Structure, Assembly and Function of Cuticle from Mechanical Perspective with Special Focus on Perianth
Abstract
1. Introduction
2. Structure, Chemical Composition and Assembly of Cuticle
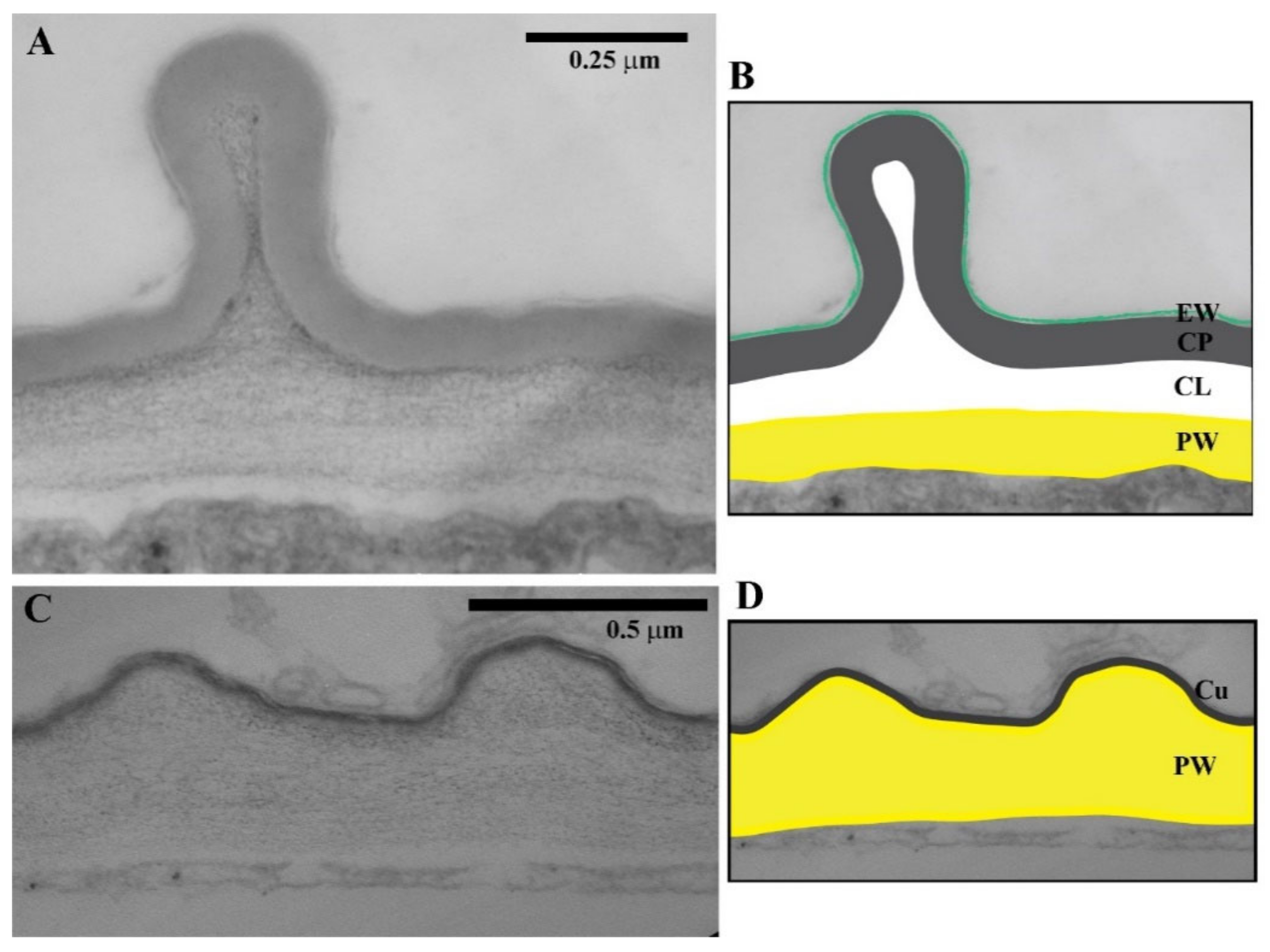
2.1. Cuticle Structure
2.2. Chemical Composition of Cuticle
2.3. Assembly of Cuticle on Organ Surface
2.3.1. Transfer of Cutin and Wax Precursors from Protoplast to Their Destination in Cell Wall
2.3.2. Final Assembly of Cuticle
2.3.3. Cutinsomes
2.3.4. Addition of New Material to Cuticle: Superimposition and Intussusception
3. Sculpture of Epidermal Surface—Its Variation and Origin
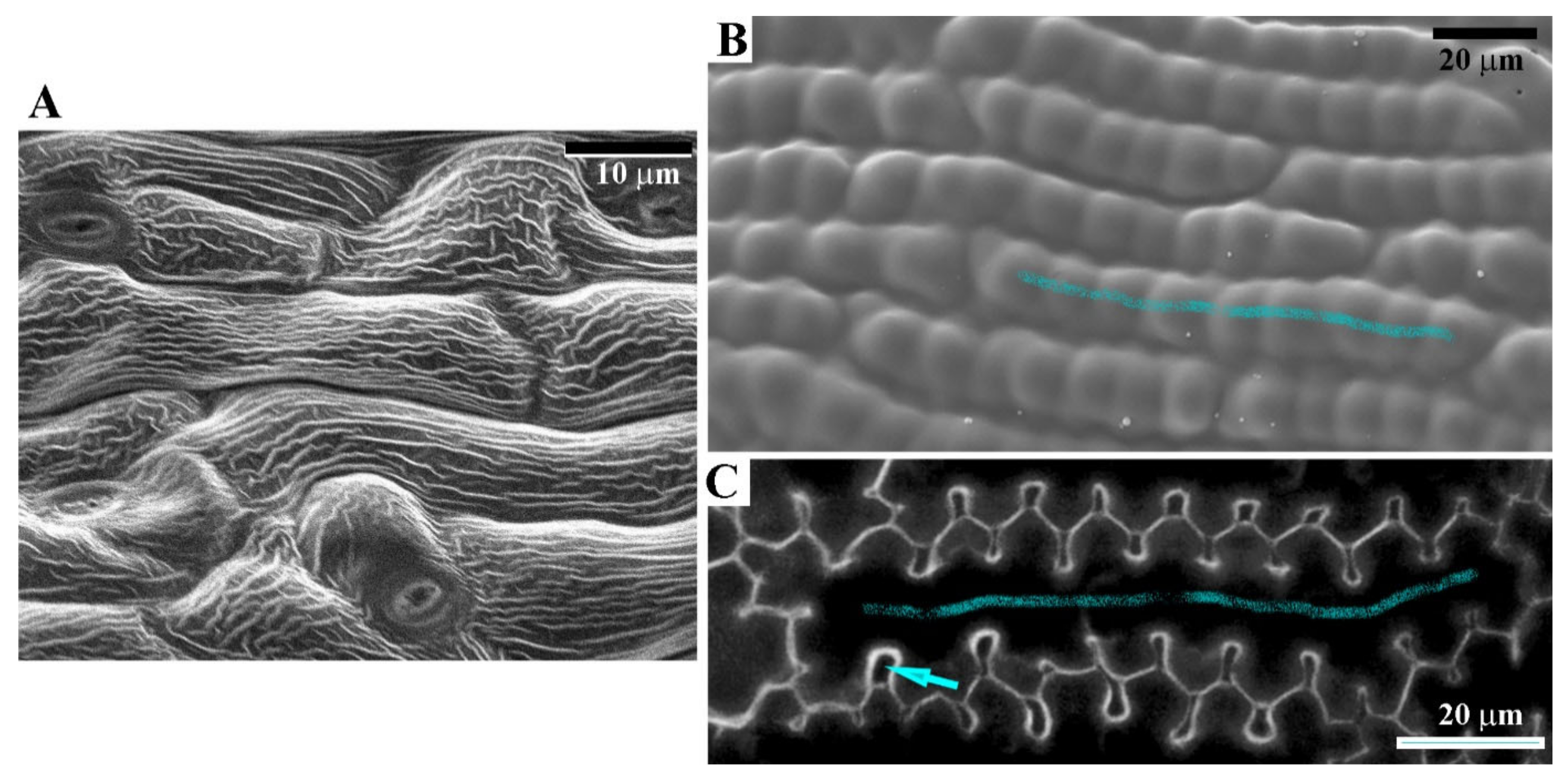
3.1. Sculpture of Perianth Surface Is Complex and Hierarchical
3.2. Empirical Studies and Modelling Suggest That Cuticle Folds Itself Due to Buckling
4. Cuticle Functions—General or Specific to Perianth Epidermis
5. Cuticle Mechanics and Mechanics-Related Functions
5.1. In Biomechanical Terms Cuticle Is a Layer of Composite Material Showing Viscoelastic Behavior
5.2. Mechanical Properties of Cuticle Depend on Its Structure and Chemical Composition and Are Changing during Organ Development
5.3. Cuticle Contributes to Stiff Faces of Plant Organs That Exhibit a Sandwich-Like Structure
5.4. Cuticle Contributes to Heterogeneity in Mechanical Status of the Epidermis Surface
6. Concluding Remarks and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niklas, K.J. Plant. Biomechanics: An Engineering Approach to Plant Form and Function; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Philippe, G.; Sorensen, I.; Jiao, C.; Sun, X.; Fei, Z.; Domozych, D.S.; Rose, J.K. Cutin and suberin: Assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef]
- Metcalfe, C.; Chalk, L. Anatomy of the Dicotyledon, Systematic Anatomy of the Leaf and Stem; Clarendon Press: Oxford, UK, 1979; Volume 1. [Google Scholar]
- Fernandez, V.; Guzman-Delgado, P.; Graca, J.; Santos, S.; Gil, L. Cuticle Structure in Relation to Chemical Composition: Re-assessing the Prevailing Model. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Jeffree, C.E. The fine structure of the plant cuticle. Annu. Plant Rev. 2006, 23, 11–125. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Guzman, P.; Fernandez, V.; Graca, J.; Cabral, V.; Kayali, N.; Khayet, M.; Gil, L. Chemical and structural analysis of Eucalyptus globulus and E. camaldulensis leaf cuticles: A lipidized cell wall region. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Guzman, P.; Fernandez, V.; Garcia, M.L.; Khayet, M.; Fernandez, A.; Gil, L. Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant. Physiol. Biochem. 2014, 76. [Google Scholar] [CrossRef]
- Philippe, G.; Geneix, N.; Petit, J.; Guillon, F.; Sandt, C.; Rothan, C.; Lahaye, M.; Marion, D.; Bakan, B. Assembly of tomato fruit cuticles: A cross-talk between the cutin polyester and cell wall polysaccharides. New Phytol. 2020, 226, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Fich, E.A.; Segerson, N.A.; Rose, J.K. The Plant Polyester Cutin: Biosynthesis, Structure, and Biological Roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef]
- Mazurek, S.; Garroum, I.; Daraspe, J.; de Bellis, D.; Olsson, V.; Mucciolo, A.; Butenko, M.A.; Humbel, B.M.; Nawrath, C. Connecting the Molecular Structure of Cutin to Ultrastructure and Physical Properties of the Cuticle in Petals of Arabidopsis. Plant Physiol. 2017, 173, 1146–1163. [Google Scholar] [CrossRef]
- Leide, J.; Nierop, K.G.J.; Deininger, A.C.; Staiger, S.; Riederer, M.; de Leeuw, J.W. Leaf cuticle analyses: Implications for the existence of cutan/non-ester cutin and its biosynthetic origin. Ann. Bot. 2020, 126, 141–162. [Google Scholar] [CrossRef]
- Boom, A.; Damsté, J.S.S.; de Leeuw, J.W. Cutan, a common aliphatic biopolymer in cuticles of drought-adapted plants. Org. Geochem. 2005, 36, 595–601. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Nawrath, C. Unraveling the complex network of cuticular structure and function. Curr. Opin. Plant Biol. 2006, 9, 281–287. [Google Scholar] [CrossRef]
