Endothelial Dysfunction and Neutrophil Degranulation as Central Events in Sepsis Physiopathology
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Endothelium and Sepsis
- Glycocalyx: It is an organized layer adhered to a surface matrix that covers the luminal surface of the endothelium, composed by glycoproteins, hyaluronan, sulphated proteoglycans and plasma proteins. It acts as a protective barrier between the blood and vessel wall, helping to regulate leucocyte adhesion, to maintain the endothelial barrier and to inhibit intravascular thrombosis [21].
Endothelial Dysfunction in Sepsis
- Systemic inflammation: A large number of mediators involved in the “molecular storm” that occurs in sepsis initiate and amplify the endothelial damage, such as pathogen-associated molecular patterns (PAMPs), cytokines, bradykinin, the platelet activating factor (PAF), vascular endothelial growth factor (VEGF), fibrin degradation products and reactive oxygen species (ROS) [24,25,26,27]. However, the endothelium is not only passive during sepsis but also stimulates the inflammatory response through the production of chemokines that attract immune cells [27].
- Glycocalyx degradation and shedding: Glycocalyx shedding occurs as a consequence of the “cocktail” of pro-oxidative and proinflammatory molecules that is generated during sepsis [16,24,28]. This deleterious response is aggravated by the release of components of neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs), such as glycocalyx degradation products themselves [21,28].
- Increased leucocyte adhesion and extravasation: The shedding of the glycocalyx exposes the endothelium to leucocyte adhesion [28]. The presence of proinflammatory cytokines during sepsis allows for the adhesion of activated immune cells to the vascular wall and promotes migration to the surrounding tissues by inducing the expression of molecules, such as selectin E (SEL-E), selectin P (SEL-P), intercellular adhesion molecule 1 (ICAM-1) or vascular adhesion molecule 1 (VCAM-1) [24]. During sepsis, disruption of the integrity of the endothelial barrier occurs as a consequence of the adhesion of activated neutrophils [29], which release proteases that contribute to the degradation of binding proteins [20].
- Destruction of intercellular junctions, disruption of the endothelial barrier and endothelial cell death: The presence of an oxidative and proinflammatory scenario during sepsis induces the disassembly of intercellular junctions, creating spaces between endothelial cells [16,24,28]. Endothelial cell death occurs as a consequence of the release of NETs—specifically, by the action of proteases and cationic proteins [23,30]. The endothelial barrier is disrupted by bacterial toxins, which can directly kill endothelial cells, weakening their cytoskeleton and breaking the intercellular junctions of these endothelial cells [26].
- Procoagulant and antifibrinolytic state induction: The production of nitric oxide (NO), a potent vasodilator, mediated by inducible nitric oxide synthase (iNOS) is increased in sepsis [24,31]. However, there is a significant reduction in NO production by endothelial synthase nitric oxide (eNOS), which causes a direct alteration of vasodilation and promotes leucocyte and platelet adhesion [25]. The downregulation of the endothelial expression of thrombomodulin and protein C receptors leads to the reduced activation of activated protein C, which plays an anticoagulant function [32]. Endothelial cells release a procoagulant glycoprotein called the tissue factor (TF), while the TF pathway inhibitor synthesis remains inhibited [23]. Platelets and the coagulation cascade activation produce microvascular thrombosis [21]. Furthermore, NETs promote hypercoagulability in patients with sepsis by providing support for the formation of thrombi [23]. Acute vascular dysfunction and leakage contribute to hypotension, local hypoxia, insufficient organ perfusion, ischemia and, ultimately, to organ failure, acute respiratory distress syndrome, shock and death in severe patients [25,33].
