Analysis of Both Lipid Metabolism and Endocannabinoid Signaling Reveals a New Role for Hypothalamic Astrocytes in Maternal Caloric Restriction-Induced Perinatal Programming
Abstract
1. Introduction
2. Results
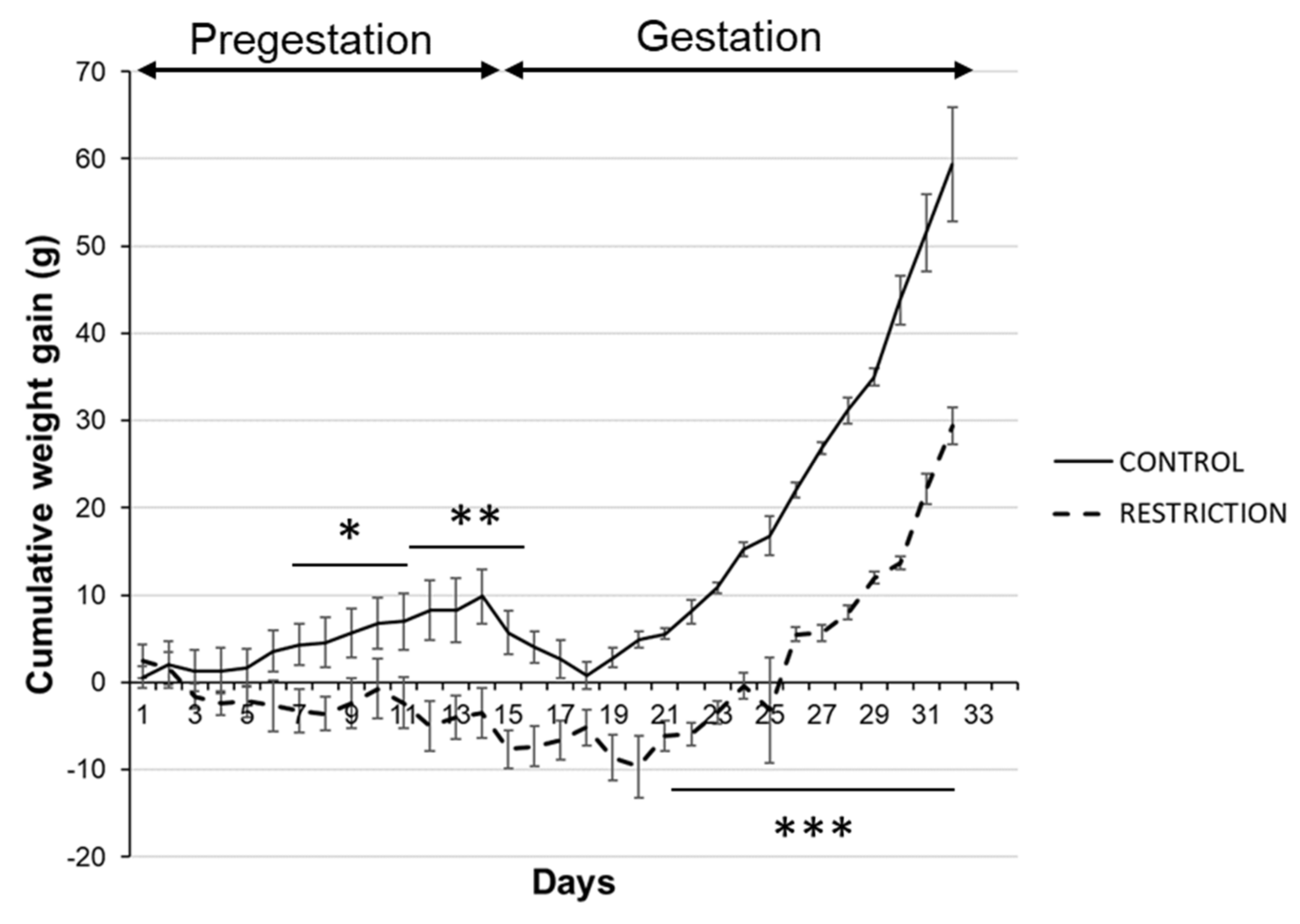
2.1. Effects of Caloric Restriction on Maternal Body Weight
2.2. Effects of Maternal Caloric Restriction on Markers of Gliosis and Neuroinflammation in PND2 Offspring Hypothalamus
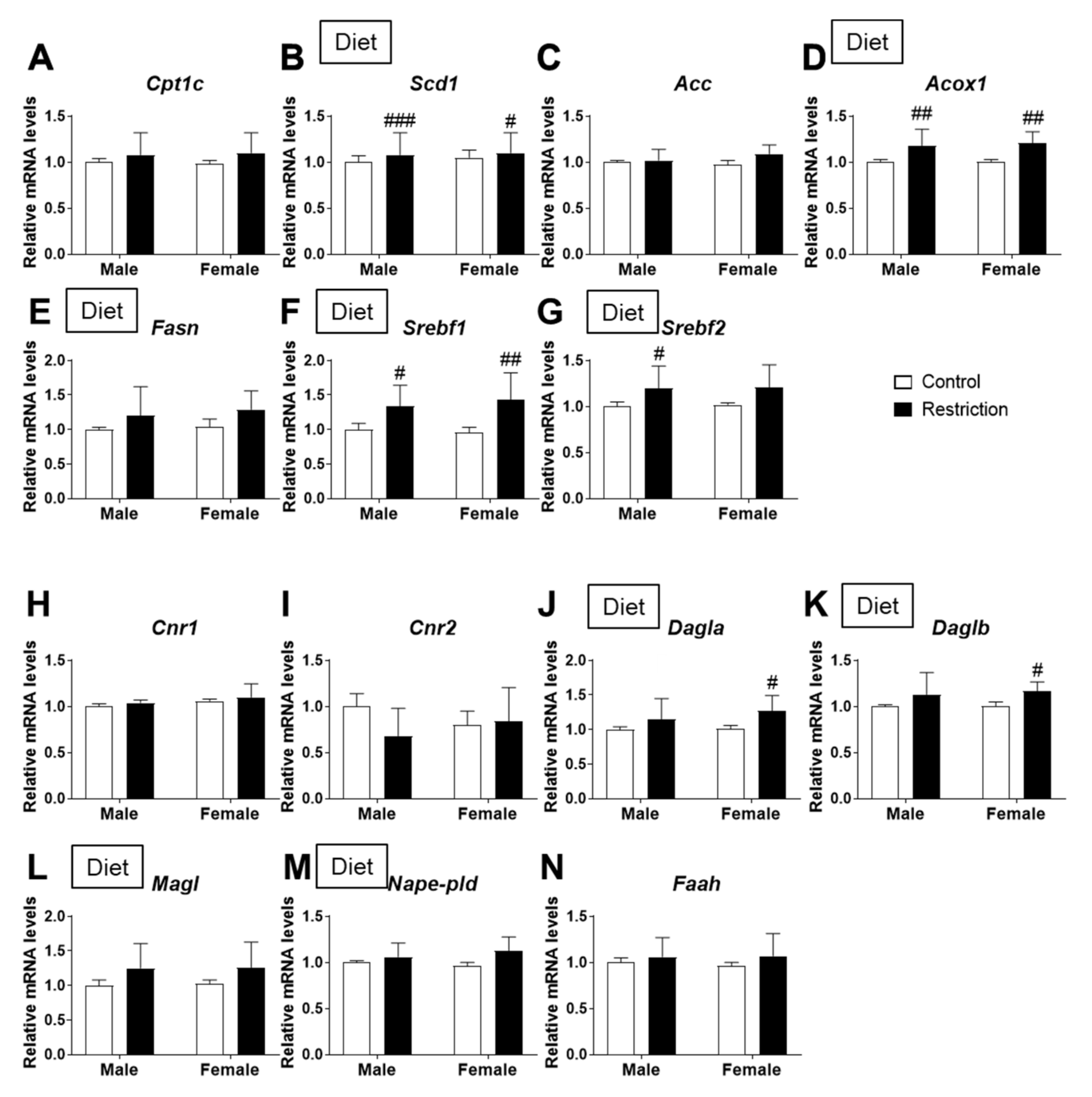
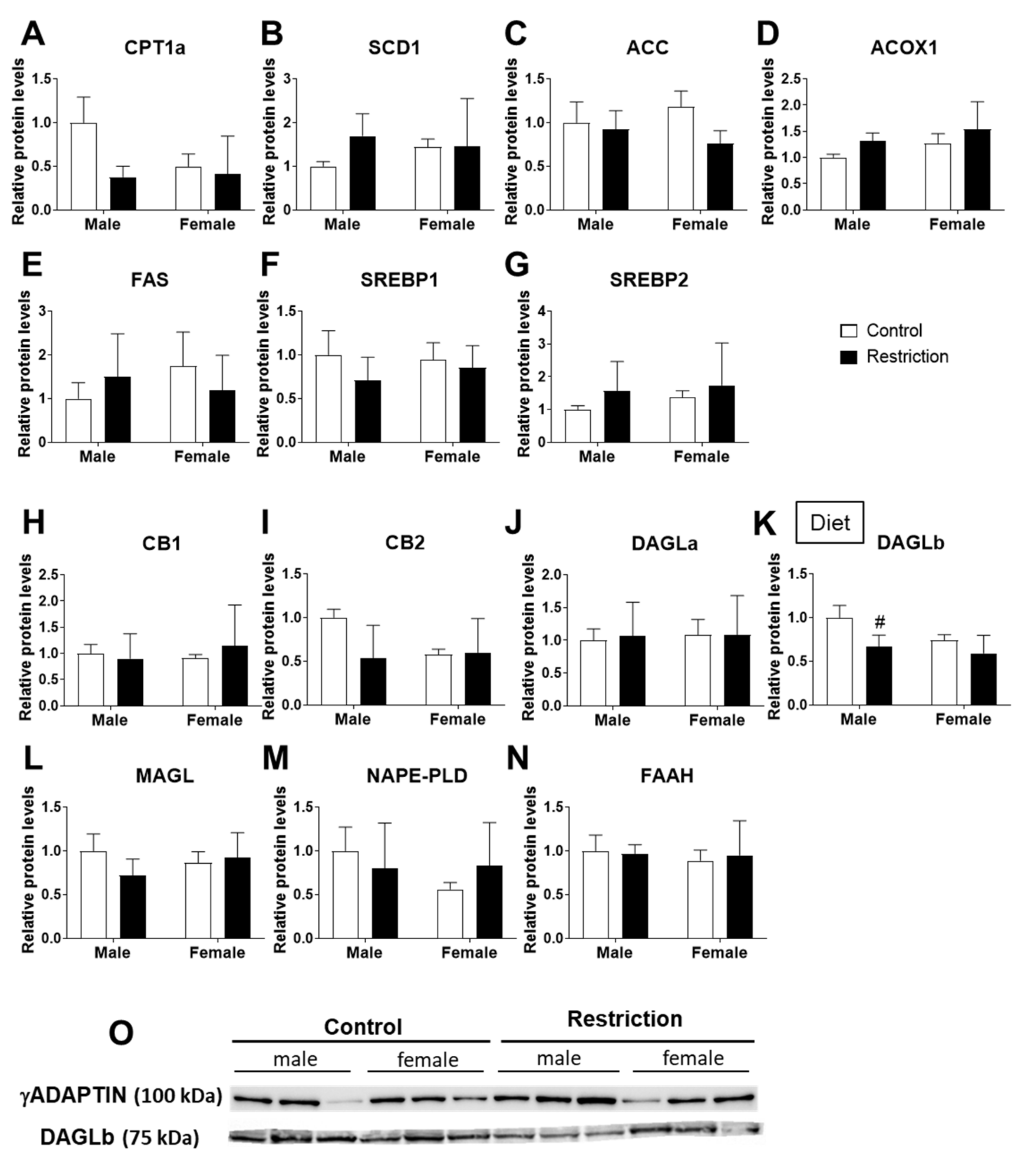
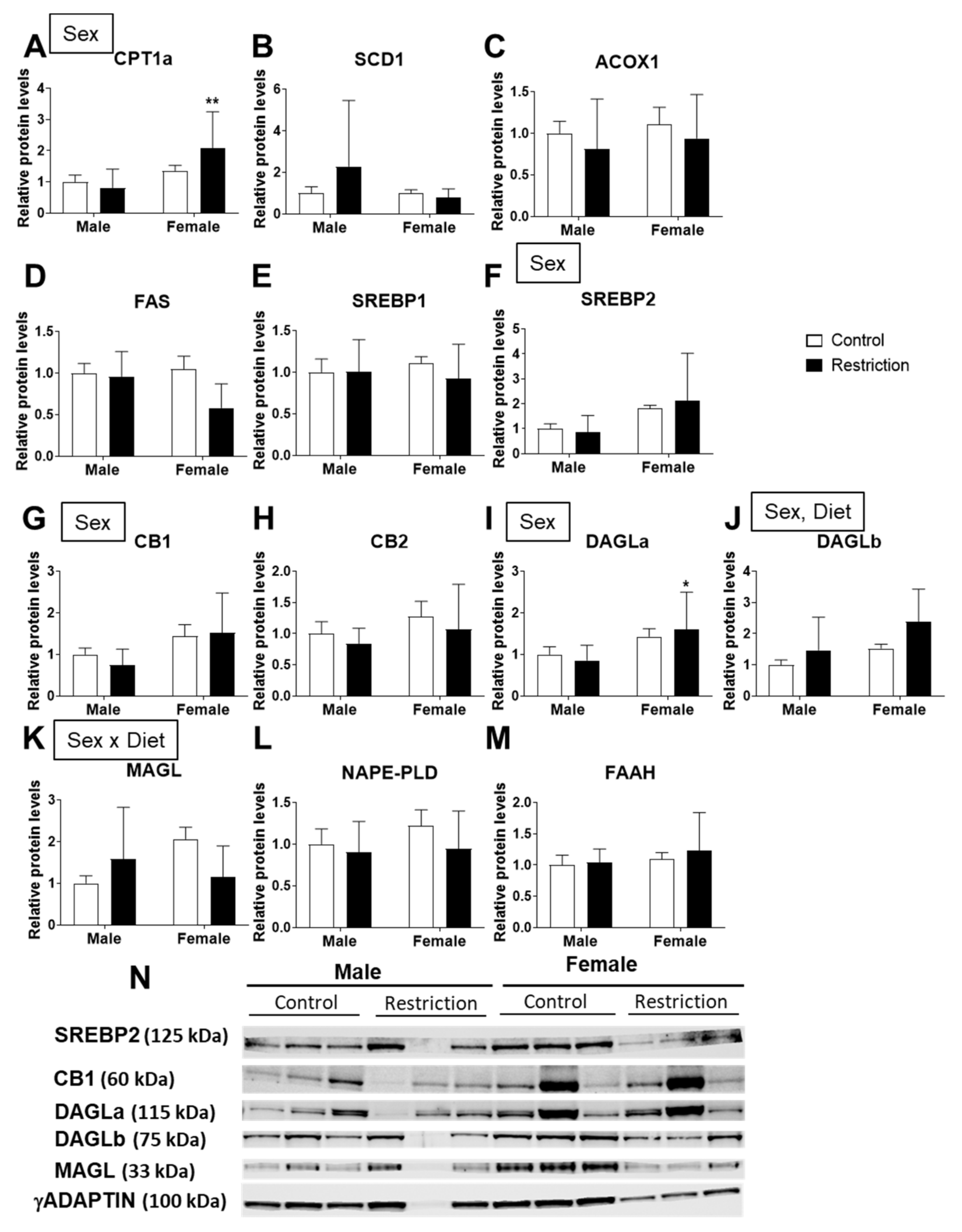
2.3. Effects of Maternal Caloric Restriction on Lipid Metabolism and Endocannabinoid Signaling in PND2 Offspring Hypothalamus
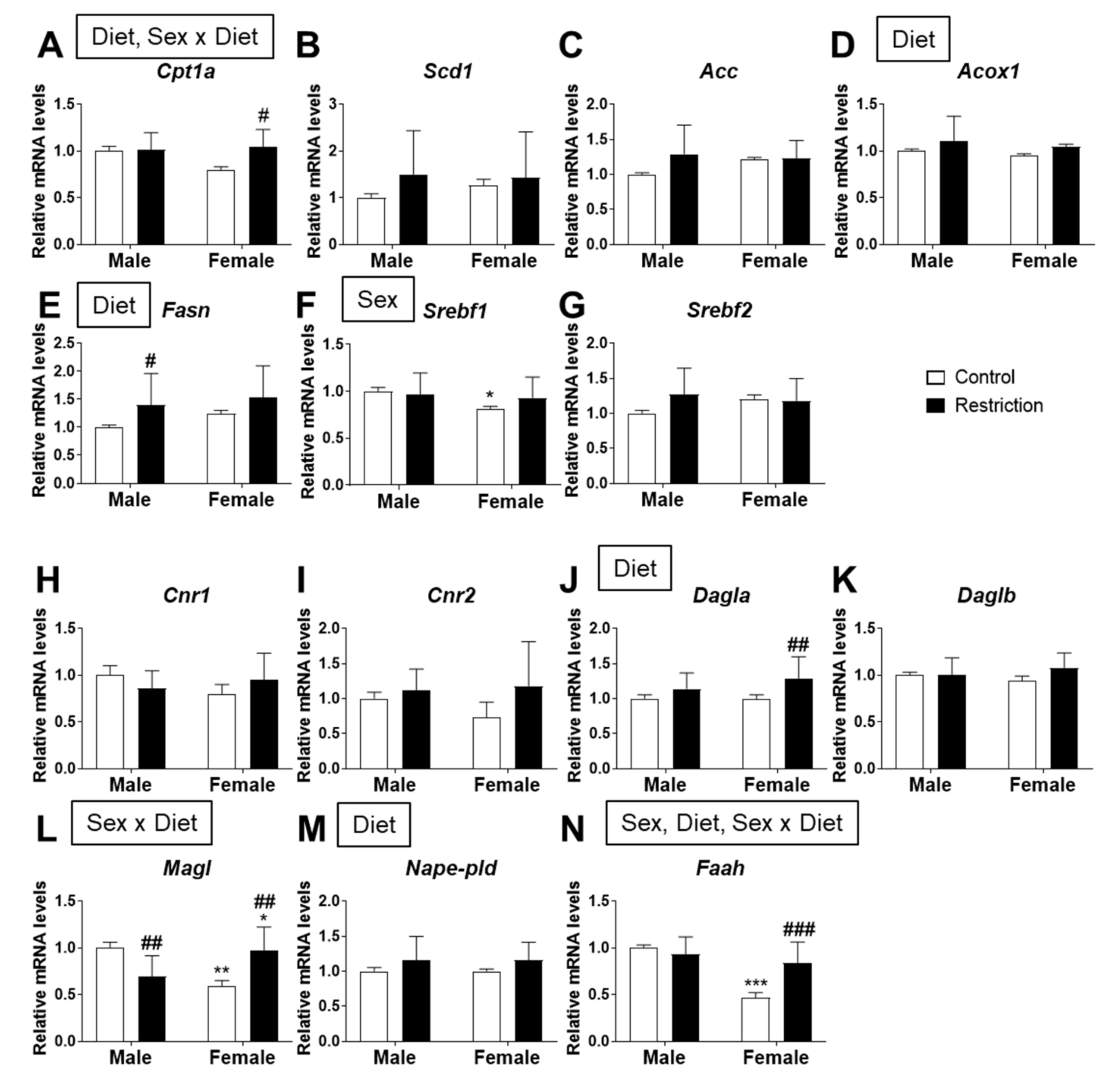
2.4. Effects of Maternal Caloric Restriction on Lipid Metabolism and Endocannabinoid Signaling in Hypothalamic Astrocytes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
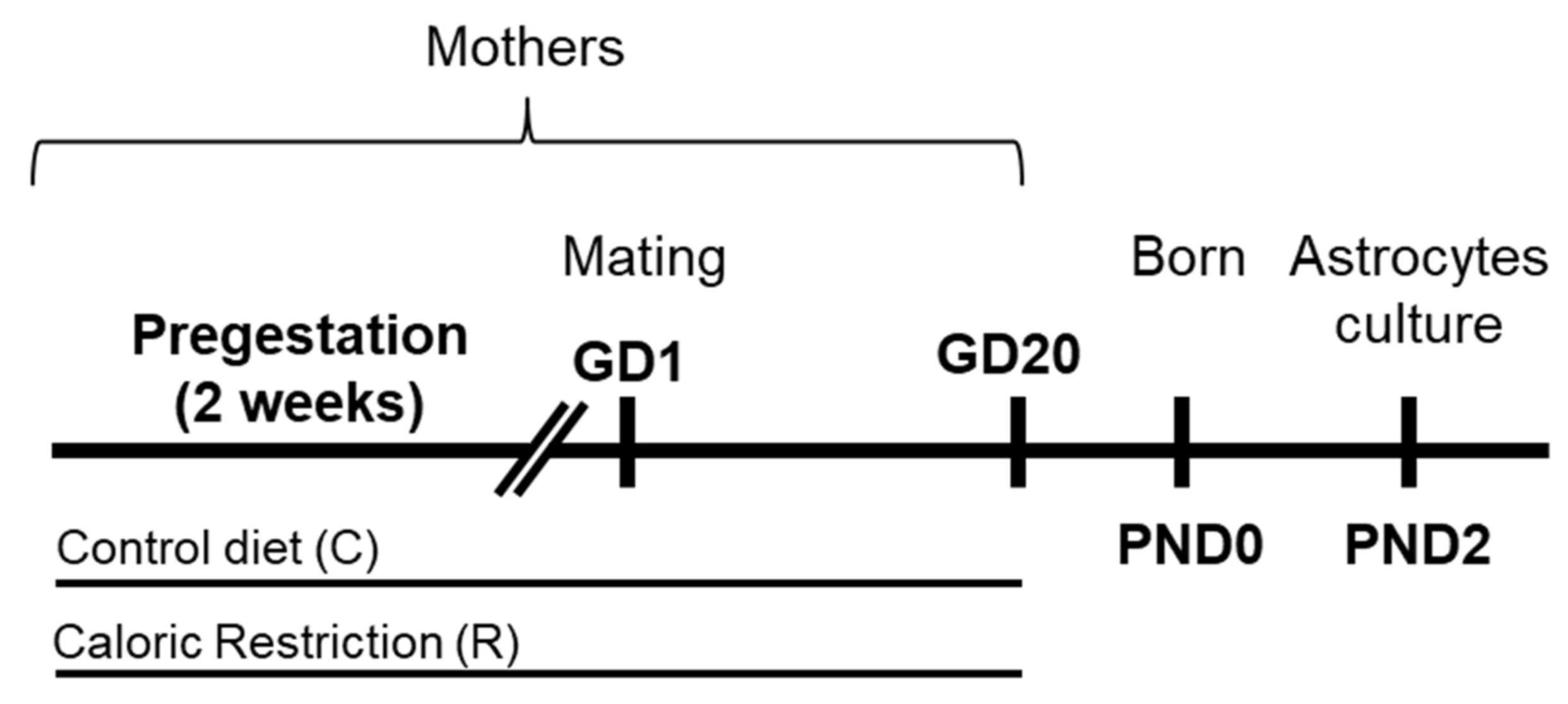
4.2. Animal Model and Diets
4.3. Sample Collection
4.4. Primary Cultures of Astrocytes
4.5. RNA Isolation and Real-Time Quantitative PCR Analysis
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, A. Programming by Early Nutrition in Man. Novartis Found. Symp. 2007, 156, 38–55. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M. The developmental origins of the metabolic syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Berrios, M.V.; González, R.A.; Velilla, R.N.B.; Del Olmo, J.M.D.; Pérez, J.S.; De Fonseca, F.R.; De Heras, R.G. The Role of Maternal Diet in Metabolic and Behavioural Programming: Review of Biologic Mechanisms Involved. Nutr. Hosp. 2015, 32, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Hauser, N.; Douglas-Escobar, M. Postnatal nutrition and adult health programming. Semin. Fetal Neonatal Med. 2007, 12, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Frazier, C.R.M.; Mason, P.; Zhuang, X.; Beeler, J.A. Sucrose Exposure in Early Life Alters Adult Motivation and Weight Gain. PLoS ONE 2008, 3, e3221. [Google Scholar] [CrossRef]
- Kwong, W.; Wild, A.; Roberts, P.; Willis, A.; Fleming, T. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000, 127, 4195–4202. [Google Scholar] [CrossRef]
