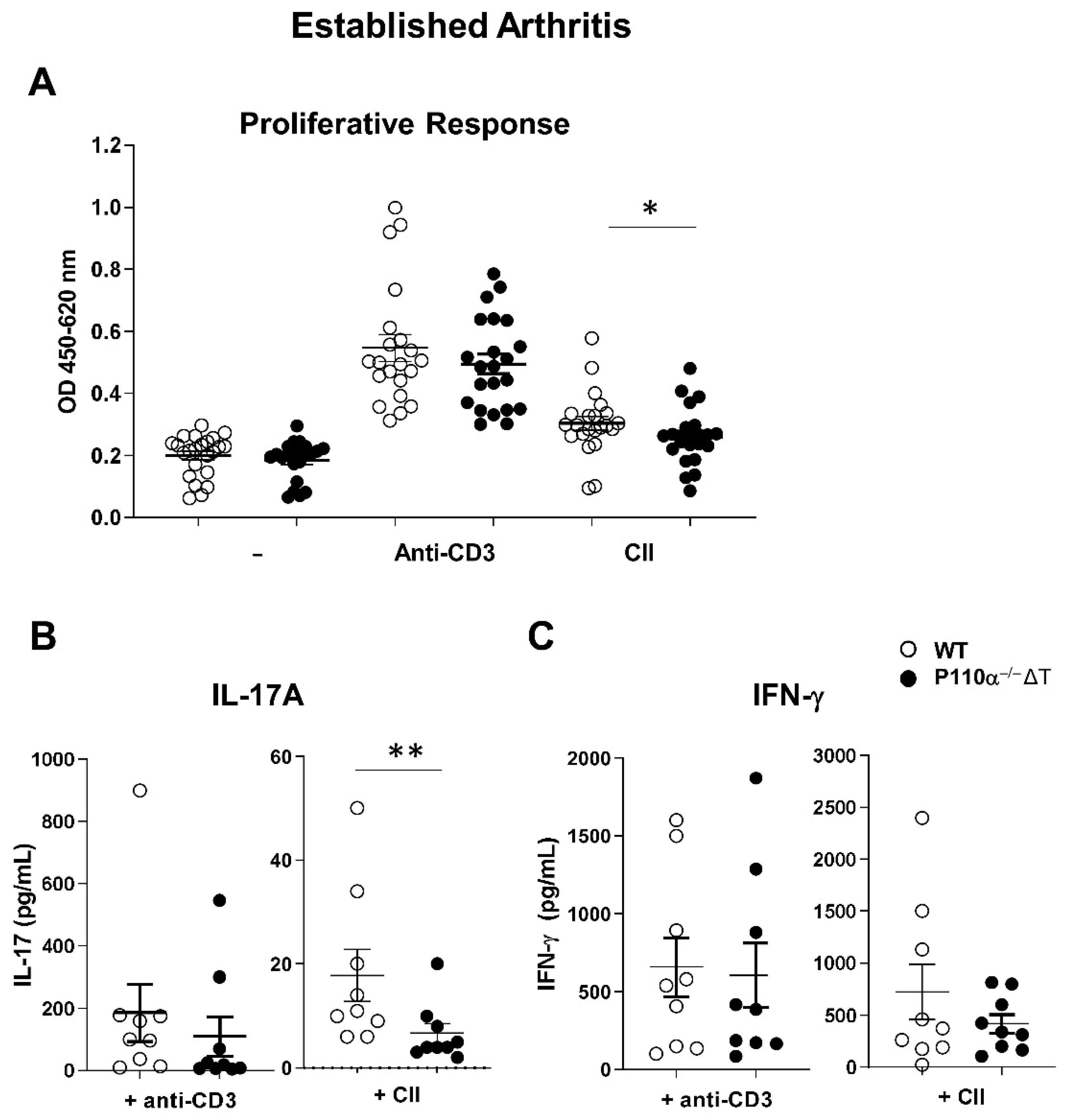
2.3. Ex Vivo Response of Lymph Node Cells in CIA in the p110α−/−ΔT Mouse Model
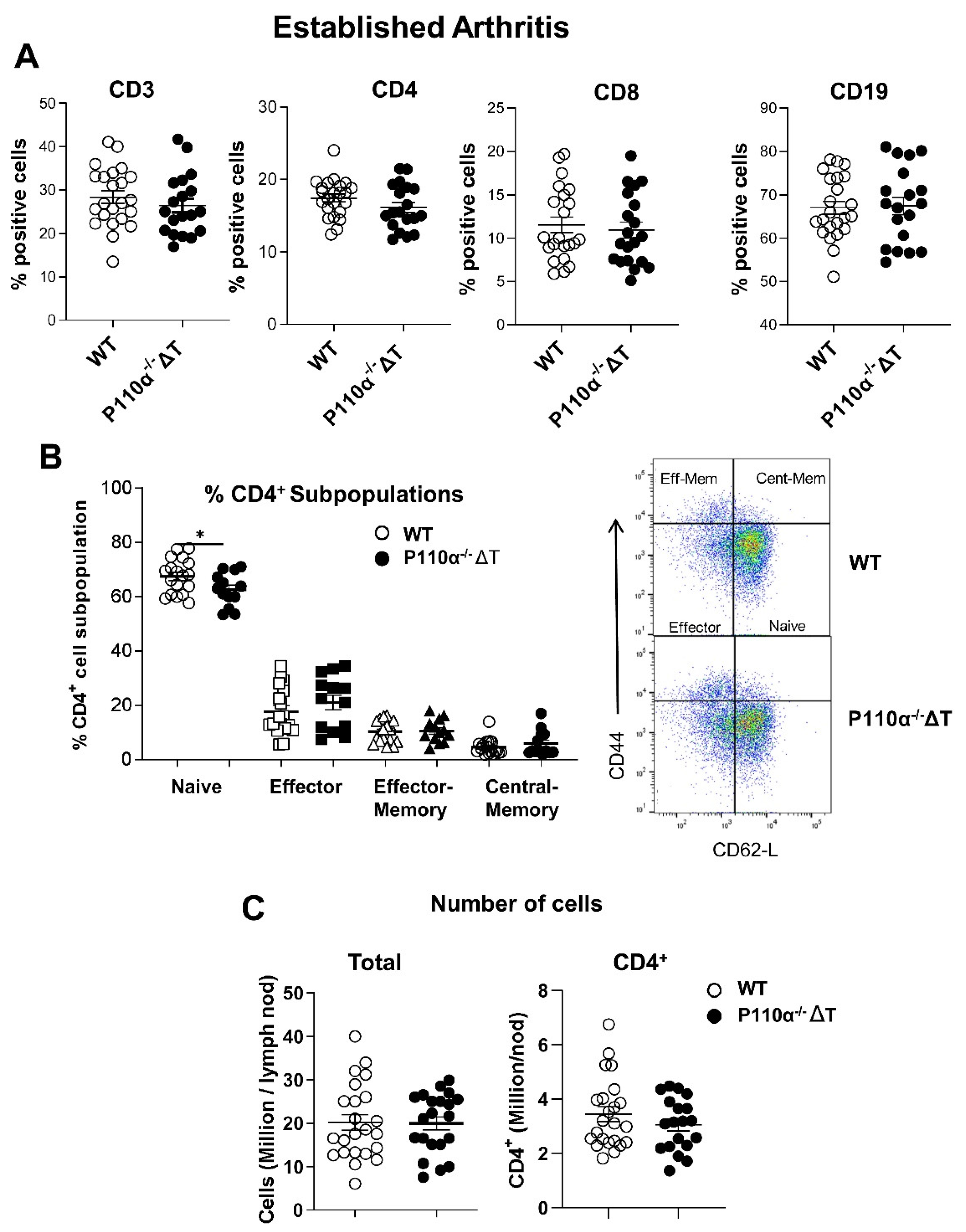
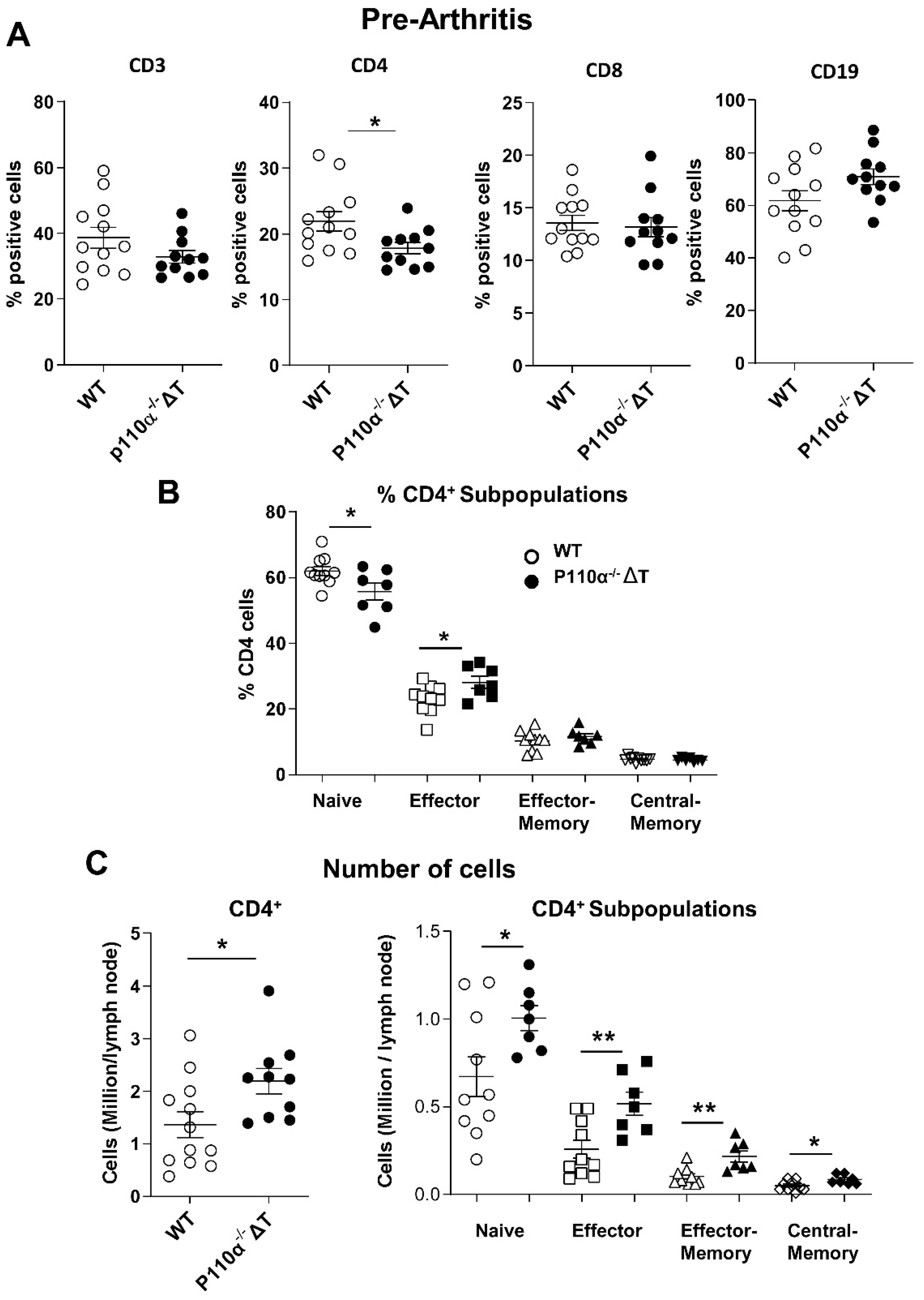
2.4. Analysis of Lymph Node Subpopulations in p110α−/−ΔT Mice under CIA
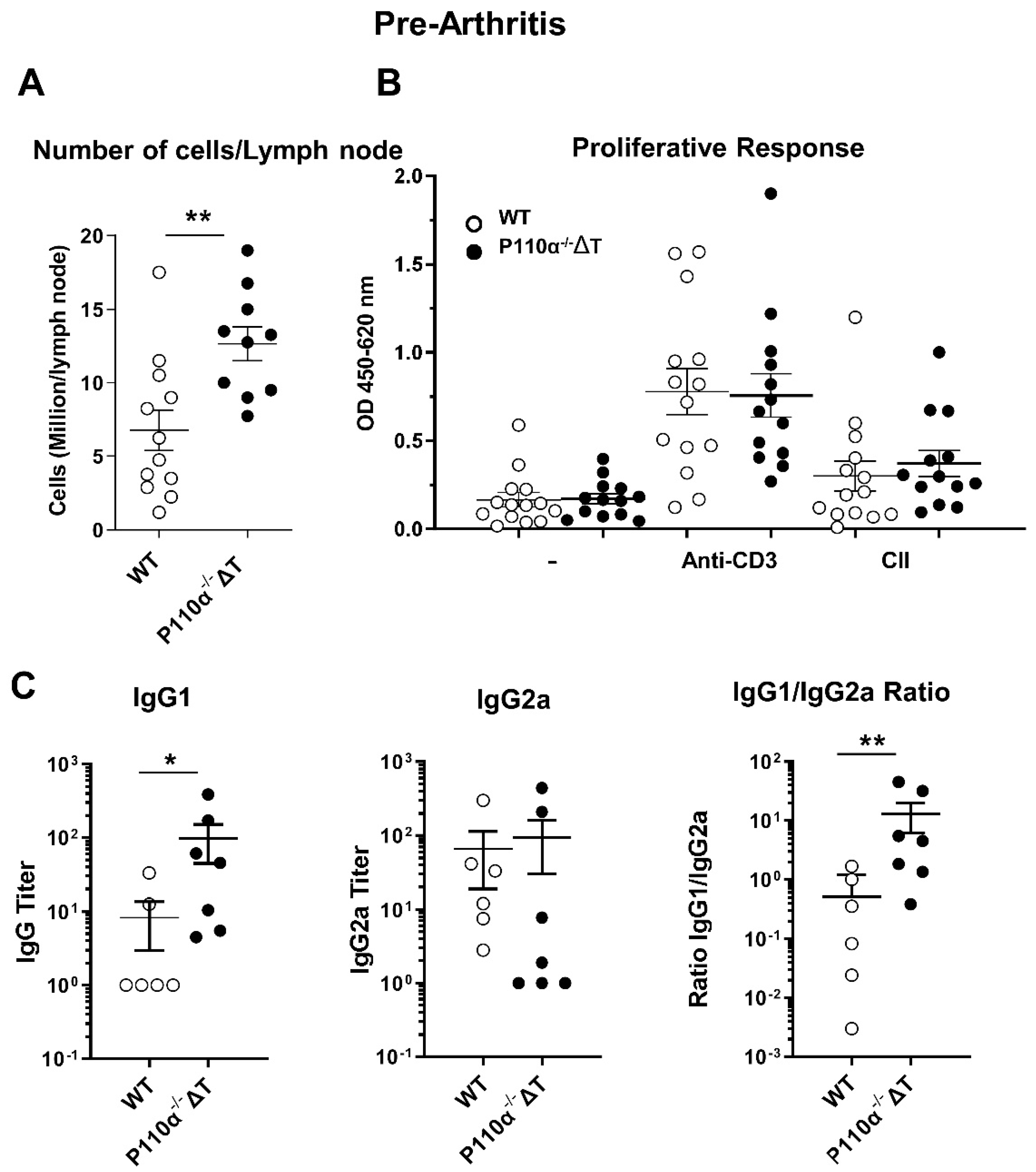
2.5. Analysis of the Immune Response to Collagen II in p110α−/−ΔT Mice before the Arthritis Onset
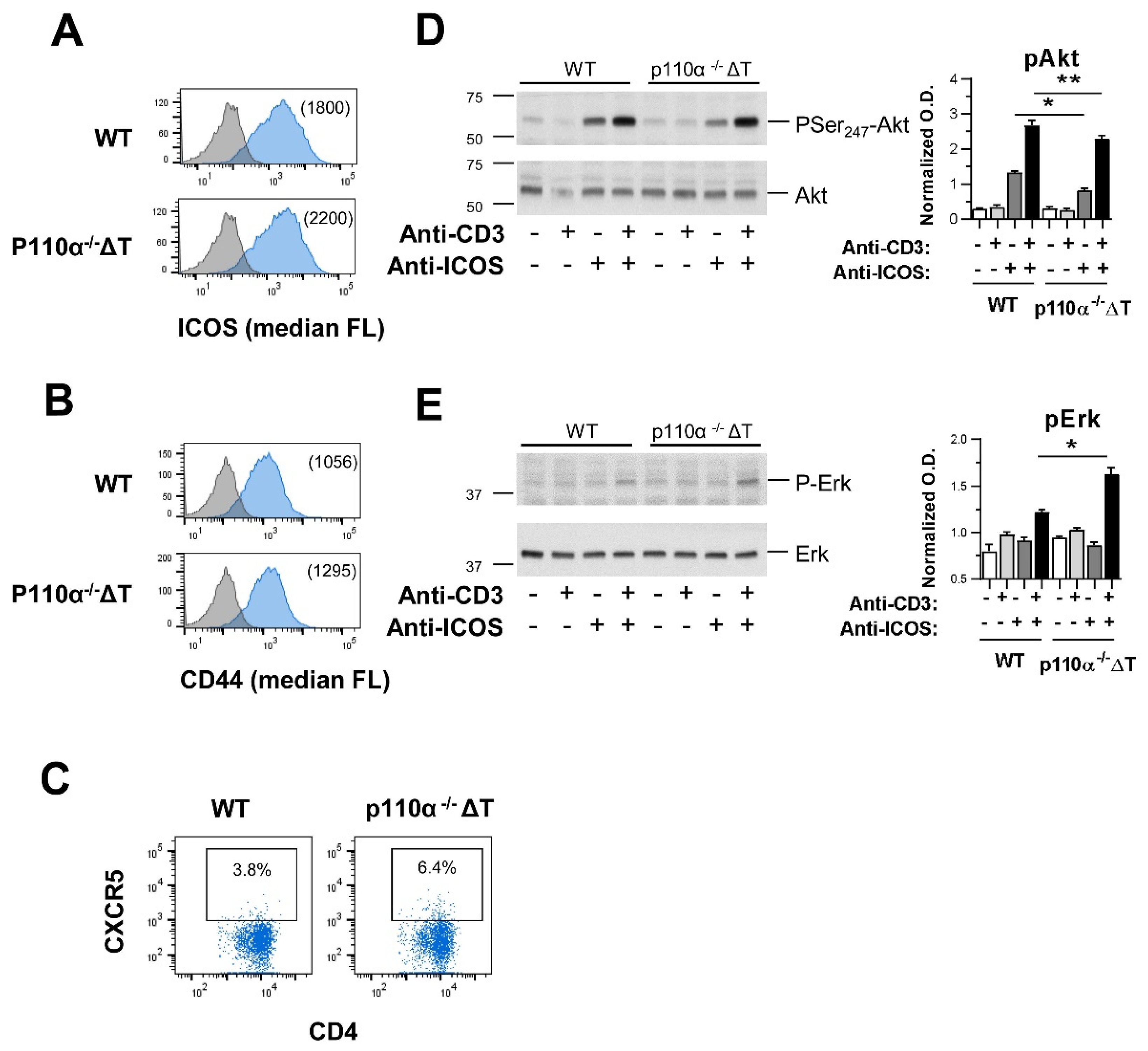
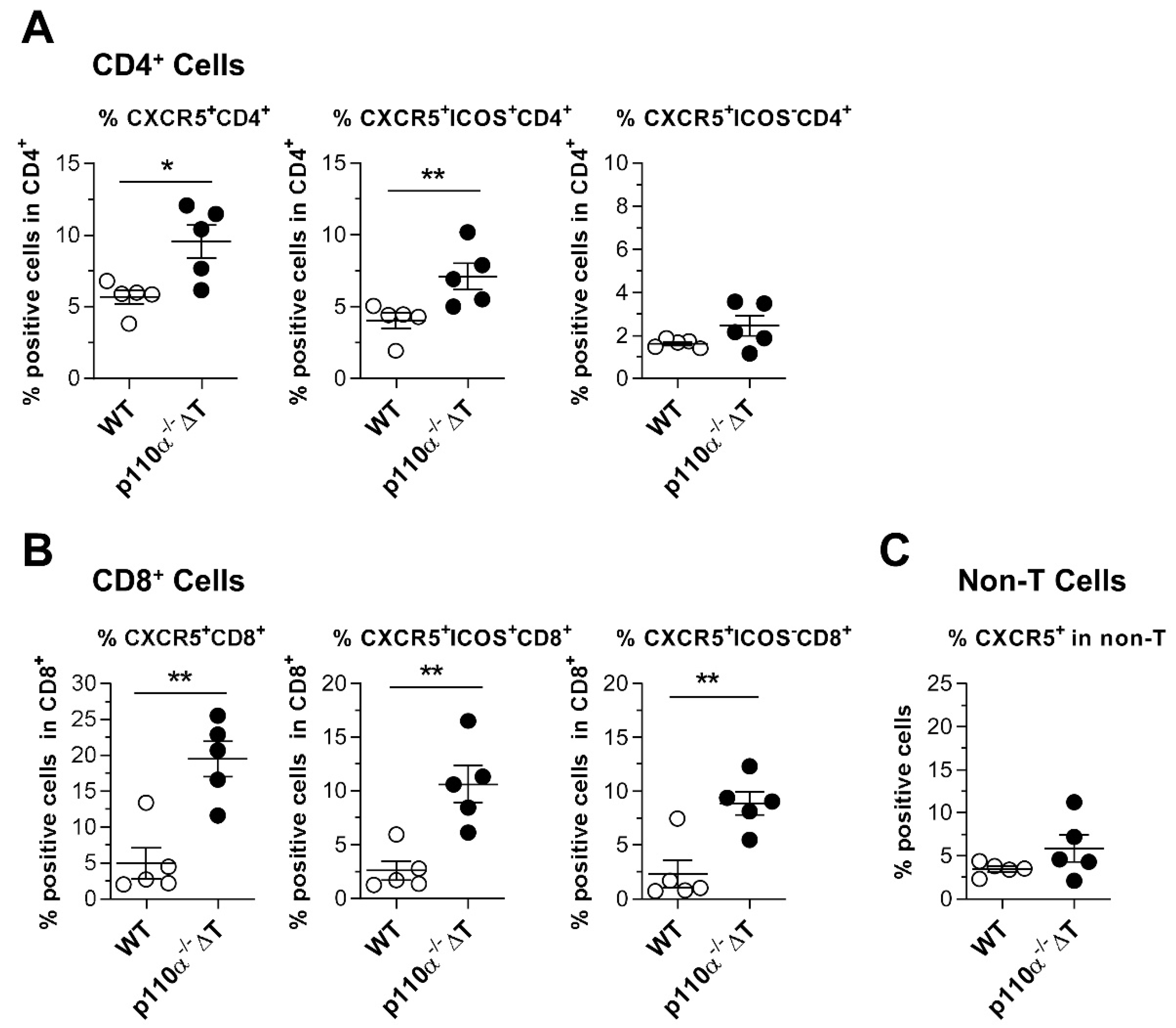
2.6. Effect of Genetic Deletion of p110α in Homing Markers Expression and ICOS-Dependent T-Cell Signaling
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Collagen-Induced Arthritis: Induction and Assessment
4.3. Analysis of T-Cell Responses
4.4. Measurement of Anti-Collagen Antibodies
4.5. Analysis of Cytokine Production
4.6. Analysis of Surface and Intracellular Markers by Flow Cytometry
4.7. CD4+ T-Cell Blasts, Generation, Activation and Western Blot
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosloniec, E.F.; Brand, D.D.; Myers, L.K.; Whittington, K.B.; Gumanovskaya, M.; Zaller, D.M.; Woods, A.; Altmann, D.; Stuart, J.M.; Kang, A.H. An HLA-DR1 Transgene Confers Susceptibility to Collagen-induced Arthritis Elicited with Human Type II Collagen. J. Exp. Med. 1997, 185, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, R.; Malmström, V.; Burkhardt, H. Autoimmune priming, tissue attack and chronic inflammation—The three stages of rheumatoid arthritis. Eur. J. Immunol. 2014, 44, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.J.; Liu, S.-C.; Su, C.-M.; Wang, Y.-H.; Tsai, C.-H.; Tang, C.