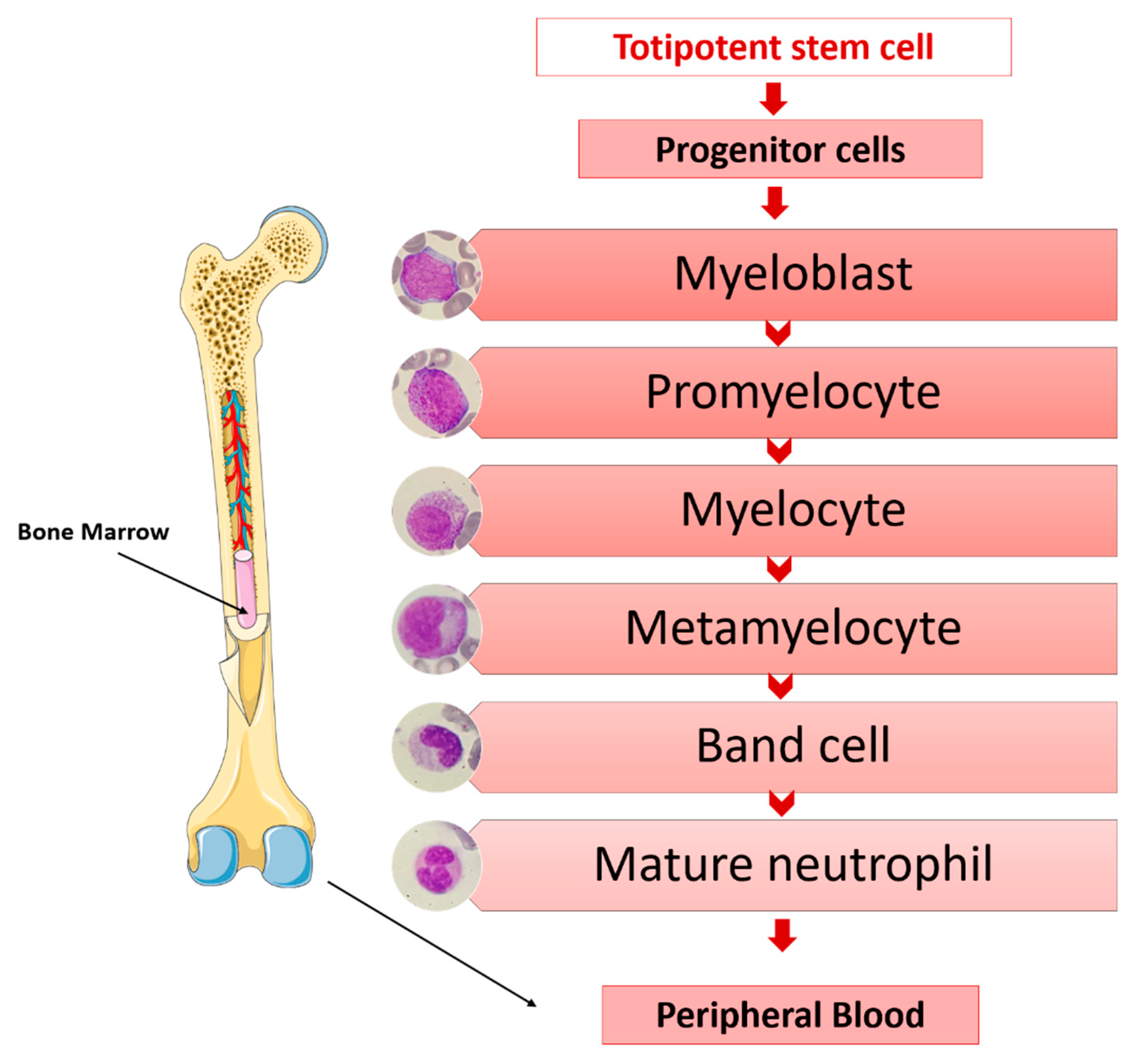
4. Neutrophils and Sepsis
- Azurophilic granules. They are lysosomes containing myeloperoxidases and powerful hydrolytic enzymes necessary for the destruction of microorganisms (acid hydrolases; proteases such as proteinase 3, cathepsin G and elastase; cationic proteins such as lysozymes, defensins, azurocidin, bactericidal permeability increasing protein (BPI); etc.) [35].
- Specific granules. They contain lysozymes; lactoferrin, which has bactericidal and bacteriostatic activity against viruses, fungi and bacteria [36]; lipocalin 2, which also has microbicidal properties; olfactomedin 4; transcobalamin I and other substances involved in the activation of phagocytosis. They are peroxidase-negative.