- Palou, M.; Priego, T.; Sánchez, J.; Palou, A.; Picó, C. Sexual dimorphism in the lasting effects of moderate caloric restriction during gestation on energy homeostasis in rats is related with fetal programming of insulin and leptin resistance. Nutr. Metab. 2010, 7, 69. [Google Scholar] [CrossRef]
- Seébert, S.P.; Hyatt, M.A.; Chan, L.L.Y.; Patel, N.; Bell, R.C.; Keisler, D.; Stephenson, T.; Budge, H.; Symonds, M.E.; Gardner, D.S. Maternal Nutrient Restriction between Early and Midgestation and Its Impact Upon Appetite Regulation after Juvenile Obesity. Endocrinology 2009, 150, 634–641. [Google Scholar] [CrossRef]
- Block, T.; El-Osta, A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis 2017, 266, 31–40. [Google Scholar] [CrossRef]
- Rivera, P.; Ramírez-López, M.T.; Vargas, A.; Decara, J.; Vázquez, M.; Arco, R.; De Heras, R.G.; Argente, J.; De Fonseca, F.R.; Chowen, J.A.; et al. Perinatal free-choice of a high-calorie low-protein diet affects leptin signaling through IRS1 and AMPK dephosphorylation in the hypothalami of female rat offspring in adulthood. Acta Physiol. 2018, 226, e13244. [Google Scholar] [CrossRef]
- Keimpema, E.; Calvigioni, D.; Harkany, T. Endocannabinoid signals in the developmental programming of delayed-onset neuropsychiatric and metabolic illnesses. Biochem. Soc. Trans. 2013, 41, 1569–1576. [Google Scholar] [CrossRef]
- Ramírez-López, M.T.; Vázquez, M.; Bindila, L.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Antón, M.; Decara, J.; Arco, R.; et al. Maternal Caloric Restriction Implemented during the Preconceptional and Pregnancy Period Alters Hypothalamic and Hippocampal Endocannabinoid Levels at Birth and Induces Overweight and Increased Adiposity at Adulthood in Male Rat Offspring. Front. Behav. Neurosci. 2016, 10, 208. [Google Scholar] [CrossRef]
- Almeida, M.M.; Dias-Rocha, C.P.; Reis-Gomes, C.F.; Wang, H.; Atella, G.C.; Cordeiro, A.; Pazos-Moura, C.C.; Joss-Moore, L.; Trevenzoli, I.H. Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats. Psychoneuroendocrinology 2019, 103, 306–315. [Google Scholar] [CrossRef]