- Diarte, C.; de Souza, A.X.; Staiger, S.; Deininger, A.C.; Bueno, A.; Burghardt, M.; Graell, J.; Riederer, M.; Lara, I.; Leide, J. Compositional, structural and functional cuticle analysis of Prunus laurocerasus L. sheds light on cuticular barrier plasticity. Plant Physiol. Biochem. 2021, 158, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.; Gray, J.E. How the stomate got his pore: Very long chain fatty acids and a structural cell wall protein sculpt the guard cell outer cuticular ledge. New Phytol. 2020, 228, 1698–1700. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tsubaki, S.; Sakamoto, M.; Watanabe, S.; Azuma, J. Growth-dependent chemical and mechanical properties of cuticular membranes from leaves of Sonneratia alba. Plant Cell Environ. 2012, 35, 1201–1210. [Google Scholar] [CrossRef]
- Shumborski, S.J.; Samuels, A.L.; Bird, D.A. Fine structure of the Arabidopsis stem cuticle: Effects of fixation and changes over development. Planta 2016, 244, 843–851. [Google Scholar] [CrossRef]
- Bourgault, R.; Matschi, S.; Vasquez, M.; Qiao, P.; Sonntag, A.; Charlebois, C.; Mohammadi, M.; Scanlon, M.J.; Smith, L.G.; Molina, I. Constructing functional cuticles: Analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves. Ann. Bot. 2020, 125, 79–91. [Google Scholar] [CrossRef]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lu, S.; Joubes, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Amid, A.; Lytovchenko, A.; Fernie, A.R.; Warren, G.; Thorlby, G.J. The sensitive to freezing3 mutation of Arabidopsis thaliana is a cold-sensitive allele of homomeric acetyl-CoA carboxylase that results in cold-induced cuticle deficiencies. J. Exp. Bot. 2012, 63, 5289–5299. [Google Scholar] [CrossRef]
- Bueno, A.; Sancho-Knapik, D.; Gil-Pelegrin, E.; Leide, J.; Peguero-Pina, J.J.; Burghardt, M.; Riederer, M. Cuticular wax coverage and its transpiration barrier properties in Quercus coccifera L. leaves: Does the environment matter? Tree Physiol. 2019, 70, 1613–1625. [Google Scholar]
- Chen, M.; Zhu, X.; Zhang, Y.; Du, Z.; Chen, X.; Kong, X.; Sun, W.; Chen, C. Drought stress modify cuticle of tender tea leaf and mature leaf for transpiration barrier enhancement through common and distinct modes. Sci. Rep. 2020, 10, 6696. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: There is more scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The formation and functions of a fundamental plant tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- Vrablova, M.; Vrabl, D.; Sokolova, B.; Markova, D.; Hronkova, M. A modified method for enzymatic isolation of and subsequent wax extraction from Arabidopsis thaliana leaf cuticle. Plant Methods 2020, 16, 129. [Google Scholar] [CrossRef]
- Suh, M.C.; Samuels, A.L.; Jetter, R.; Kunst, L.; Pollard, M.; Ohlrogge, J.; Beisson, F. Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol. 2005, 139, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues-a typical suberin and a particular cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef]
- Fabre, G.; Garroum, I.; Mazurek, S.; Daraspe, J.; Mucciolo, A.; Sankar, M.; Humbel, B.M.; Nawrath, C. The ABCG transporter PEC1/ABCG32 is required for the formation of the developing leaf cuticle in Arabidopsis. New Phytol. 2016, 209, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol. 2009, 151, 1773–1789. [Google Scholar] [CrossRef]
- Mazurek, S.; Mucciolo, A.; Humbel, B.M.; Nawrath, C. Transmission Fourier transform infrared microspectroscopy allows simultaneous assessment of cutin and cell-wall polysaccharides of Arabidopsis petals. Plant J. 2013, 74, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Bakan, B.; Marion, D. Assembly of the Cutin Polyester: From Cells to Extracellular Cell Walls. Plants 2017, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Dominguez, E.; Heredia-Guerrero, J.A.; Heredia, A. Plant cutin genesis: Unanswered questions. Trends Plant Sci. 2015, 20, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Anderson, C.T. We be jammin’: An update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot. 2016, 67, 495–502. [Google Scholar] [CrossRef]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373. [Google Scholar] [CrossRef]