-H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Lin, G.-J.; Chen, J.-W.; Wang, K.-C.; Tien, C.-H.; Hu, C.-F.; Chang, C.-N.; Hsu, W.-F.; Fan, H.-C.; Sytwu, H.-K. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 1332. [Google Scholar] [CrossRef]
- Deane, J.A.; Fruman, D.A. Phosphoinositide3-Kinase: Diverse Roles in Immune Cell Activation. Annu. Rev. Immunol. 2004, 22, 563–598. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.T.; Anderson, K.E.; Davidson, K.; Stephens, L.R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006, 34, 647–662. [Google Scholar] [CrossRef]
- Stark, A.-K.; Davenport, E.C.M.; Patton, D.T.; Scudamore, C.L.; Vanhaesebroeck, B.; Veldhoen, M.; Garden, O.A.; Okkenhaug, K. Loss of Phosphatidylinositol 3-Kinase Activity in Regulatory T Cells Leads to Neuronal Inflammation. J. Immunol. 2020, 205, 78–89. [Google Scholar] [CrossRef]
- Rommel, C.; Camps, M.; Ji, H. PI3Kδ and PI3Kγ: Partners in crime in inflammation in rheumatoid arthritis and beyond? Nat. Rev. Immunol. 2007, 7, 191–201. [Google Scholar] [CrossRef]
- Banham-Hall, E.; Clatworthy, M.R.; Okkenhaug, K. The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases. Open Rheumatol. J. 2012, 6, 245–258. [Google Scholar] [CrossRef]
- Hayer, S.; Pundt, N.; Peters, M.A.; Wunrau, C.; Kühnel, I.; Neugebauer, K.; Strietholt, S.; Zwerina, J.; Korb, A.; Penninger, J.; et al. PI3Kγ regulates cartilage damage in chronic inflammatory arthritis. FASEB J. 2009, 23, 4288–4298. [Google Scholar] [CrossRef]