- Ramírez-López, M.T.; Arco, R.; Decara, J.; Vázquez, M.; Rivera, P.; Blanco, R.N.; Alén, F.; De Heras, R.G.; Suárez, J.; De Fonseca, F.R. Long-Term Effects of Prenatal Exposure to Undernutrition on Cannabinoid Receptor-Related Behaviors: Sex and Tissue-Specific Alterations in the mRNA Expression of Cannabinoid Receptors and Lipid Metabolic Regulators. Front. Behav. Neurosci. 2016, 10, 241. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Rosell-Valle, C.; López-Gambero, A.; Navarro, J.; Zambrana-Infantes, E.; Rivera, P.; Santín, L.; Suarez, J.; De Fonseca, F.R. Imbalance of Endocannabinoid/Lysophosphatidylinositol Receptors Marks the Severity of Alzheimer’s Disease in a Preclinical Model: A Therapeutic Opportunity. Biology 2020, 9, 377. [Google Scholar] [CrossRef]
- Berger, A.; Crozier, G.; Bisogno, T.; Cavaliere, P.; Innis, S.; Di Marzo, V. Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc. Natl. Acad. Sci. USA 2001, 98, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Lesage, J.; De Petrocellis, L.; Dupouy, J.-P.; Vieau, D.; Di Marzo, V. Effect of maternal under-nutrition on pup body weight and hypothalamic endocannabinoid levels. Cell. Mol. Life Sci. 2003, 60, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A. Diet, endocannabinoids, and health. Nutr. Res. 2019, 70, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Vázquez, M.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Anton, M.; Decara, J.; Arco, R.; Orio, L.; et al. A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 2017, 12, e0174307. [Google Scholar] [CrossRef]
- Hansen, H.S.; Artmann, A. Endocannabinoids and Nutrition. J. Neuroendocr. 2008, 20, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Guerra-Cantera, S.; Vargas, A.; Díaz, F.; García-Úbeda, R.; Tovar, R.; Ramírez-López, M.T.; Argente, J.; De Fonseca, F.R.; Suárez, J.; et al. Maternal hypercaloric diet affects factors involved in lipid metabolism and the endogenous cannabinoid systems in the hypothalamus of adult offspring: Sex-specific response of astrocytes to palmitic acid and anandamide. Nutr. Neurosci. 2020, 1–14. [Google Scholar] [CrossRef]
- Morita, M.; Ikeshima-Kataoka, H.; Kreft, M.; Vardjan, N.; Zorec, R.; Noda, M. Metabolic Plasticity of Astrocytes and Aging of the Brain. Int. J. Mol. Sci. 2019, 20, 941. [Google Scholar] [CrossRef]
- Chowen, J.A.; Frago, L.M.; Fernandez-Alfonso, M.S. Physiological and pathophysiological roles of hypothalamic astrocytes in metabolism. J. Neuroendocr. 2018, 31, e12671. [Google Scholar] [CrossRef] [PubMed]
