Effect of Exogenous Auxin Treatment on Cell Wall Polymers of Strawberry Fruit
Abstract
1. Introduction
2. Results and Discussion
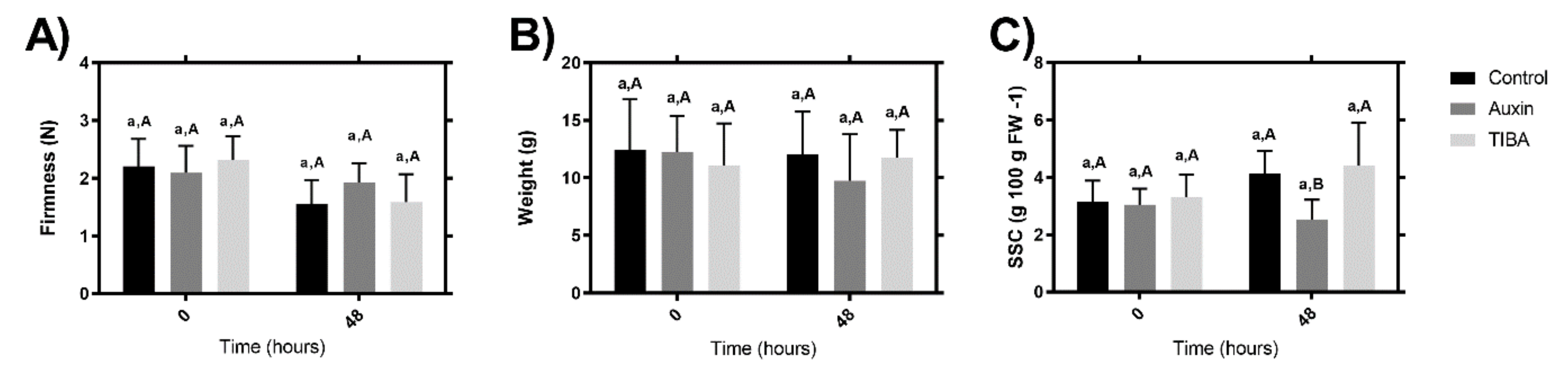
2.1. Fruit Quality Determinations
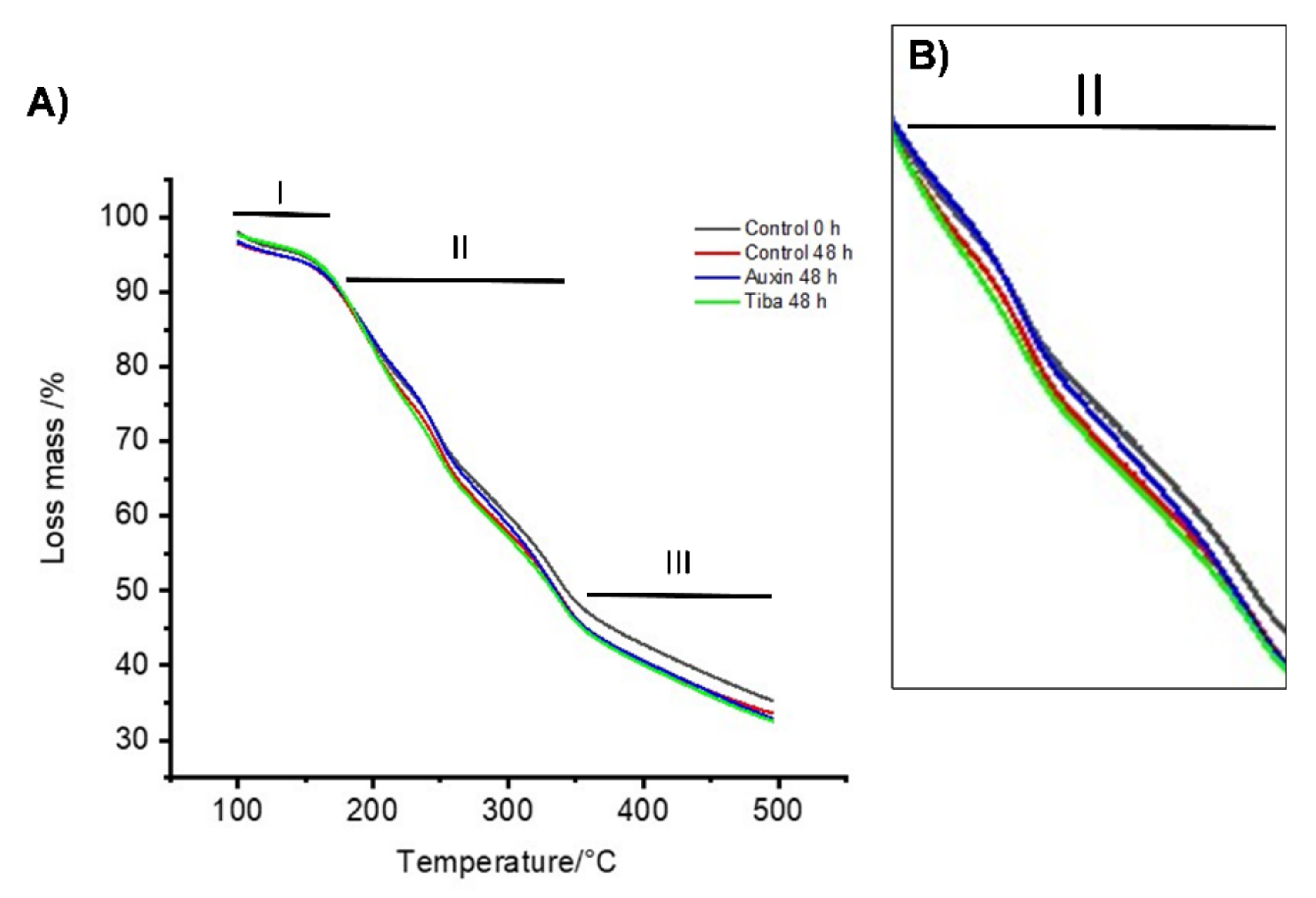
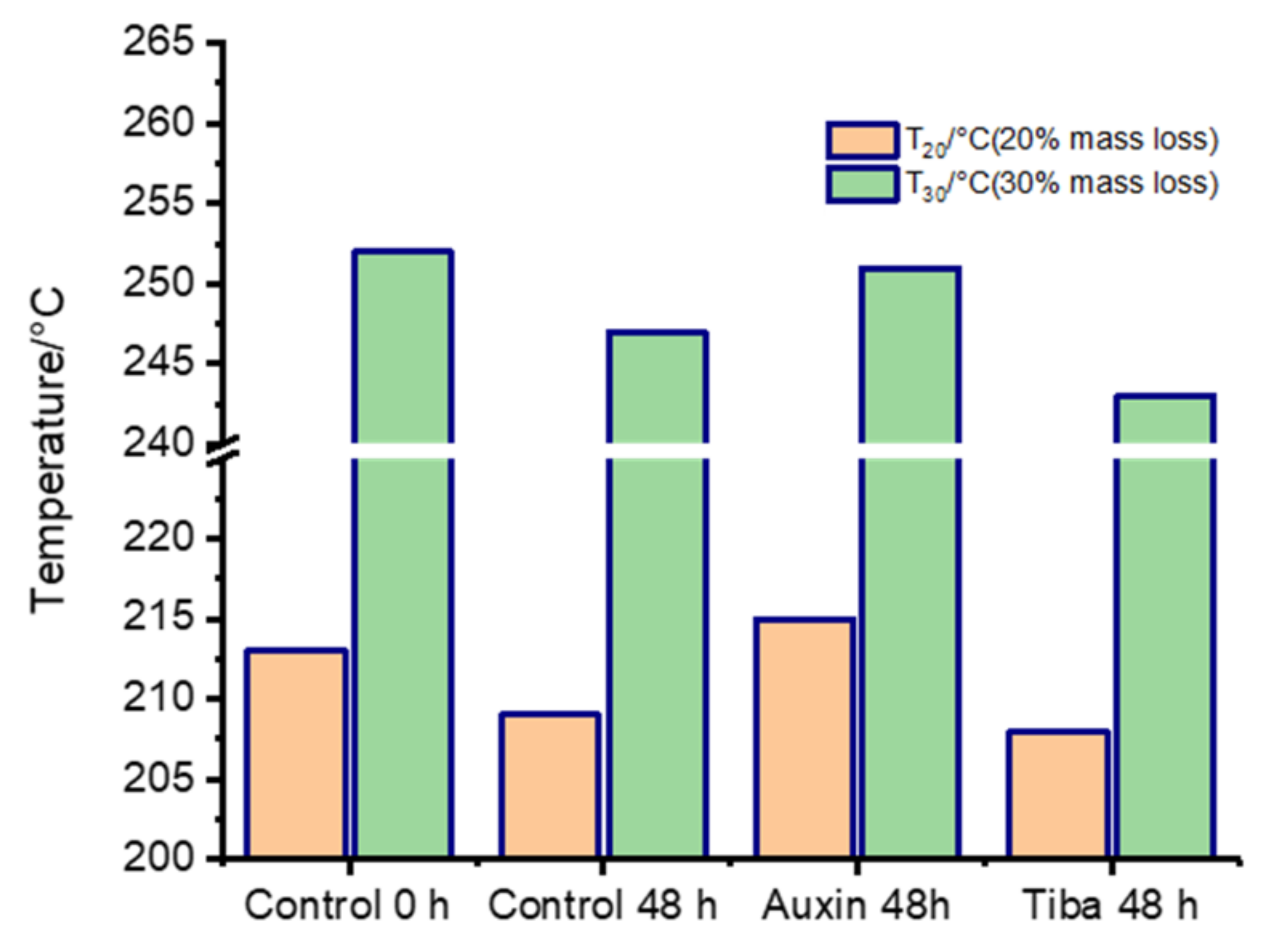
2.2. Thermal Stability Analysis of the Treated Fruits
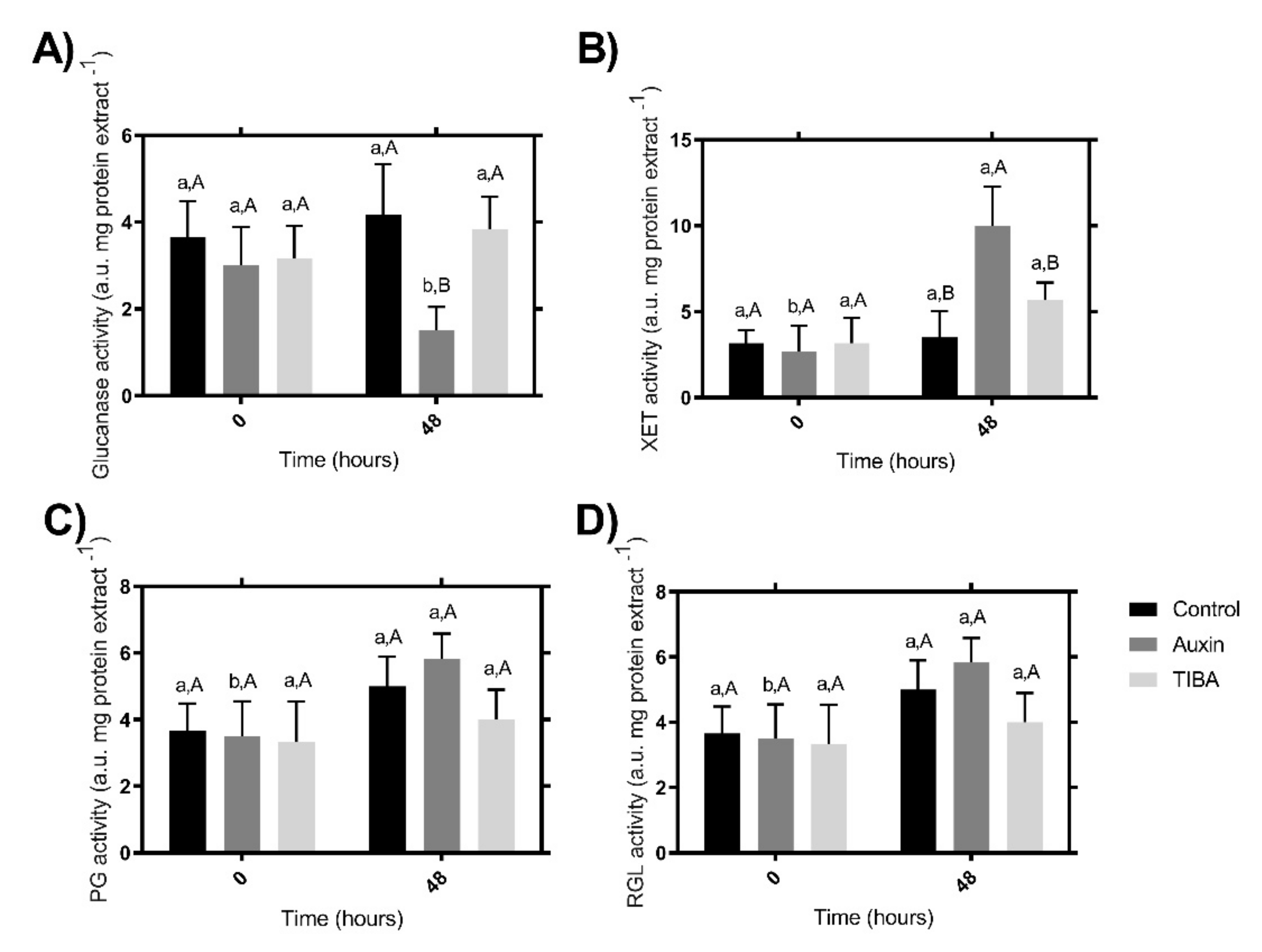
2.3. Enzymatic Activity
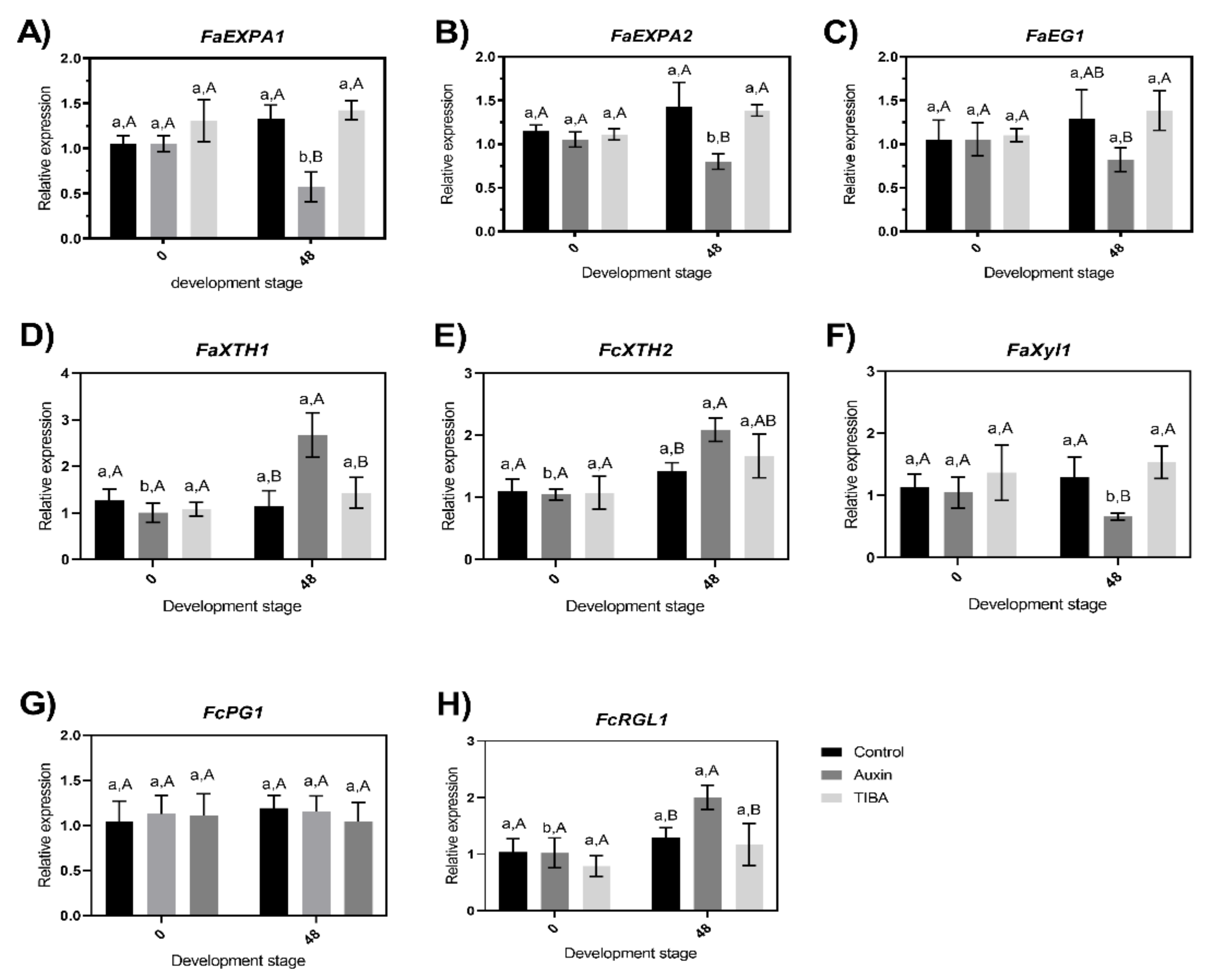
2.4. Evaluation of Changes in the Transcriptional Accumulation Level of Cell Wall-Related Genes
3. Materials and Methods
3.1. Plant Material
3.2. Auxin and TIBA Treatments
3.3. Determination of Fruit Quality Parameters
3.4. Evaluation of the Thermal Stability Using Thermogravimetric Analysis (TGA)
3.5. RNA Isolation, Reverse Transcription, and RT-qPCR Analysis
3.6. Total Enzymatic Activity Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pattison, R.J.; Csukasi, F.; Catalá, C. Mechanisms regulating auxin action during fruit development. Physiol. Plant. 2014, 151, 62–72. [Google Scholar] [CrossRef]
- Estrada-Johnson, E.; Csukasi, F.; Pizarro, C.M.; Vallarino, J.G.; Kiryakova, Y.; Vioque, A.; Brumos, J.; Medina-Escobar, N.; Botella, M.A.; Alonso, J.M.; et al. Transcriptomic Analysis in Strawberry Fruits Reveals Active Auxin Biosynthesis and Signaling in the Ripe Receptacle. Front. Plant Sci. 2017, 8, 889. [Google Scholar] [CrossRef]
- Trainotti, L.; Tadiello, A.; Casadoro, G. The involvement of auxin in the ripening of climacteric fruits comes of age: The hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 2007, 58, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, J.P. Growth and morphogenesis of the strawberry as related to auxin. Am. J. Bot. 1950, 37, 211–215. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef]
- Davies, C.; Boss, P.K.; Robinson, S.P. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997, 115, 1155–1161. [Google Scholar] [CrossRef]