- Swan, D.J.; Aschenbrenner, D.; Lamb, C.A.; Chakraborty, K.; Clark, J.; Pandey, S.; Engelhardt, K.R.; Chen, R.; Cavounidis, A.; Ding, Y.; et al. Immunodeficiency, autoimmune thrombocytopenia and enterocolitis caused by autosomal recessive deficiency of PIK3CD-encoded phosphoinositide 3-kinase δ. Haematologica 2019, 104, e483–e486. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; et al. Impaired B and T Cell Antigen Receptor Signaling in p110delta PI 3-Kinase Mutant Mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.T.; Garden, O.A.; Pearce, W.P.; Clough, L.E.; Monk, C.R.; Leung, E.; Rowan, W.C.; Sancho, S.; Walker, L.S.K.; Vanhaesebroeck, B.; et al. Cutting Edge: The Phosphoinositide 3-Kinase p110δ Is Critical for the Function of CD4+CD25+Foxp3+ Regulatory T Cells. J. Immunol. 2006, 177, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Sogkas, G.; Fedchenko, M.; Dhingra, A.; Jablonka, A.; Schmidt, R.E.; Atschekzei, F. Primary immunodeficiency disorder caused by phosphoinositide 3–kinase δ deficiency. J. Allergy Clin. Immunol. 2018, 142, 1650–1653.e2. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.-K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Acosta, Y.Y.; Zafra, M.P.; Ojeda, G.; Bernardone, I.S.; Dianzani, U.; Portolés, P.; Rojo, J.M. Biased binding of class IA phosphatidyl inositol 3-kinase subunits to inducible costimulator (CD278). Cell. Mol. Life Sci. 2010, 68, 3065–3079. [Google Scholar] [CrossRef]
- Graupera, M.; Guillermet-Guibert, J.; Foukas, L.C.; Phng, L.-K.; Cain, R.J.; Salpekar, A.; Pearce, W.; Meek, S.; Millan, J.A.; Cutillas, P.R.; et al. Angiogenesis selectively requires the p110α isoform of PI3K to control endothelial cell migration. Nat. Cell Biol. 2008, 453, 662–666. [Google Scholar] [CrossRef]