- Gelatinase granules. These types of granules are mobilized when neutrophils contact the activated endothelium for the first time. They contain matrix-degrading enzymes, such as gelatinase, and membrane receptors, such as macrophage receptor 1 (MAC-1), CD177 Molecule (CD177), Carcinoembryonic Antigen-Related Cell Adhesion Molecule 8 (CEACAM8), etc., which are essential in the early phases of the inflammatory response of neutrophils and their extravasation into inflamed tissues [37].
- Secretory vesicles. They are not considered true neutrophil granules, being significantly smaller. They constitute an important reservoir for membrane-associated receptors, such as Matrix Metallopeptidase 25 (MMP25), lymphocyte function-associated antigen-1 (LFA-1) and MAC-1, as well as actin, actin-binding proteins and alkaline phosphatase, which are essential for the establishment of firm contact of the neutrophil with the endothelium-activated vascular system and to complete diapedesis towards inflamed tissues where, through chemotaxis, it locates and eradicates the responsible pathogen [37,38].
- Adhesion: Neutrophil migration from blood to tissues is an active process involving a complex set of adhesion molecules on the membrane of the leucocyte that are sequentially activated and have their corresponding receptors on the vascular endothelium. This mechanism allows neutrophils to roll and adhere with progressive firmness to the endothelial surface by selectins, integrins and other molecules and allows their receptors to finally cross the endothelial barrier [34]. Neutrophils are first captured onto the endothelial cell surfaces by the upregulation of adhesive molecules on the endothelial luminal surface in response to inflammatory cytokines and bacteria-derived peptides. Leukocyte selectins mediate these early adhesive interactions, which are transient and weak, promoting the “rolling” of neutrophils on endothelial cells [39]. Upon activation via chemokine receptorsLFA-1, MAC-1 and very late antigen-4 (VLA-4) bind to members of the immunoglobulin superfamily present on endothelial cell membranes, such as intercellular adhesion molecules 1 and 2 (ICAM-1 and ICAM-2) and VCAM-1, respectively [40].
- Chemotaxis: It is the mechanism by which multiple chemotactic factors (products released by microorganisms, damaged cells, C-X-C Motif Chemokine Ligand 8 (IL-8) and complement fractions) form a chemical gradient that directs the diapedesis of neutrophils to tissues in the precise direction of the focus of infection or inflammation, where they accumulate after passing between the endothelial cells of the microcirculation [34].
- Phagocytosis: The bacterium or foreign material is recognized and consequently ingested during this process. The membrane then invaginates and simultaneously emits pseudopods, encompassing the particle in a phagosome [34].
- Bacteriolysis: The formation of the phagosome attracts the granules of the neutrophils, which bind to it, degranulating themselves. The killing of microorganisms occurs, in part, due to the lytic action of the different granular enzymes, but the most important mechanism consists in the generation of oxygen metabolites, with great microbicidal power. Oxygen is reduced by nicotinamide adenine dinucleotide phosphate (NADPH), forming superoxide radicals (O2−) and generating hydrogen peroxide (H2O2), which acts as a substrate for myeloperoxidase, which oxidizes halides into hypochlorous acids and chloramines, the latter being powerful microbicides. There is a detoxification mechanism that prevents the excess H2O2 generated from destroying the granulocytes and damaging the adjacent tissues [34].