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Leng, P.; Zhang, G.; Li, X. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J. Plant Physiol. 2009, 166, 1241–1252. [Google Scholar] [CrossRef]
- Jia, H.-F.; Chai, Y.-M.; Li, C.-L.; Lu, D.; Luo, J.-J.; Qin, L.; Shen, Y.-Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-F.; Songtao, J.; Cheng, Z.; Chen, W.; Pervaiz, T.; Zhongjie, L.; Baoju, W.; Liwen, C.; Jinggui, F. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Zhang, Q.; Xing, Y.; Yan, J.; Liu, L.; Nie, K. A development-associated decrease in osmotic potential contributes to fruit ripening initiation in strawberry (Fragaria x ananassa). Front. Plant Sci. 2020, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, e11542–e11550. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Jia, S.; Huang, X.; Wang, L.; Fu, W.; Huo, G.; Ding, J.; Li, Y. Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening. Planta 2019, 250, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Riffo, F.; Parra-Palma, C.; Ramos, P.; Morales-Quintana, L. Molecular and structural insights into FaEXPA5, an alpha-expansin protein related with cell wall disassembly during ripening of strawberry fruit. Plant Physiol. Biochem. 2020, 154, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.I.; Gonzalez-Feliu, A.; Valenzuela-Riffo, F.; Parra-Palma, C.; Morales-Quintana, L. Changes in the cell wall components produced by exogenous abscisic acid treatment in strawberry fruit. Cellulose 2021, 28, 1555–1570. [Google Scholar] [CrossRef]
- Li, H.; Mao, W.J.; Liu, W.; Dai, H.Y.; Liu, Y.X.; Ma, Y.; Zhang, Z.H. Deep sequencing discovery of novel and conserved microRNAs in wild type and a white-flesh mutant strawberry. Planta 2013, 238, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Parra-Palma, C.; Figueroa, C.R.; Zuñiga, P.E.; Valenzuela-Riffo, F.; Gonzalez, J.; Gaete-Eastman, C.; Morales-Quintana, L. Cell wall-related enzymatic activities and transcriptional profiles in four strawberries (Fragaria x ananassa) cultivars during fruit development and ripening. Sci. Hortic. 