- Perez-Catalan, N.A.; Doe, C.Q.; Ackerman, S.D. The role of astrocyte-mediated plasticity in neural circuit development and function. Neural Dev. 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Setkowicz, Z.; Kosonowska, E.; Janeczko, K. Inflammation in the developing rat modulates astroglial reactivity to seizures in the mature brain. J. Anat. 2017, 231, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Silva, R.H.; De Moura, A.B.; Presa, J.F.; Abelaira, H.M.; Abatti, M.; Vieira, A.; Pescador, B.; Michels, M.; Ignácio, Z.M.; et al. Early Maternal Deprivation Induces Microglial Activation, Alters Glial Fibrillary Acidic Protein Immunoreactivity and Indoleamine 2,3-Dioxygenase during the Development of Offspring Rats. Mol. Neurobiol. 2019, 56, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Debarba, L.K.; Marangon, P.B.; Borges, B.C.; Veida-Silva, H.; Venancio, J.; Almeida-Pereira, G.; Antunes-Rodrigues, J.; Elias, L.L.K. Neonatal nutritional programming induces gliosis and alters the expression of T-cell protein tyrosine phosphatase and connexins in male rats. Horm. Behav. 2020, 120, 104690. [Google Scholar] [CrossRef] [PubMed]
- Abbink, M.R.; van Deijk, A.-L.F.; Heine, V.M.; Verheijen, M.H.; Korosi, A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia 2019, 67, 1637–1653. [Google Scholar] [CrossRef]
- Navarrete, M.; Díez, A.; Araque, A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130599. [Google Scholar] [CrossRef]
- Grabner, G.F.; Eichmann, T.O.; Wagner, B.; Gao, Y.; Farzi, A.; Taschler, U.; Radner, F.; Schweiger, M.; Lass, A.; Holzer, P.; et al. Deletion of Monoglyceride Lipase in Astrocytes Attenuates Lipopolysaccharide-induced Neuroinflammation. J. Biol. Chem. 2016, 291, 913–923. [Google Scholar] [CrossRef]
- Suzuki, M.; Shibanuma, M.; Kimura, S. Effect of Severe Maternal Dietary Restriction on Growth and Intra-Abdominal Adipose Tissue Weights in Offspring Rats. J. Nutr. Sci. Vitaminol. 2010, 56, 293–298. [Google Scholar] [CrossRef]
- Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetology 2008, 51, 1356–1367. [Google Scholar] [CrossRef]