5. Sepsis Biomarkers
6. Clinical Practice Implications
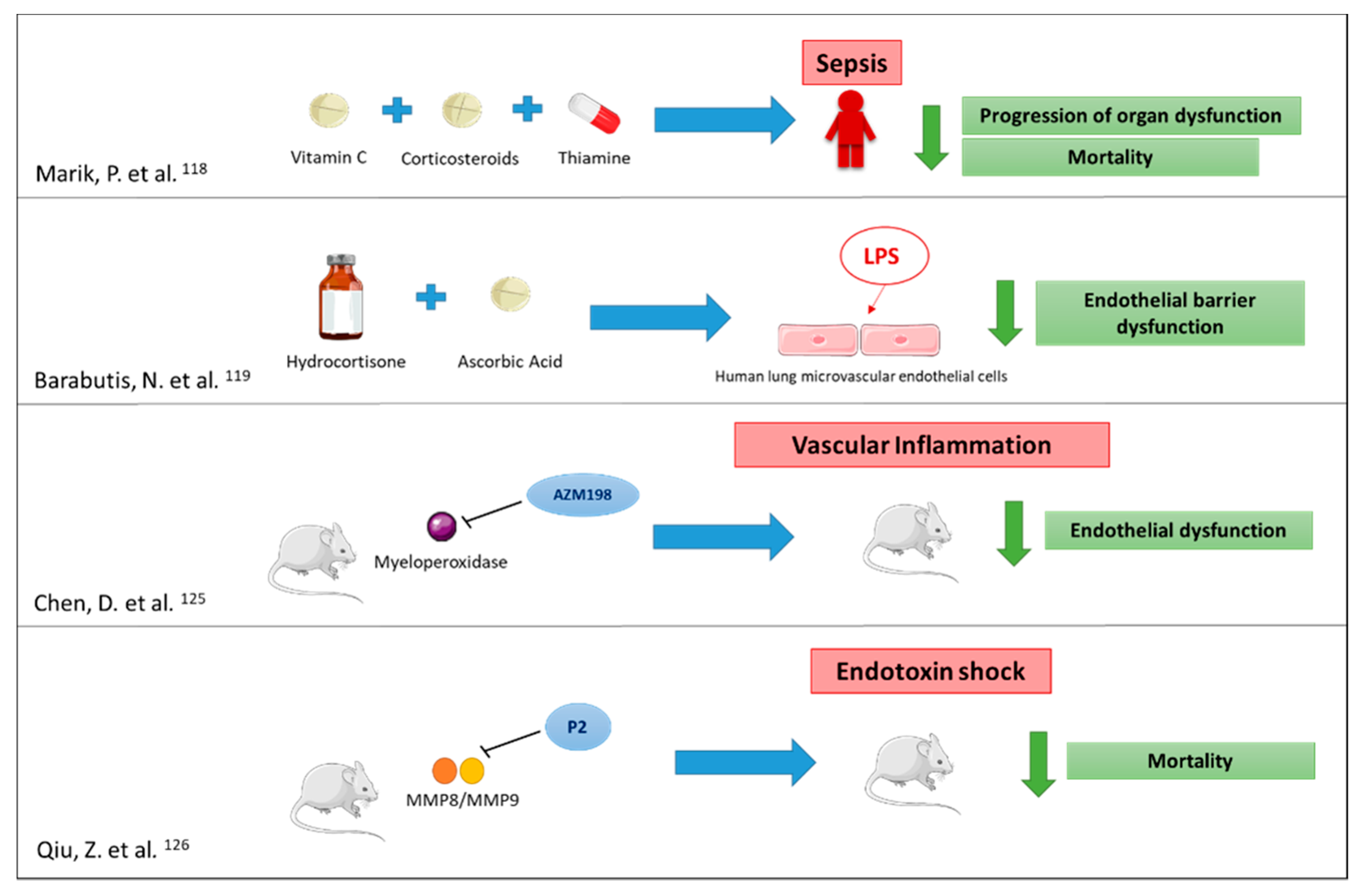
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2015, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 7 May 2021).