- Polko, J.K.; Kieber, J.J. The Regulation of Cellulose Biosynthesis in Plants. Plant Cell 2019, 31, 282–296. [Google Scholar] [CrossRef]
- Bird, D.A. The role of ABC transporters in cuticular lipid secretion. Plant Sci. 2008, 174, 563–569. [Google Scholar] [CrossRef]
- Hurlock, A.K.; Roston, R.L.; Wang, K.; Benning, C. Lipid trafficking in plant cells. Traffic 2014, 15, 915–932. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K. The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Sci. 2008, 17, 191–198. [Google Scholar] [CrossRef]
- Benitez, J.J.; Heredia-Guerrero, J.A.; Heredia, A. Self-assembly of carboxylic acids and hydroxyl derivatives on mica. A qualitative AFM study. J. Phys. Chem. C 2007, 111, 9465–9470. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; San-Miguel, M.A.; Sansom, M.S.; Heredia, A.; Benitez, J.J. Chemical reactions in 2D: Self-assembly and self-esterification of 9(10),16-dihydroxypalmitic acid on mica surface. Langmuir 2009, 25, 6869–6874. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Dominguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef]
- Stępiński, D.; Kwiatkowska, M.; Wojtczak, A.; Polit, J.T.; Dominguez, E.; Heredia, A.; Popłońska, K. The Role of Cutinsomes in Plant Cuticle Formation. Cells 2020, 9, 1778. [Google Scholar] [CrossRef]
- Segado, P.; Heredia-Guerrero, J.A.; Heredia, A.; Dominguez, E. Cutinsomes and CUTIN SYNTHASE1 Function Sequentially in Tomato Fruit Cutin Deposition. Plant Physiol. 2020, 183, 1622–1637. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.P.; Knoche, M. Mechanical properties of cuticles and their primary determinants. J. Exp. Bot. 2017, 68, 5351–5367. [Google Scholar] [CrossRef]
- Hong, L.; Brown, J.; Segerson, N.A.; Rose, J.K.; Roeder, A.H. CUTIN SYNTHASE 2 Maintains Progressively Developing Cuticular Ridges in Arabidopsis Sepals. Mol. Plant. 2017, 10, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Rounds, C.M.; Winship, L.J. Control of cell wall extensibility during pollen tube growth. Mol. Plant 2013, 6, 998–1017. [Google Scholar] [CrossRef]
- Dumais, J. Modes of deformation of walled cells. J. Exp. Bot. 2013, 64, 4681–4695. [Google Scholar] [CrossRef]
- Wiedemann, P.; Neinhuis, C. Biomechanics of isolated plant cuticles. Bot. Acta 1998, 111, 28–34. [Google Scholar] [CrossRef]
- Lai, X.; Khanal, B.P.; Knoche, M. Mismatch between cuticle deposition and area expansion in fruit skins allows potentially catastrophic buildup of elastic strain. Planta 2016, 244, 1145–1156. [Google Scholar] [CrossRef]
- Koch, K.; Neinhuis, C.; Ensikat, H.J.; Barthlott, W. Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM). J. Exp. Bot. 2004, 55, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.J.; Droste, D.M.; Koch, K.; Barthlott, W. Hierarchically structured superhydrophobic flowers with low hysteresis of the wild pansy (Viola tricolor)—New design principles for biomimetic materials. Beilstein J. Nanotechnol. 2011, 2, 228–236. [Google Scholar] [CrossRef]
- Kay, Q.O.N.; Daoud, H.S.; Stirton, C.H. Pigment Distribution, Light-Reflection and Cell Structure in Petals. Bot. J. Linn. Soc. 1981, 83, 57–83. [Google Scholar] [CrossRef]
- Ojeda, I.; Francisco-Ortega, J.; Cronk, Q.C. Evolution of petal epidermal micromorphology in Leguminosae and its use as a marker of petal identity. Ann. Bot. 2009, 104, 1099–1110. [Google Scholar] [CrossRef]
- Martin, C.; Glover, B.J. Functional aspects of cell patterning in aerial epidermis. Curr. Opin. Plant Biol. 2007, 10, 70–82. [Google Scholar] [CrossRef]
- Quintana, A.; Albrechtová, J.; Griesbach, R.J.; Freyre, R. Anatomical and biochemical studies of anthocyanidins in flowers of Anagallis monelli L. (Primulaceae) hybrids. Sci. Hortic. Amst. 2007, 112, 413–421. [Google Scholar] [CrossRef]
- Thomas, M.M.; Rudall, P.J.; Ellis, A.G.; Savolainen, V.; Glover, B.J. Development of a complex floral trait: The pollinator-attracting petal spots of the beetle daisy, Gorteria diffusa (Asteraceae). Am. J. Bot. 2009, 96, 2184–2196. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Martens, P. Recherches sur la cuticule. Protoplasma 1933, 20, 483–515. [Google Scholar] [CrossRef]