- Bermudez-Silva, F.-J.; Cardinal, P.; Cota, D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J. Psychopharmacol. 2011, 26, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.P.; Palou, M.; Priego, T.; Sánchez, J.; Palou, A.; Pico, C. Moderate caloric restriction during gestation results in lower arcuate nucleus NPY- and αMSH-neurons and impairs hypothalamic response to fed/fasting conditions in weaned rats. Diabetes Obes. Metab. 2010, 12, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Li, T.; Ross, M.G. Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: Neurotrophic effects of leptin and insulin. Brain Res. 2011, 1378, 29–42. [Google Scholar] [CrossRef]
- Karelis, A.D. Metabolically healthy but obese individuals. Lancet 2008, 372, 1281–1283. [Google Scholar] [CrossRef]
- Song, L.; Cui, J.; Wang, N.; Wang, R.; Yan, J.; Sun, B. Maternal exercise during gestation and lactation decreases high-fat diet preference by altering central reward system gene expression in adult female offspring from high-fat fed dams. Behav. Brain Res. 2020, 390, 112660. [Google Scholar] [CrossRef]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef]
- Bermudez-Silva, F.J.; Viveros, M.P.; McPartland, J.M.; Rodriguez de Fonseca, F. The endocannabinoid system, eating behavior and energy homeostasis: The end or a new beginning? Pharmacol. Biochem. Behav. 2010, 95, 375–382. [Google Scholar] [CrossRef]
- Viveros, M.P.; Llorente, R.; Suarez, J.; Llorente-Berzal, A.; Gallardo, M.L.; De Fonseca, F.R. The endocannabinoid system in critical neurodevelopmental periods: Sex differences and neuropsychiatric implications. J. Psychopharmacol. 2011, 26, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.; Rivera, P.; Llorente, R.; Romero-Zerbo, S.Y.; Bermudez-Silva, F.-J.; De Fonseca, F.R.; Viveros, M.-P. Early maternal deprivation induces changes on the expression of 2-AG biosynthesis and degradation enzymes in neonatal rat hippocampus. Brain Res. 2010, 1349, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.; Llorente, R.; Gallardo, M.L.; Suarez, J.; Bermúdez-Silva, F.; De La Fuente, M.; De Fonseca, F.R.; Garcia-Segura, L. Sex-dependent alterations in response to maternal deprivation in rats. Psychoneuroendocrinology 2009, 34, S217–S226. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.; Llorente, R.; Romero-Zerbo, S.Y.; Mateos, B.; Bermúdez-Silva, F.J.; de Fonseca, F.R.; Viveros, M.