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital Deaths in Patients with Sepsis From 2 Independent Cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Winters, B.D.; Eberlein, M.; Leung, J.; Needham, D.M.; Pronovost, P.J.; Sevransky, J.E. Long-Term Mortality and Quality of Life in Sepsis: A Systematic Review. Crit. Care Med. 2010, 38, 1276–1283. [Google Scholar] [CrossRef]
- Lund-Sørensen, H.; Benros, M.E.; Madsen, T.; Sørensen, H.J.; Eaton, W.W.; Postolache, T.T.; Nordentoft, M.; Erlangsen, A. A Nationwide Cohort Study of the Association Between Hospitalization with Infection and Risk of Death by Suicide. JAMA Psychiatry 2016, 73, 912–919. [Google Scholar] [CrossRef]
- Torio, C.M.; Moore, B.J. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality (US): Rockville MD, USA, 2006. [Google Scholar]
- Arefian, H.; Heublein, S.; Scherag, A.; Brunkhorst, F.M.; Younis, M.Z.; Moerer, O.; Fischer, D.; Hartmann, M. Hospital-Related Cost of Sepsis: A Systematic Review. J. Infect. 2017, 74, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Pfuntner, A.; Wier, L.M.; Steiner, C. Costs for Hospital Stays in the United States, 2010: Statistical Brief #146. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2006. [Google Scholar]
- Vincent, J.-L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.-R.; Payen, D.; et al. Sepsis in European Intensive Care Units: Results of the SOAP Study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Lefrant, J.-Y.; Kotfis, K.; Nanchal, R.; Martin-Loeches, I.; Wittebole, X.; Sakka, S.G.; Pickkers, P.; Moreno, R.; Sakr, Y. Comparison of European ICU Patients in 2012 (ICON) versus 2002 (SOAP). Intensive Care Med. 2018, 44, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Frutos-Vivar, F.; Ferguson, N.D.; Peñuelas, O.; Lorente, J.A.; Gordo, F.; Honrubia, T.; Algora, A.; Bustos, A.; García, G.; et al. Sepsis Incidence and Outcome: Contrasting the Intensive Care Unit with the Hospital Ward. Crit. Care Med. 2007, 35, 1284–1289. [Google Scholar] [CrossRef]
- Darbà, J.; Marsà, A. Epidemiology, Management and Costs of Sepsis in Spain (2008–2017): A Retrospective Multicentre Study. Curr. Med. Res. Opin. 2020, 36, 1089–1095. [Google Scholar] [CrossRef]
- Álvaro-Meca, A.; Jiménez-Sousa, M.A.; Micheloud, D.; Sánchez-Lopez, A.; Heredia-Rodríguez, M.; Tamayo, E.; Resino, S. Group of Biomedical Research in Critical Care Medicine (BioCritic) Epidemiological Trends of Sepsis in the Twenty-First Century (2000–2013): An Analysis of Incidence, Mortality, and Associated Costs in Spain. Popul. Health Metr. 2018, 16, 4. [Google Scholar] [CrossRef]
- Lee, W.L.; Slutsky, A.S. Sepsis and Endothelial Permeability. N. Engl. J. Med. 2010, 363, 689–691. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Spiller, F.; Cunha, F.Q. Neutrophil paralysis in sepsis. Shock 2010, 34, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; Martín-Fernandez, M.; López-Mestanza, C.; Duque, P.; Almansa, R. Shared Features of Endothelial Dysfunction between Sepsis and Its Preceding Risk Factors (Aging and Chronic Disease). J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Radeva, M.Y.; Waschke, J. Mind the Gap: Mechanisms Regulating the Endothelial Barrier. Acta Physiol. 2018, 222. [Google Scholar] [CrossRef] [PubMed]
- Gane, J.; Stockley, R. Mechanisms of Neutrophil Transmigration across the Vascular Endothelium in COPD. Thorax 2012, 67, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Colbert, J.F.; Schmidt, E.P. Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin. Chest Med. 2016, 37, 263–275. [Google Scholar] [CrossRef]
- Wallez, Y.; Huber, P. Endothelial Adherens and Tight Junctions in Vascular Homeostasis, Inflammation and Angiogenesis. Biochim. Biophys. Acta 2008, 1778, 794–809. [Google Scholar] [CrossRef]
- Hattori, Y.; Hattori, K.; Suzuki, T.; Matsuda, N. Recent Advances in the Pathophysiology and Molecular Basis of Sepsis-Associated Organ Dysfunction: Novel Therapeutic Implications and Challenges. Pharmacol. Ther. 2017, 177, 56–66. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. ADQI XIV Workgroup. The endothelium in sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2018, 34, 63–80. [Google Scholar] [CrossRef]