2018, 238, 325–332. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Ramos, P. Chilean strawberry (Fragaria chiloensis): An integrative and comprehensive review. Food Res. Int. 2019, 119, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jung, H.; Win, N.M.; Kwon, J.-G.; Cho, Y.-J.; Jung, H.-Y.; Lee, D.H.; Kang, I.-K. Changes in Fruit Quality Attributes, Cell Wall Materials, and Related Hydrolases Activities in 1-Methylcyclopropene (1-MCP)-treated ‘Honggeum’ Apples during Cold Storage. Hortic. Sci. Technol. 2020, 38, 870–879. [Google Scholar] [CrossRef]
- Nardi, C.F.; Villareal, N.M.; Rossi, F.R.; Martinez, S.; Martinez, G.A.; Civello, P.M. Overexpression of the carbohydrate binding module of strawberry expansin2 in Arabidopsis thaliana modifies plant growth and cell wall metabolism. Plant Mol. Biol. 2015, 88, 101–117. [Google Scholar] [CrossRef]
- Gilbert, H.J. The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol. 2010, 153, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Brumer, H., III; Baumann, M.J.; Kallas, A.M.; Henriksson, H.; Denman, S.E.; Teeri, T.T.; Jones, T.A. Crystal structures of a poplar xyloglucan endotransglycosylase reveal details of transglycosylation acceptor binding. Plant Cell 2004, 16, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Farkaš, V.; Hrmova, M.; Fincher, G.B. Xyloglucan xyloglucosyl transferases from barley (Hordeum vulgare L.) bind oligomeric and polymeric xyloglucan molecules in their acceptor binding sites. Biochim. Biophys. Acta 2010, 1800, 674–684. [Google Scholar] [CrossRef]
- Pilling, E.; Höfte, H. Feedback from the wall. Curr. Opin. Plant Biol. 2003, 6, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Enzymes and other agents that enhance cell wall extensibility. Annu. Rev. Plant Physiol. 1999, 50, 391–417. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Posé, S.; Paniagua, C.; Matas, A.J.