-P. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB1and CB2cannabinoid receptors of neonatal rats. Hippocampus 2009, 19, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.A.; De Almeida, M.M.; Da Rocha, C.P.D.; Fassarella, L.D.B.; Souza, L.; De Souza, A.F.P.; De Andrade, C.B.V.; Fortunato, R.S.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Ramírez-López, M.T.; Vázquez, M.; Bindila, L.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alen, F.; Anton, M.; Decara, J.; Ouro, D.; et al. Exposure to a Highly Caloric Palatable Diet During Pregestational and Gestational Periods Affects Hypothalamic and Hippocampal Endocannabinoid Levels at Birth and Induces Adiposity and Anxiety-Like Behaviors in Male Rat Offspring. Front. Behav. Neurosci. 2016, 9. [Google Scholar] [CrossRef]
- De Almeida, M.M.; Dias-Rocha, C.P.; Reis-Gomes, C.F.; Wang, H.; Cordeiro, A.; Pazos-Moura, C.C.; Joss-Moore, L.; Trevenzoli, I.H. Maternal high-fat diet up-regulates type-1 cannabinoid receptor with estrogen signaling changes in a sex- and depot- specific manner in white adipose tissue of adult rat offspring. Eur. J. Nutr. 2021, 60, 1313–1326. [Google Scholar] [CrossRef]
- Gandhi, K.; Li, C.; German, N.; Skobowiat, C.; Carrillo, M.; Kallem, R.R.; Larumbe, E.; Martinez, S.; Chuecos, M.; Ventolini, G.; et al. Effect of maternal high-fat diet on key components of the placental and hepatic endocannabinoid system. Am. J. Physiol. Metab. 2018, 314, E322–E333. [Google Scholar] [CrossRef]
- Rivera, P.; Tovar, R.; Ramírez-López, M.T.; Navarro, J.A.; Vargas, A.; Suárez, J.; De Fonseca, F.R. Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model. Nutrients 2020, 12, 1829. [Google Scholar] [CrossRef]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef]
- Berrendero, F.; Sepe, N.; Di Marzo, V. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 1999, 33, 181–191. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial Fibrillary Acidic Protein: GFAP-Thirty-One Years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Tanaka, H.; Katoh, A.; Oguro, K.; Shimazaki, K.; Gomi, H.; Itohara, S.; Masuzawa, T.; Kawai, N. Disturbance of hippocampal long-term potentiation after transient ischemia in GFAP deficient mice. J. Neurosci. Res. 2002, 67, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Metna-Laurent, M.; Marsicano, G. Rising stars: Modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia 2014, 63, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nat. Cell Biol. 2020, 583, 603–608. [Google Scholar] [CrossRef]