- Lubkin, A.; Torres, V.J. Bacteria and Endothelial Cells: A Toxic Relationship. Curr. Opin. Microbiol. 2017, 35, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine Storm and Sepsis Disease Pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Koczera, P.; Zechendorf, E.; Schuerholz, T. The Endothelial Glycocalyx: New Diagnostic and Therapeutic Approaches in Sepsis. BioMed Res. Int. 2016, 2016, 3758278. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.D.; Heffernan, D.S.; Cioffi, W.G.; Reichner, J.S. Neutrophils from Critically Ill Septic Patients Mediate Profound Loss of Endothelial Barrier Integrity. Crit. Care 2013, 17, R226. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; McDonald, P.P.; Fülöp, T.; Lesur, O. Sepsis, Leukocytes, and Nitric Oxide (NO): An Intricate Affair. Shock 2010, 33, 344–352. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and Coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef]
- Crouser, E.D.; Matthay, M.A. Endothelial Damage During Septic Shock: Significance and Implications for Future Therapies. Chest 2017, 152, 1–3. [Google Scholar] [CrossRef]
- García Hernández, A.M.; Jarque Ramos, I. LEUCOCITOS. PATOLOGÍA DE LOS GRANULOCITOS. AGRANULOCITOSIS. In Pregrado de Hematología; Luzán 5: Madrid, Spain, 2017; ISBN 978-84-7989-874-8. [Google Scholar]
- Lawrence, S.M.; Corriden, R.; Nizet, V. The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis. Microbiol. Mol. Biol. Rev. 2018, 82. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2576. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and Granules of Human Neutrophils. Immunol. Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Rørvig, S.; Østergaard, O.; Heegaard, N.H.H.; Borregaard, N. Proteome Profiling of Human Neutrophil Granule Subsets, Secretory Vesicles, and Cell Membrane: Correlation with Transcriptome Profiling of Neutrophil Precursors. J. Leukoc. Biol. 2013, 94, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.-D. Neutrophil Transendothelial Migration: Updates and New Perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; Andaluz-Ojeda, D.; Almansa, R.; Gandía, F.; Gómez-Herreras, J.I.; Gomez-Sanchez, E.; Heredia-Rodríguez, M.; Eiros, J.M.; Kelvin, D.J.; Tamayo, E. Defining Immunological Dysfunction in Sepsis: A Requisite Tool for Precision Medicine. J. Infect. 2016. [Google Scholar] [CrossRef]
- Sônego, F.; e Silva, C.F.V.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.V.G.; Nascimento, D.C.; Colón, D.F.; Borges, V.d.F.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Bermejo-Martín, J.F.; Tamayo, E.; Ruiz, G.; Andaluz-Ojeda, D.; Herrán-Monge, R.; Muriel-Bombín, A.; Muñoz, M.F.; Heredia-Rodríguez, M.; Citores, R.; Gómez-Herreras, J.I.; et al. Circulating Neutrophil Counts and Mortality in Septic Shock. Crit. Care 2014, 18, 407. [Google Scholar] [CrossRef]
- Brown, K.A.; Treacher, D.F. Neutrophils as Potential Therapeutic Targets in Sepsis. Discov. Med. 2006, 6, 118–122. [Google Scholar]
- Mare, T.A.; Treacher, D.F.; Shankar-Hari, M.; Beale, R.; Lewis, S.M.; Chambers, D.J.; Brown, K.A. The Diagnostic and Prognostic Significance of Monitoring Blood Levels of Immature Neutrophils in Patients with Systemic Inflammation. Crit. Care 2015, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Demaret, J.; Venet, F.; Friggeri, A.; Cazalis, M.-A.; Plassais, J.; Jallades, L.; Malcus, C.; Poitevin-Later, F.; Textoris, J.; Lepape, A.; et al. Marked Alterations of Neutrophil Functions during Sepsis-Induced Immunosuppression. J. Leukoc. Biol. 2015, 98, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Kalupov, T.; Brillard-Bourdet, M.; Dadé, S.; Serrano, H.; Wartelle, J.; Guyot, N.; Juliano, L.; Moreau, T.; Belaaouaj, A.; Gauthier, F. Structural Characterization of Mouse Neutrophil Serine Proteases and Identification of Their Substrate Specificities: Relevance to Mouse Models of Human Inflammatory Diseases. J. Biol. Chem. 2009, 284, 34084–34091. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, M.L.; Trevani, A.S.; Sabatté, J.; Geffner, J. Mechanisms Regulating Neutrophil Survival and Cell Death. Semin. Immunopathol. 2013, 35, 423–437. [Google Scholar] [CrossRef]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in Development of Multiple Organ Failure in Sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Wolf, D.; Anto-Michel, N.; Blankenbach, H.; Wiedemann, A.; Buscher, K.; Hohmann, J.D.; Lim, B.; Bäuml, M.; Marki, A.; Mauler, M.; et al. A Ligand-Specific Blockade of the Integrin Mac-1 Selectively Targets Pathologic Inflammation While Maintaining Protective Host-Defense. Nat. Commun. 2018, 9, 525. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in Tissue Injury and Repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The Glycocalyx: A Novel Diagnostic and Therapeutic Target in Sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Almansa, R.; Heredia-Rodríguez, M.; Gomez-Sanchez, E.; Andaluz-Ojeda, D.; Iglesias, V.; Rico, L.; Ortega, A.; Gomez-Pesquera, E.; Liu, P.; Aragón, M.; et al. Transcriptomic Correlates of Organ Failure Extent in Sepsis. J. Infect. 2015, 70, 445–456. [Google Scholar] [CrossRef]
- van den Berg, C.W.; Tambourgi, D.V.; Clark, H.W.; Hoong, S.J.; Spiller, O.B.; McGreal, E.P. Mechanism of Neutrophil Dysfunction: Neutrophil Serine Proteases Cleave and Inactivate the C5a Receptor. J. Immunol. 2014, 192, 1787–1795. [Google Scholar] [CrossRef]
- Xu, R.; Lin, F.; Bao, C.; Huang, H.; Ji, C.; Wang, S.; Jin, L.; Sun, L.; Li, K.; Zhang, Z.; et al. Complement 5a Receptor-Mediated Neutrophil Dysfunction Is Associated with a Poor Outcome in Sepsis. Cell Mol. Immunol. 2016, 13, 103–109. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U.; Wiedow, O. Neutrophil Serine Proteases: Mediators of Innate Immune Responses. Curr. Opin. Hematol. 2011, 18, 19–24. [Google Scholar] [CrossRef]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New Approaches to Sepsis: Molecular Diagnostics and Biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Shidham, A.; Wong, H.R.; Khatri, P. A Comprehensive Time-Course-Based Multicohort Analysis of Sepsis and Sterile Inflammation Reveals a Robust Diagnostic Gene Set. Sci. Transl. Med. 2015, 7, 287ra71. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Wong, H.R.; Khatri, P. Robust Classification of Bacterial and Viral Infections via Integrated Host Gene Expression Diagnostics. Sci. Transl. Med. 2016, 8, 346ra91. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Perumal, T.M.; Henao, R.; Nichols, M.; Howrylak, J.A.; Choi, A.M.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Davenport, E.E.; et al. A Community Approach to Mortality Prediction in Sepsis via Gene Expression Analysis. Nat. Commun. 2018, 9, 694. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis Across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.; Hassan, U.; Seymour, C.; Angus, D.C.; Isbell, T.S.; White, K.; Weir, W.; Yeh, L.; Vincent, A.; Bashir, R. Point-of-Care Sensors for the Management of Sepsis. Nat. Biomed. Eng. 2018, 2, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Vincent, J.-L. Sepsis Biomarkers: A Review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Samraj, R.S.; Zingarelli, B.; Wong, H.R. Role of Biomarkers in Sepsis Care. Shock 2013, 40, 358–365. [Google Scholar] [CrossRef]
- Bloos, F.; Reinhart, K. Rapid Diagnosis of Sepsis. Virulence 2014, 5, 154–160. [Google Scholar] [CrossRef]
- Lobo, S.M.A.; Lobo, F.R.M.; Bota, D.P.; Lopes-Ferreira, F.; Soliman, H.M.; Mélot, C.; Vincent, J.-L. C-Reactive Protein Levels Correlate with Mortality and Organ Failure in Critically Ill Patients. Chest 2003, 123, 2043–2049. [Google Scholar] [CrossRef]