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci. Technol. 2019, 87, 47–58. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.I.; Muñoz-Vera, M.; Morales-Quintana, L. Evaluation of Cell Wall Modification in Two Strawberry Cultivars with Contrasted Softness. Agronomy 2021, 11, 1100. [Google Scholar] [CrossRef]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [PubMed]
- Woodward, W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Mravec, J.; Kračun, S.K.; Zemlyanskaya, E.; Rydahl, M.G.; Guo, X.; Pičmanová, M.; Sørensen, K.K.; Růžička, K.; Willats, W.G.T. Click chemistry-based tracking reveals putative cell wall-located auxin binding sites in expanding cells. Sci. Rep. 2017, 7, 15988. [Google Scholar] [CrossRef]
- Medina-Escobar, N.; Cárdenas, J.; Moyano, E.; Caballero, J.L.; Muñoz-Blanco, J. Cloning, molecular characterization and expression pattern of a strawberry ripeningspecific cDNA with sequence homology to pectate lyase from higher plants. Plant. Mol. Biol. 1997, 34, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Nevado, J.; Moyano, E.; Medina-Escobar, N.; Caballero, J.L.; Muñoz-Blanco, J. A fruit-specific and developmentally regulated endopolygalacturonase gene strawberry (Fragaria × ananassa cv. Chandler). J. Exp. Bot. 2001, 52, 1941–1945. [Google Scholar] [CrossRef]
- Jiménez-Bermúdez, S.; Redondo-Nevado, J.; Muñoz-Blanco, J.; Caballero, J.L.; López-Aranda, J.M.; Valpuesta, V.; Pliego-Alfaro, F.; Quesada, M.A.; Mercado, J.A. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant. Physiol. 2002, 128, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Burraco, A.; Blanco-Portales, R.; Redondo-Nevado, J.; Bellido, M.L.; Caballero, J.L.; Muñoz-Blanco, J. Cloning and characterization of two ripening-related strawberry (Fragaria × ananassa c.v. Chandler) pectate lyase genes. J. Exp. Bot. 2003, 5, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef]