- Terry, K.; Chatman, L.; Foley, G.; Kadyszewski, E.; Fleeman, T.; Hurtt, M.; Chapin, R. Effects of feed restriction on fertility in female rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2005, 74, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780125476126. [Google Scholar]
- Fuente-Martín, E.; García-Cáceres, C.; Granado, M.; De Ceballos, M.L.; Sánchez-Garrido, M.Á.; Sarman, B.; Liu, Z.-W.; Dietrich, M.; Tena-Sempere, M.; Argente-Arizón, P.; et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J. Clin. Investig. 2012, 122, 3900–3913. [Google Scholar] [CrossRef]

| Gene Symbol | Assay ID | GenBank Accession Number | Amplicon Length (bp) |
|---|---|---|---|
| B2M | Rn00560865_m1 | NM_012512.2 | 58 |
| Actb | Rn00667869_m1 | NM_031144.3 | 91 |
| Srebf1 | Rn01495769_m1 | NM_001276707.1 | 79 |
| Srebf2 | Rn01502638_m1 | NM_001033694.1 | 61 |
| Cpt1c | Rn01475546_m1 | NM_001034925.2 | 88 |
| Cpt1a | Rn00580702_m1 | NM_031559.2 | 64 |
| Insr | Rn00573576_m1 | NM_022212.2 | 65 |
| Acox1 | Rn01460628_m1 | NM_017340.2 | 63 |
| Acaca | Rn00573474_m1 | NM_022193.1 | 60 |
| Scd1 | Rn00594894_g1 | NM_139192.2 | 86 |
| LepR | Rn01433205_m1 | NM_012596.1 | 94 |
| Fasn | Rn01463550_m1 | NM_017332.1 | 148 |
| Cnr1 | Rn02758689_s1 | NM_012784.4 | 92 |
| Cnr2 | Rn01637601_m1 | NM_020543.4 | 68 |
| Dagla | Rn01454303_m1 | NM_001005886.1 | 61 |
| Daglb | Rn01453770_m1 | NM_001107120.1 | 57 |
| Mgll | Rn00593297_m1 | NM_138502.2 | 78 |
| Napepld | Rn01786262_m1 | NM_199381.1 | 71 |
| Faah | Rn00577086_m1 | NM_024132.3 | 63 |
| Vimentin | Rn00667825_m1 | NM_031140.1 | 83 |
| Gfap | Rn01253033_m1 | NM_017009.2 | 75 |
| Aif1 | Rn03993468_g1 | NM_017196.3 | 82 |
| Antigen | Manufacturing | Dilution |
|---|---|---|
| ACC | Cell signal (C83B10) Rabbit mAb | 1/1000 |
| ACOX1 | Abcam [EPR19038] ab184032 | 1/500 |
| γ-adaptina | BD Biosciences. Mouse Adaptin 610385 | 1/2000 |
| CPT1a | Abccam (ab102679) rabbit | 1/1000 |
| FAS | Cell Signaling (C18C12) Rabbit mAb | 1/1000 |