- Acosta, Y.; Montes-Casado, M.; Aragoneses-Fenoll, L.; Dianzani, U.; Portolés, P.; Rojo, J. Suppression of CD4+ T Lymphocyte Activation in Vitro and Experimental Encephalomyelitis in Vivo by the Phosphatidyl Inositol 3-Kinase Inhibitor PIK-75. Int. J. Immunopathol. Pharmacol. 2014, 27, 53–67. [Google Scholar] [CrossRef]
- Aragoneses-Fenoll, L.; Montes-Casado, M.; Ojeda, G.; Acosta-Ampudia, Y.; Herranz, J.; Martínez, S.; Blanco-Aparicio, C.; Criado, G.; Pastor, J.; Dianzani, U.; et al. ETP-46321, a dual p110α/δ class IA phosphoinositide 3-kinase inhibitor modulates T lymphocyte activation and collagen-induced arthritis. Biochem. Pharmacol. 2016, 106, 56–69. [Google Scholar] [CrossRef]
- Ramadani, F.; Bolland, D.J.; Garcon, F.; Emery, J.L.; Vanhaesebroeck, B.; Corcoran, A.E.; Okkenhaug, K. The PI3K Isoforms p110 and p110 Are Essential for Pre-B Cell Receptor Signaling and B Cell Development. Sci. Signal. 2010, 3, ra60. [Google Scholar] [CrossRef]
- Greaves, S.A.; Peterson, J.N.; Strauch, P.; Torres, R.M.; Pelanda, R. Active PI3K abrogates central tolerance in high-avidity autoreactive B cells. J. Exp. Med. 2019, 216, 1135–1153. [Google Scholar] [CrossRef]
- Aragoneses-Fenoll, L.; Ojeda, G.; Montes-Casado, M.; Acosta-Ampudia, Y.; Dianzani, U.; Portolés, P.; Rojo, J.M. T-Cell-Specific Loss of the PI-3-Kinase p110α Catalytic Subunit Results in Enhanced Cytokine Production and Antitumor Response. Front. Immunol. 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Davila, E.; Kang, Y.M.; Park, Y.W.; Sawai, H.; He, X.; Pryshchep, S.; Goronzy, J.J.; Weyand, C.M. Cell-Based Immunotherapy with Suppressor CD8+ T Cells in Rheumatoid Arthritis. J. Immunol. 2005, 174, 7292–7301. [Google Scholar] [CrossRef] [PubMed]