- Smyth, D.R. Wrinkles on Sepals: Cuticular Ridges Form when Cuticle Production Outpaces Epidermal Cell Expansion. Mol. Plant 2017, 10, 540–541. [Google Scholar] [CrossRef][Green Version]
- Shi, J.X.; Malitsky, S.; de Oliveira, S.; Branigan, C.; Franke, R.B.; Schreiber, L.; Aharoni, A. SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs. PLoS Genet. 2011, 7, e1001388. [Google Scholar] [CrossRef]
- Khanal, B.P.; Grimm, E.; Finger, S.; Blume, A.; Knoche, M. Intracuticular wax fixes and restricts strain in leaf and fruit cuticles. New Phytol. 2013, 200, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kourounioti, R.L.A.; Band, L.R.; Fozard, J.A.; Hampstead, A.; Lovrics, A.; Moyroud, E.; Vignolini, S.; King, J.R.; Jensen, O.E.; Glover, B.J. Buckling as an origin of ordered cuticular patterns in flower petals. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hai, Y.; Xie, W.-H. Anisotropic cell growth-regulated surface micropatterns in flower petals. Theor. Appl. Mech. Lett. 2017, 7, 169–174. [Google Scholar] [CrossRef]
- Dumais, J.; Shaw, S.L.; Steele, C.R.; Long, S.R.; Ray, P.M. An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. Int. J. Dev. Biol. 2006, 50, 209–222. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nanomicro Lett. 2017, 9, 23. [Google Scholar] [CrossRef]
- Krauss, P.; Markstadter, C.; Riederer, M. Attenuation of UV radiation by plant cuticles from woody species. Plant Cell Environ. 1997, 20, 1079–1085. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef] [PubMed]
- Bargel, H.; Neinhuis, C. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Metraux, J.-P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Sieber, P.; Schorderet, M.; Ryser, U.; Buchala, A.; Kolattukudy, P.; Metraux, J.P.; Nawrath, C. Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 2000, 12, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Pollard, M.; Sauveplane, V.; Pinot, F.; Ohlrogge, J.; Beisson, F. Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. USA 2009, 106, 22008–22013. [Google Scholar] [CrossRef]
- Isaacson, T.; Kosma, D.K.; Matas, A.J.; Buda, G.J.; He, Y.; Yu, B.; Pravitasari, A.; Batteas, J.D.; Stark, R.E.; Jenks, M.A.; et al. Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. Plant J. 2009, 60, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Reussing, K.; Riederer, M.; Vogg, G. The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: Effects of a deficiency in a beta-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol. 2007, 144, 1667–1679. [Google Scholar] [CrossRef]
- Bräuer, P.; Neinhuis, C.; Voigt, D. Attachment of honeybees and greenbottle flies to petal surfaces. Arthropod Plant Interact. 2016, 11, 171–192. [Google Scholar] [CrossRef]
- Whitney, H.M.; Federle, W. Biomechanics of plant-insect interactions. Curr. Opin. Plant Biol. 2013, 16, 105–111. [Google Scholar] [CrossRef]
- Gorb, E.V.; Gorb, S.N. Anti-adhesive effects of plant wax coverage on insect attachment. J. Exp. Bot. 2017, 68, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Matschi, S.; Vasquez, M.F.; Bourgault, R.; Steinbach, P.; Chamness, J.; Kaczmar, N.; Gore, M.A.; Molina, I.; Smith, L.G. Structure-function analysis of the maize bulliform cell cuticle and its potential role in dehydration and leaf rolling. Plant Direct 2020, 4, e00282. [Google Scholar] [CrossRef]
- Leyton, L.; Juniper, B.E. Cuticle Structure and Water Relations of Pine Needles. Nature 1963, 198, 770–771. [Google Scholar] [CrossRef]
- Weng, H.; Molina, I.; Shockey, J.; Browse, J. Organ fusion and defective cuticle function in a lacs1 lacs2 double mutant of Arabidopsis. Planta 2010, 231, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, A.; Leide, J.; Riederer, M. Deficiency in a very-long-chain fatty acid beta-ketoacyl-coenzyme a synthase of tomato impairs microgametogenesis and causes floral organ fusion. Plant Physiol. 2013, 161, 196–209. [Google Scholar] [CrossRef]