| SCD1 | Abcam [CD.E10] ab19862 | 1/250 |
| Daglα | Biorbyt #orb-158553 | 1/100 |
| Daglβ | Biorbyt #orb-182975 | 1/100 |
| CB1 | Abcam #Ab23703 | 1/200 |
| CB2 | Abcam #Ab3561 | 1/200 |
| NAPEPLD | Abcam #Ab95397 | 1/1000 |
| FAAH | CAYMAN #101600 | 1/100 |
| GFAP | Thermofisher #MA5-12023 | 1/100 |
| VIMENTINE | Thermofisher #MA5-11883 | 1/100 |
| SBREP1 | Santa Cruz #sc-365513 | 1/100 |
| SBREP2 | Santa Cruz #sc-13552 | 1/100 |
| IBA1 | Wako | 1/500 |
| MGLL | Abcam #Ab24701 | 1/200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovar, R.; Vargas, A.; Aranda, J.; Sánchez-Salido, L.; González-González, L.; Chowen, J.A.; Rodríguez de Fonseca, F.; Suárez, J.; Rivera, P. Analysis of Both Lipid Metabolism and Endocannabinoid Signaling Reveals a New Role for Hypothalamic Astrocytes in Maternal Caloric Restriction-Induced Perinatal Programming. Int. J. Mol. Sci. 2021, 22, 6292. https://doi.org/10.3390/ijms22126292
Tovar R, Vargas A, Aranda J, Sánchez-Salido L, González-González L, Chowen JA, Rodríguez de Fonseca F, Suárez J, Rivera P. Analysis of Both Lipid Metabolism and Endocannabinoid Signaling Reveals a New Role for Hypothalamic Astrocytes in Maternal Caloric Restriction-Induced Perinatal Programming. International Journal of Molecular Sciences. 2021; 22(12):6292. https://doi.org/10.3390/ijms22126292
Chicago/Turabian StyleTovar, Rubén, Antonio Vargas, Jesús Aranda, Lourdes Sánchez-Salido, Laura González-González, Julie A. Chowen, Fernando Rodríguez de Fonseca, Juan Suárez, and Patricia Rivera. 2021. "Analysis of Both Lipid Metabolism and Endocannabinoid Signaling Reveals a New Role for Hypothalamic Astrocytes in Maternal Caloric Restriction-Induced Perinatal Programming" International Journal of Molecular Sciences 22, no. 12: 6292. https://doi.org/10.3390/ijms22126292
APA StyleTovar, R., Vargas, A., Aranda, J., Sánchez-Salido, L., González-González, L., Chowen, J. A., Rodríguez de Fonseca, F., Suárez, J., & Rivera, P. (2021). Analysis of Both Lipid Metabolism and Endocannabinoid Signaling Reveals a New Role for Hypothalamic Astrocytes in Maternal Caloric Restriction-Induced Perinatal Programming. International Journal of Molecular Sciences, 22(12), 6292. https://doi.org/10.3390/ijms22126292