- Mijnheer, G.; Van Wijk, F. T-Cell Compartmentalization and Functional Adaptation in Autoimmune Inflammation: Lessons from Pediatric Rheumatic Diseases. Front. Immunol. 2019, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Van Wijk, F. CD8+ T cells in human autoimmune arthritis: The unusual suspects. Nat. Rev. Rheumatol. 2016, 12, 421–428. [Google Scholar] [CrossRef]
- Iwai, H.; Kozono, Y.; Hirose, S.; Akiba, H.; Yagita, H.; Okumura, K.; Kohsaka, H.; Miyasaka, N.; Azuma, M. Amelioration of Collagen-Induced Arthritis by Blockade of Inducible Costimulator-B7 Homologous Protein Costimulation. J. Immunol. 2002, 169, 4332–4339. [Google Scholar] [CrossRef]
- Panneton, V.; Yazdchi, S.B.; Witalis, M.; Chang, J.; Suh, W.-K. ICOS Signaling Controls Induction and Maintenance of Collagen-Induced Arthritis. J. Immunol. 2018, 200, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Treuting, P.; Duong, J.; Flavell, R.A.; Dong, C. Inducible costimulator is essential for collagen-induced arthritis. J. Clin. Investig. 2003, 111, 701–706. [Google Scholar] [CrossRef]
- Akiba, H.; Takeda, K.; Kojima, Y.; Usui, Y.; Harada, N.; Yamazaki, T.; Ma, J.; Tezuka, K.; Yagita, H.; Okumura, K. The Role of ICOS in the CXCR5+Follicular B Helper T Cell Maintenance In Vivo. J. Immunol. 2005, 175, 2340–2348. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.-H.; Watowich, S.S.; Jetten, A.; Tian, Q.; et al. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef]