- Figueroa, C.; Gaete-Eastman, C.; Moya, M.; Herrera, R.; Caligari, P.; Moya-León, M.A. Softening rate of the Chilean strawberry (Fragaria chiloensis) fruit reflects the expression of polygalacturonase and pectate lyase genes. Postharvest Biol. Technol. 2008, 49, 210–220. [Google Scholar] [CrossRef]
- Han, Y.; Ban, Q.; Li, H.; Hou, Y.; Jin, M.; Han, S.; Rao, J. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Mattus, E.; Herrera, R. Molecular events occurring during softening of strawberry fruit. Front. Plant Sci. 2019, 10, 615. [Google Scholar] [CrossRef]
- Jara, K.; Castro, R.I.; Ramos, P.; Parra-Palma, P.; Valenzuela-Riffo, F.; Morales-Quintana, L. Molecular insights into FaEG1, a strawberry endoglucanase enzyme expressed during strawberry fruit ripening. Plants 2019, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, C.A.; Civello, P.M.; Martínez, G.A. Cloning of the promoter region of β-xylosidase (FaXyl1) gene and effect of plant growth regulators on the expression of FaXyl1 in strawberry fruit. Plant Sci. 2009, 177, 49–56. [Google Scholar] [CrossRef]
- Trainotti, L.; Pavanello, A.; Casadoro, G. Different ethylene receptors show an increased expression during the ripening of strawberries: Does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J. Exp. Bot. 2005, 56, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, N.M.; Bustamante, C.A.; Civello, P.M.; Martínez, G.A. Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J. Sci. Food Agric. 2010, 90, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, N.; Marina, M.; Nardi, C.F.; Civello, P.M.; Martínez, G. Novel insights of ethylene role in strawberry cell wall metabolism. Plant Sci. 2016, 252, 1–11. [Google Scholar] [CrossRef]
- Liu, C.; Wyman, C.E. Partial flow of compressed-hot water through corn stover to enhance hemicellulose sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2005, 96, 1978–1985. [Google Scholar] [CrossRef]