- Borodich, F.M.; Gorb, E.V.; Gorb, S.N. Fracture behaviour of plant epicuticular wax crystals and its role in preventing insect attachment: A theoretical approach. Appl. Phys. A 2010, 100, 63–71. [Google Scholar] [CrossRef]
- Jetter, R. Examination of the processes involved in the emission of scent volatiles from flowers. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2006; pp. 125–144. [Google Scholar]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Hager, D.; Jetter, R. Wax layers on Cosmos bipinnatus petals contribute unequally to total petal water resistance. Plant Physiol. 2015, 167, 80–88. [Google Scholar] [CrossRef]
- Buschhaus, C.; Peng, C.; Jetter, R. Very-long-chain 1,2- and 1,3-bifunctional compounds from the cuticular wax of Cosmos bipinnatus petals. Phytochemistry 2013, 91, 249–256. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, H.; Zhou, L.; He, S.; Zhang, Y.; Cheng, X. Chemical composition and water permeability of the cuticular wax barrier in rose leaf and petal: A comparative investigation. Plant Physiol. Biochem. 2019, 135, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202. [Google Scholar] [CrossRef]
- Neinhuis, C. Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Agati, G.; Cerovic, Z.G. Optical properties of plant surfaces. In Annual Plant Reviews; Riederer, M., Muller, C., Eds.; Blackwell Publishing: Oxford, UK, 2008; Volume 3, pp. 216–249. [Google Scholar]
- Goodwin, S.M.; Kolosova, N.; Kish, C.M.; Wood, K.V.; Dudareva, N.; Jenks, M.A. Cuticle characteristics and volatile emissions of petals in Antirrhinum majus. Physiol. Plant. 2003, 117, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Glover, B.J.; Whitney, H.M. Structural colour and iridescence in plants: The poorly studied relations of pigment colour. Ann. Bot. 2010, 105, 505–511. [Google Scholar] [CrossRef]
- Whitney, H.M.; Kolle, M.; Andrew, P.; Chittka, L.; Steiner, U.; Glover, B.J. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 2009, 323, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Whitney, H.M.; Bennett, K.M.; Dorling, M.; Sandbach, L.; Prince, D.; Chittka, L.; Glover, B.J. Why do so many petals have conical epidermal cells? Ann. Bot. 2011, 108, 609–616. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Z.; Yan, W.; Xie, G.; Lu, J.; Wang, N.; Lu, Q.; Yao, N.; Yang, G.; Xia, J.; et al. An ABC transporter, OsABCG26, is required for anther cuticle and pollen exine formation and pollen-pistil interactions in rice. Plant Sci. 2016, 253, 21–30. [Google Scholar] [CrossRef]
- Verbeke, J.A. Fusion Events during Floral Morphogenesis. Annu. Rev. Plant Phys. 1992, 43, 583–598. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S.; Hinz, M.; Bukovac, M.J. Studies on water transport through the sweet cherry fruit surface: II. Conductance of the cuticle in relation to fruit development. Planta 2001, 213, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Peschel, S.; Beyer, M.; Knoche, M. Surface characteristics of sweet cherry fruit: Stomata-number, distribution, functionality and surface wetting. Sci. Hortic. Amst. 2003, 97, 265–278. [Google Scholar] [CrossRef]
- Veraverbeke, E.A.; Verboven, P.; Scheerlinck, N.; lan Hoang, M.; Nicolaï, B.M. Determination of the diffusion coefficient of tissue, cuticle, cutin and wax of apple. J. Food Eng. 2003, 58, 285–294. [Google Scholar] [CrossRef]
- Lechaudel, M.; Lopez-Lauri, F.; Vidal, V.; Sallanon, H.; Joas, J. Response of the physiological parameters of mango fruit (transpiration, water relations and antioxidant system) to its light and temperature environment. J. Plant. Physiol. 2013, 170, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lu, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiol. Plant 2012, 146, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Vogg, G.; Fischer, S.; Leide, J.; Emmanuel, E.; Jetter, R.; Levy, A.A.; Riederer, M. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: Functional characterization of a mutant deficient in a very-long-chain fatty acid beta-ketoacyl-CoA synthase. J. Exp. Bot. 2004, 55, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.; Riederer, M. Ecophysiology of cuticular transpiration: Comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 1996, 107, 426–432. [Google Scholar] [CrossRef]
- Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 1996, 47, 1813–1832. [Google Scholar] [CrossRef]
- Bessire, M.; Borel, S.; Fabre, G.; Carraca, L.; Efremova, N.; Yephremov, A.; Cao, Y.; Jetter, R.; Jacquat, A.C.; Metraux, J.P.; et al. A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the formation of a functional cuticle in Arabidopsis. Plant Cell 2011, 23, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, V.; Bahamonde, H.A.; Peguero-Pina, J.J.; Gil-Pelegrin, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef]
- Schreiber, L.; Bach, S.; Kirsch, T.; Knoll, D.; Schalz, K.; Riederer, M. A simple photometric device analysing cuticular transport physiology: Surfactant effect on permeability of isolated cuticular membranes of Prunus laurocerasus L. J. Exp. Bot. 1995, 46, 1915–1921. [Google Scholar] [CrossRef]
- Bueno, A.; Alfarhan, A.; Arand, K.; Burghardt, M.; Deininger, A.C.; Hedrich, R.; Leide, J.; Seufert, P.; Staiger, S.; Riederer, M. Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies. J. Exp. Bot. 2019, 70, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Heredia-Guerrero, J.A.; Dominguez, E.; Benitez, J.J. Cutin synthesis: A slippery paradigm. Biointerphases 2009, 4. [Google Scholar] [CrossRef]
- Riedel, M.; Eichner, A.; Meimberg, H.; Jetter, R. Chemical composition of epicuticular wax crystals on the slippery zone in pitchers of five Nepenthes species and hybrids. Planta 2007, 225, 1517–1534. [Google Scholar] [CrossRef]
- Tenberge, K.B. Morphology and Cellular Organisation in Botrytis Interactions with Plants. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 67–84. [Google Scholar]
- Bessire, M.; Chassot, C.; Jacquat, A.C.; Humphry, M.; Borel, S.; Petetot, J.M.; Metraux, J.P.; Nawrath, C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007, 26, 2158–2168. [Google Scholar] [CrossRef]
- Deising, H.B.; Werner, S.; Wernitz, M. The role of fungal appressoria in plant infection. Microbes Infect. 2000, 2, 1631–1641. [Google Scholar] [CrossRef]
- Meyer, S.; Cartelat, A.; Moya, I.; Cerovic, Z.G. UV-induced blue-green and far-red fluorescence along wheat leaves: A potential signature of leaf ageing. J. Exp. Bot. 2003, 54, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.; Iwasaki, M.; Kojima, S.; Ueno, Y.; Soma, T.; Tanaka, H.; Semiarti, E.; Machida, Y.; Machida, C. Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. Plant J. 2007, 51, 173–184. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, H.; Machida, C.; Watanabe, M.; Machida, Y. A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 2004, 37, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Meder, F.; Must, I.; Sadeghi, A.; Mondini, A.; Filippeschi, C.; Beccai, L.; Mattoli, V.; Pingue, P.; Mazzolai, B. Energy Conversion at the Cuticle of Living Plants. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Ginzberg, I.; Stern, R.A. Control of Fruit Cracking by Shaping Skin Traits—Apple as a Model. Crit. Rev. Plant Sci. 2019, 38, 401–410. [Google Scholar] [CrossRef]
- Matas, A.J.; Cobb, E.D.; Paolillo, D.J.; Niklas, K.J. Crack resistance in cherry tomato fruit correlates with correlates with cuticular membrane thickness. Hortscience 2004, 39, 1354–1358. [Google Scholar] [CrossRef]
- Koch, K.; Bennemann, M.; Bohn, H.F.; Albach, D.C.; Barthlott, W. Surface microstructures of daisy florets (Asteraceae) and characterization of their anisotropic wetting. Bioinspir. Biomim. 2013, 8, 036005. [Google Scholar] [CrossRef]
- Petracek, P.D.; Bukovac, M.J. Rheological Properties of Enzymatically Isolated Tomato Fruit Cuticle. Plant Physiol. 1995, 109, 675–679. [Google Scholar] [CrossRef]
- Burgert, I.; Fratzl, P. Mechanics of the Expanding Cell Wall. In The Expanding Cell; Verbelen, J.-P., Vissenberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 5, pp. 191–215. [Google Scholar]