- Cao, G.; Chi, S.; Wang, X.; Sun, J.; Zhang, Y. CD4+CXCR5+PD-1+ T Follicular Helper Cells Play a Pivotal Role in the Development of Rheumatoid Arthritis. Med. Sci. Monit. 2019, 25, 3032–3040. [Google Scholar] [CrossRef]
- Leavenworth, J.W.; Verbinnen, B.; Yin, J.; Huang, H.; Cantor, H. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 2015, 16, 96–106. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Shih, C.; Wan, Z.; Ma, X.; Ma, W.; Luo, D.; Qi, H. T–B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nat. Cell Biol. 2014, 517, 214–218. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Liu, D.; Li, J.; Zhang, X.; Chen, X.; Hou, S.; Peng, L.; Xu, C.; Liu, W.; et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nat. Cell Biol. 2013, 496, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Valentine, K.M.; Davini, D.; Lawrence, T.J.; Mullins, G.N.; Manansala, M.; Al-Kuhlani, M.; Pinney, J.M.; Davis, J.K.; Beaudin, A.E.; Sindi, S.S.; et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J. Immunol. 2018, 201, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Valentine, K.M.; Hoyer, K.K. CXCR5+ CD8 T Cells: Protective or Pathogenic? Front. Immunol. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- So, L.; Fruman, D.A. PI3K signalling in B- and T-lymphocytes: New developments and therapeutic advances. Biochem. J. 2012, 442, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.D.; Gold, M.R. Selective inhibitors of phosphoinositide 3-kinase delta: Modulators of B-cell function with potential for treating autoimmune inflammatory diseases and B-cell malignancies. Front. Immunol. 2012, 3, 256. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, G.; Montes-Casado, M.; Sancho-Navarro, J.C.; Martínez, S.; Blanco-Aparicio, S.; García Gonzalez, J.; Criado, G.; Galindo, M.; Pastor, J.; Rojo, J.M.; et al. Effect of PI3 Kinase p110a and p110d inhibitors on cytokine secretion. Implication in rheumatoid arthritis. In Proceedings of the 42 Congreso de la Sociedad Española de Inmunología, Madrid, Spain, 4–6 June 2020. [Google Scholar]
- Haylock-Jacobs, S.; Comerford, I.; Bunting, M.; Kara, E.; Townley, S.; Klingler-Hoffmann, M.; Vanhaesebroeck, B.; Puri, K.D.; McColl, S.R. PI3Kd drives the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting effector T cell apoptosis and promoting Th17 differentiation. J. Autoimmun. 2011, 36, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.; Fitzpatrick, D.R.; Beard, C.; Jessup, H.K.; Lehar, S.; Makar, K.W.; Pérez-Melgosa, M.; Sweetser, M.T.; Schlissel, M.S.; Nguyen, S.; et al. A Critical Role for Dnmt1 and DNA Methylation in T Cell Development, Function, and Survival. Immunity 2001, 15, 763–774. [Google Scholar] [CrossRef]
- Inglis, J.J.; Šimelyte, E.; McCann, F.E.; Criado, G.; Williams, R.O. Protocol for the induction of arthritis in C57BL/6 mice. Nat. Protoc. 2008, 3, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Portoles, P.; Rojo, J.; Golby, A.; Bonneville, M.; Gromkowski, S.; Greenbaum, L.; Janeway, C.A.; Murphy, D.B.; Bottomly, K. Monoclonal antibodies to murine CD3 epsilon define distinct epitopes, one of which may interact with CD4 during T cell activation. J. Immunol. 1989, 142, 4169–4175. [Google Scholar] [PubMed]