- Villarreal, N.; Martínez, G.A.; Civello, P.M. Influence of plant growth regulators on polygalacturonase expression in strawberry fruit. Plant Sci. 2009, 176, 749–757. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Bennett, A.B. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999, 4, 176–183. [Google Scholar] [CrossRef]
- Castro, R.I.; Muñoz-Vera, M.; Parra-Palma, C.; Valenzuela-Riffo, F.; Figueroa, C.R.; Morales-Quintana, L. Characterization of cell wall modification through thermogravimetric analysis during ripening of Chilean strawberry (Fragaria chiloensis) fruit. Cellulose 2021. [Google Scholar] [CrossRef]
- Ghaffari, A.; Navaee, K.; Oskoui, M.; Bayati, K.; Rafiee-Tehrani, M. Preparation and characterization of free mixed-film of pectin/chitosan/Eudragit® RS intended for sigmoidal drug delivery. Eur. J. Pharm. Biopharm. 2007, 67, 175–186. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Wang, S.R.; Fang, M.X. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit development in capsicum a nnuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, M.; Fimognari, L.; Sakuragi, Y. Interplays between the cell wall and phytohormones in interaction between plants and necrotrophic pathogens. Phytochemistry 2015, 112, 63–71. [Google Scholar] [CrossRef]
- Verma, D.P.; Maclachlan, G.A.; Byrne, H.; Ewings, D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea doiepicotyls. J. Biol. Chem. 1975, 250, 1019–1026. [Google Scholar] [CrossRef]
- Paniagua, C.; Ric-Varas, P.; García-Gago, J.A.; López-Casado, G.; Blanco-Portales, R.; Muñoz-Blanco, J.; Schückel, J.; Knox, J.P.; Matas, A.J.; Quesada, M.A.; et al. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J. Exp. Bot. 2020, 71, 7103–7117. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Langer, S.E.; Marina, M.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Villarreal, N.M. Expression profiling of endo-xylanases during ripening of strawberry cultivars with contrasting softening rates. Influence of postharvest and hormonal treatments. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hidalgo, F.J.; Franco, A.R.; Villatoro, C.; Medina-Puche, L.; Mercado, J.A.; Hidalgo, M.A.; Monfort, A.; Caballero, J.L.; Muñoz-Blanco, J.; Blanco-Portales, R. The strawberry (Fragaria x ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell wall middle lamellae. J. Exp. Bot. 2013, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, N.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biol. Technol. 2008, 47, 141–150. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Expansive growth of plant cell walls. Plant Physiol. Biochem. 2000, 38, 109–124. [Google Scholar] [CrossRef]
- Opazo, M.C.; Lizana, R.; Stappung, Y.; Davis, T.M.; Herrera, R.; Moya-León, M.A. XTHs from Fragaria vesca: Genomic structure and transcriptomic analysis in ripening fruit and other tissues. BMC Genomics 2017, 18, 852. [Google Scholar] [CrossRef]
- McQueen-Mason, S.; Cosgrove, D.J. Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proc. Natl. Acad. Sci. USA 1994, 91, 6574–6578. [Google Scholar] [CrossRef]
- Nardi, C.F.; Villarreal, N.M.; Opazo, M.C.; Martínez, G.A.; Moya-León, M.A.; Civello, P.M. Expression of FaXTH1 and FaXTH2 genes in strawberry fruit. Cloning of promoter regions and effect of plant growth regulators. Sci. Hortic. 2014, 165, 111–122. [Google Scholar] [CrossRef]
- Spolaore, S.; Trainotti, L.; Pavanello, A.; Casadoro, G. Isolation and promoter analysis of two genes encoding different endo-β-1,4-glucanases in the non-climacteric strawberry1. J. Exp. Bot. 2003, 54, 271–277. [Google Scholar] [CrossRef][Green Version]
- Castro, R.I.; Morales-Quintana, L. Study of the cell wall components produced during different ripening stages through thermogravimetric analysis. Cellulose 2019, 26, 3009–3020. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of realtime quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Accession Number | Primer Forward/Reverse |
|---|---|---|
| FaEXPA1 | AF163812 | 5′-AACTTCTGCCCTCCCAACTT-3′ |