- Roelofsen, P.A. On the Submicroscopic Structure of Cuticular Cell Walls. Acta Bot. Neerl. 1952, 1, 99–114. [Google Scholar] [CrossRef]
- Matas, A.J.; Lopez-Casado, G.; Cuartero, J.; Heredia, A. Relative humidity and temperature modify the mechanical properties of isolated tomato fruit cuticles. Am. J. Bot. 2005, 92, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Round, A.N.; Yan, B.; Dang, S.; Estephan, R.; Stark, R.E.; Batteas, J.D. The influence of water on the nanomechanical behavior of the plant biopolyester cutin as studied by AFM and solid-state NMR. Biophys. J. 2000, 79, 2761–2767. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Guzman-Puyol, S.; Benitez, J.J.; Athanassiou, A.; Heredia, A.; Dominguez, E. Plant cuticle under global change: Biophysical implications. Glob. Chang. Biol. 2018, 24, 2749–2751. [Google Scholar] [CrossRef]
- Bargel, H.; Koch, K.; Cerman, Z.; Neinhuis, C. Structure-function relationships of the plant cuticle and cuticular waxes—A smart material? Funct. Plant Biol. 2006, 33, 893–910. [Google Scholar] [CrossRef]
- Knoche, M.; Lang, A. Ongoing Growth Challenges Fruit Skin Integrity. Crit. Rev. Plant Sci. 2017, 36, 190–215. [Google Scholar] [CrossRef]
- Onoda, Y.; Schieving, F.; Anten, N.P. A novel method of measuring leaf epidermis and mesophyll stiffness shows the ubiquitous nature of the sandwich structure of leaf laminas in broad-leaved angiosperm species. J. Exp. Bot. 2015, 66, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Mahadevan, L. The shape of a long leaf. Proc. Natl. Acad. Sci. USA 2009, 106, 22049–22054. [Google Scholar] [CrossRef]
- Hiller, G.H. Untersuchungen über die Epidermis der Blüthenblätter. Jahrb. Wiss. Bot. 1884, 15, 411–451. [Google Scholar]
- Borowska-Wykręt, D.; Kwiatkowska, D. Folding, Wrinkling, and Buckling in Plant Cell Walls. In Plant Biomechanics: From Structure to Function at Multiple Scales; Geitmann, A., Gril, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 209–233. [Google Scholar]
- Wright, H.D.; Hossain, K.M.A. In-plane shear behaviour of profiled steel sheeting. Thin Walled Struct. 1997, 29, 79–100. [Google Scholar] [CrossRef]
- King, M.J.; Vincent, J.F.V.; Harris, W. Curling and folding of leaves of monocotyledons—A strategy for structural stiffness. N. Z. J. Bot. 1996, 34, 411–416. [Google Scholar] [CrossRef]
- Hejnowicz, Z.; Borowska-Wykręt, D. Buckling of inner cell wall layers after manipulations to reduce tensile stress: Observations and interpretations for stress transmission. Planta 2005, 220, 465–473. [Google Scholar] [CrossRef]
- Lipowczan, M.; Borowska-Wykręt, D.; Natonik-Białoń, S.; Kwiatkowska, D. Growing cell walls show a gradient of elastic strain across their layers. J. Exp. Bot. 2018, 69, 4349–4362. [Google Scholar] [CrossRef] [PubMed]
- Natonik-Białoń, S.; Borowska-Wykręt, D.; Mosca, G.; Grelowski, M.; Wrzalik, R.; Smith, R.S.; Kwiatkowska, D. Deformation of a cell monolayer due to osmotic treatment: A case study of onion scale epidermis. Botany 2020, 98, 21–36. [Google Scholar] [CrossRef]
- Khanal, B.P.; Knoche, M.; Bussler, S.; Schluter, O. Evidence for a radial strain gradient in apple fruit cuticles. Planta 2014, 240, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Routier-Kierzkowska, A.L.; Weber, A.; Kochova, P.; Felekis, D.; Nelson, B.J.; Kuhlemeier, C.; Smith, R.S. Cellular force microscopy for in vivo measurements of plant tissue mechanics. Plant Physiol. 2012, 158, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Michels, L.; Gorelova, V.; Harnvanichvech, Y.; Borst, J.W.; Albada, B.; Weijers, D.; Sprakel, J. Complete microviscosity maps of living plant cells and tissues with a toolbox of targeting mechanoprobes. Proc. Natl. Acad. Sci. USA 2020, 117, 18110–18118. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Heredia, A.; Dominguez, E.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Benitez, J.J. Cutin from agro-waste as a raw material for the production of bioplastics. J. Exp. Bot. 2017, 68, 5401–5410. [Google Scholar] [CrossRef]