- Feito, M.J.; Vaschetto, R.; Criado, G.; Sánchez, A.; Chiocchetti, A.; Jiménez-Periáñez, A.; Dianzani, U.; Portoles, P.; Rojo, J.M. Mechanisms of H4/ICOS costimulation: Effects on proximal TCR signals and MAP kinase pathways. Eur. J. Immunol. 2002, 33, 204–214. [Google Scholar] [CrossRef]
- Redoglia, V.; Dianzani, U.; Rojo, J.M.; Portolés, P.; Bragardo, M.; Wolff, H.; Buonfiglio, D.; Bonissoni, S.; Janeway, C.A. Characterization of H4: A mouse T lymphocyte activation molecule functionally associated with the CD3/T cell receptor. Eur. J. Immunol. 1996, 26, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Casado, M.; Ojeda, G.; Criado, G.; Rojo, J.M.; Portolés, P. The PI-3-Kinase P110α Catalytic Subunit of T Lymphocytes Modulates Collagen-Induced Arthritis. Int. J. Mol. Sci. 2021, 22, 6405. https://doi.org/10.3390/ijms22126405
Montes-Casado M, Ojeda G, Criado G, Rojo JM, Portolés P. The PI-3-Kinase P110α Catalytic Subunit of T Lymphocytes Modulates Collagen-Induced Arthritis. International Journal of Molecular Sciences. 2021; 22(12):6405. https://doi.org/10.3390/ijms22126405
Chicago/Turabian StyleMontes-Casado, María, Gloria Ojeda, Gabriel Criado, José M. Rojo, and Pilar Portolés. 2021. "The PI-3-Kinase P110α Catalytic Subunit of T Lymphocytes Modulates Collagen-Induced Arthritis" International Journal of Molecular Sciences 22, no. 12: 6405. https://doi.org/10.3390/ijms22126405
APA StyleMontes-Casado, M., Ojeda, G., Criado, G., Rojo, J. M., & Portolés, P. (2021). The PI-3-Kinase P110α Catalytic Subunit of T Lymphocytes Modulates Collagen-Induced Arthritis. International Journal of Molecular Sciences, 22(12), 6405. https://doi.org/10.3390/ijms22126405