| 5′-TGAACCTGATCCCACCCTTC-3′ | ||
| FaEXPA2a | AF159563 | 5′-CCGAGTTACTATTTGCGGTGA-3′ |
| 5′-CACGTTGCCTCTCCCTAATC-3′ | ||
| FaXTH1b | GQ367550 | 5′-ACTCTGCTCTTGAGCATAGTGC-3′ |
| 5′-GAGCTGAATCTCATTGCCACC3-3′ | ||
| FaXTH2b | GQ367551 | 5′-AGCTTTCTTTTGGGTTCTCTCTC-3′ |
| 5′-CCTTAACAACCAAAGCAGATGGT-3′ | ||
| FaPG1a | AY282613 | 5′-CGCCTCTTGCTTGTGCTAC-3′ |
| 5′-TCACACTGCATTGATCTCACC-3′ | ||
| FaEG1c | AJ414709 | 5′-CCACGGGCTCTATCAAAATC-3 |
| 5′-TGGCCTTCGAAGAAGAGG-3′ | ||
| FaRGL1d | CO381780.1 | 5′-TCCCTGATCGCTCAGCTGCCGA-3′ |
| 5′-TCGTGAGAGTTGGATCCTCGTGCCG-3′ | ||
| FaXyl1 | AY486104 | 5′-ATGTACAATGGAGGCCAAGC-3′ |
| 5′-GCCATTCCAATTGTCGAGAT-3′ | ||
| FaGAPDH1b | AB363963 | 5′-TCCATCACTGCCACCCAGAAGACTG-3′ |
| 5′-AGCAGGCAGAACCTTTCCGACAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, R.I.; González-Feliu, A.; Muñoz-Vera, M.; Valenzuela-Riffo, F.; Parra-Palma, C.; Morales-Quintana, L. Effect of Exogenous Auxin Treatment on Cell Wall Polymers of Strawberry Fruit. Int. J. Mol. Sci. 2021, 22, 6294. https://doi.org/10.3390/ijms22126294
Castro RI, González-Feliu A, Muñoz-Vera M, Valenzuela-Riffo F, Parra-Palma C, Morales-Quintana L. Effect of Exogenous Auxin Treatment on Cell Wall Polymers of Strawberry Fruit. International Journal of Molecular Sciences. 2021; 22(12):6294. https://doi.org/10.3390/ijms22126294
Chicago/Turabian StyleCastro, Ricardo I., Ana González-Feliu, Marcelo Muñoz-Vera, Felipe Valenzuela-Riffo, Carolina Parra-Palma, and Luis Morales-Quintana. 2021. "Effect of Exogenous Auxin Treatment on Cell Wall Polymers of Strawberry Fruit" International Journal of Molecular Sciences 22, no. 12: 6294. https://doi.org/10.3390/ijms22126294
APA StyleCastro, R. I., González-Feliu, A., Muñoz-Vera, M., Valenzuela-Riffo, F., Parra-Palma, C., & Morales-Quintana, L. (2021). Effect of Exogenous Auxin Treatment on Cell Wall Polymers of Strawberry Fruit. International Journal of Molecular Sciences, 22(12), 6294. https://doi.org/10.3390/ijms22126294