- Benitez, J.J.; Osbild, S.; Guzman-Puyol, S.; Heredia, A.; Heredia-Guerrero, J.A. Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin. Polymers 2020, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889. [Google Scholar] [CrossRef]
| Organ | Function | Species | Source |
|---|---|---|---|
| Flower organs | Prevention of organ fusion | Arabidopsis thaliana | [95] |
| Solanum lycopersicon | [96] | ||
| Protection against insects | Aristolochia fimbriata | [97] | |
| Perianth | Control of organ adhesion | A. thaliana | [75,85] |
| Releasing floral scent | Clarkia breweri | [98] | |
| Antirrhinum majus | [99] | ||
| Petals | Protection against water loss | Cosmos bipinnatus | [100,101] |
| Rosa chinensis (Movie star, Tineke) | [102] | ||
| Self-cleaning | Mutisia decurrens | [103,104] | |
| Protection against UV radiation | [105] | ||
| Emission of volatiles to attract insect and enhance pollination | Antirrhinum majus | [106] | |
| Facilitation of pollinator attachment | [90] | ||
| Attraction of pollinators by optical effects (structural color, iridescence) | [107,108,109] | ||
| Pistil | Control of pollen/pistil interactions | Brassica, Arabidopsis thaliana | [82] |
| Oryza sativa | [110] | ||
| Carpel | Prevention of organ fusion | Catharanthus roseus | [111] |
| Fruit | Control of nonstomatal transpiration | Prunus avium cv. Sam | [112] |
| Control of stomatal transpiration | Prunus avium cv. Sam | [113] | |
| Protection against water loss | Malus domestica (Jonagold, Jonagored, Elstar) | [114] | |
| Mangifera Indica cv. Cogshall | [115] | ||
| Capsicum | [116] | ||
| Solanum lycopersicon | [89,117] | ||
| Malus domestica | [118] | ||
| Protection against UV radiation | Cydonia oblonga | [81] | |
| Protection against pathogens | Solanum lycopersicon | [87] | |
| Protection against insects | Prunus domestica | [97] | |
| Leaf | Protection against water loss (transpiration barrier) | [80,119] | |
| Arabidopsis thaliana | [120] | ||
| Zea mays | [121] | ||
| Olea europaea | |||
| Triticum aestivum | |||
| Citrus sinensis | |||
| Prunus laurocerasus | [122] | ||
| Vanilla planifolia | |||
| Ruellia | [118] | ||
| Protection against high temperature | Citrullus colocynthis | [123] | |
| Phoenix dactylifera | |||
| Providing superhydrophobic surface | Salvinia natans | [80] | |
| Aponogeton madagascariensis | |||
| Protection against insects | Arabidopsis thaliana | [124] | |
| Chenopodium album | [97] | ||
| Nepenthes albomarginata | [125] | ||
| Prunus avium | |||
| Protection against pathogens | Cucumis sativus | [126] | |
| Phaseolus vulgaris | |||
| Arabidopsis thaliana | [127] | ||
| [128] | |||
| Ilex aquifolium | [81] | ||
| Prunus avium | |||
| Protection against UV radiation | [105] | ||
| Triticum aestivum cv. Shango | [129] | ||
| Self-cleaning | Mutisia decurrens | [103] | |
| Nelumbo nucifera | |||
| Colocasia esculenta | |||
| Prevention of organ fusion | Arabidopsis thaliana | [130,131] | |
| Triboelectric effect | Rhododendron | [30,132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzydeł, J.; Borowska-Wykręt, D.; Kwiatkowska, D. Structure, Assembly and Function of Cuticle from Mechanical Perspective with Special Focus on Perianth. Int. J. Mol. Sci. 2021, 22, 4160. https://doi.org/10.3390/ijms22084160
Skrzydeł J, Borowska-Wykręt D, Kwiatkowska D. Structure, Assembly and Function of Cuticle from Mechanical Perspective with Special Focus on Perianth. International Journal of Molecular Sciences. 2021; 22(8):4160. https://doi.org/10.3390/ijms22084160
Chicago/Turabian StyleSkrzydeł, Joanna, Dorota Borowska-Wykręt, and Dorota Kwiatkowska. 2021. "Structure, Assembly and Function of Cuticle from Mechanical Perspective with Special Focus on Perianth" International Journal of Molecular Sciences 22, no. 8: 4160. https://doi.org/10.3390/ijms22084160
APA StyleSkrzydeł, J., Borowska-Wykręt, D., & Kwiatkowska, D. (2021). Structure, Assembly and Function of Cuticle from Mechanical Perspective with Special Focus on Perianth. International Journal of Molecular Sciences, 22(8), 4160. https://doi.org/10.3390/ijms22084160