- Baidoshvili, A.; Nijmeijer, R.; Lagrand, W.K.; Hack, C.E.; Niessen, H.W.M. Localisation of C Reactive Protein in Infarcted Tissue Sites of Multiple Organs during Sepsis. J. Clin. Pathol. 2002, 55, 152–153. [Google Scholar] [CrossRef][Green Version]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Póvoa, P.; Coelho, L.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. C-Reactive Protein as a Marker of Infection in Critically Ill Patients. Clin. Microbiol. Infect. 2005, 11, 101–108. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Kontakiotis, T.; Lazaridis, L.; Tsotsolis, N.; Koumis, J.; Kyriazis, G.; Bitzani, M. Inflammatory Markers in Patients with Severe Burn Injury. What Is the Best Indicator of Sepsis? Burns 2007, 33, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Fraunberger, P.; Wang, Y.; Holler, E.; Parhofer, K.G.; Nagel, D.; Walli, A.K.; Seidel, D. Prognostic Value of Interleukin 6, Procalcitonin, and C-Reactive Protein Levels in Intensive Care Unit Patients during First Increase of Fever. Shock 2006, 26, 10–12. [Google Scholar] [CrossRef]
- Almansa, R.; Martín, S.; Martin-Fernandez, M.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Aragón, M.; Andrés, C.; Calvo, D.; Rico-Feijoo, J.; Esteban-Velasco, M.C.; et al. Combined Quantification of Procalcitonin and HLA-DR Improves Sepsis Detection in Surgical Patients. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Update on Procalcitonin Measurements. Ann. Lab Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; White, J.C.; Nylén, E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous Expression of the Calcitonin-i Gene in Multiple Tissues in Response to Sepsis. J. Clin. Endocrinol. Metab. 2001, 86, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Wada, H.; Ikejiri, M.; Hatada, T.; Sakurai, H.; Matsushima, Y.; Nishioka, J.; Maruyama, K.; Isaji, S.; Takeda, T.; et al. Efficacy of Procalcitonin in the Early Diagnosis of Bacterial Infections in a Critical Care Unit. Shock 2009, 31, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a Diagnostic Marker for Sepsis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Yunus, I.; Fasih, A.; Wang, Y. The Use of Procalcitonin in the Determination of Severity of Sepsis, Patient Outcomes and Infection Characteristics. PLoS ONE 2018, 13, e0206527. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Becker, K.L.; Schächinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin Precursors Are Reliable Markers of Sepsis in a Medical Intensive Care Unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of Procalcitonin-Guided Antibiotic Treatment on Clinical Outcomes in Intensive Care Unit Patients with Infection and Sepsis Patients: A Patient-Level Meta-Analysis of Randomized Trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Sepsis Definitions Task Force Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate Measurements in Sepsis-Induced Tissue Hypoperfusion: Results from the Surviving Sepsis Campaign Database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; Mcguire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of Cytokines as a Double-Edged Sword in Sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Andaluz-Ojeda, D.; Bobillo, F.; Iglesias, V.; Almansa, R.; Rico, L.; Gandía, F.; Resino, S.; Tamayo, E.; de Lejarazu, R.O.; Bermejo-Martin, J.F. A Combined Score of Pro- and Anti-Inflammatory Interleukins Improves Mortality Prediction in Severe Sepsis. Cytokine 2012, 57, 332–336. [Google Scholar] [CrossRef]
- Mebazaa, A.; Geven, C.; Hollinger, A.; Wittebole, X.; Chousterman, B.G.; Blet, A.; Gayat, E.; Hartmann, O.; Scigalla, P.; Struck, J.; et al. Circulating Adrenomedullin Estimates Survival and Reversibility of Organ Failure in Sepsis: The Prospective Observational Multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) Study. Crit. Care 2018, 22, 354. [Google Scholar] [CrossRef]
- Bełtowski, J.; Jamroz, A. Adrenomedullin—What Do We Know 10 Years since Its Discovery? Pol. J. Pharmacol. 2004, 56, 5–27. [Google Scholar]
- Andaluz-Ojeda, D.; Cicuéndez, R.; Calvo, D.; Largo, E.; Nogales, L.; Muñoz, M.F.; Bueno, P.; Eiros, J.M.; Gandía, F.; Bermejo-Martín, J.F. Sustained Value of Proadrenomedullin as Mortality Predictor in Severe Sepsis. J. Infect. 2015, 71, 136–139. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Vaquero-Roncero, L.M.; Almansa, R.; Gómez-Sánchez, E.; Martín, S.; Tamayo, E.; Esteban-Velasco, M.C.; Ruiz-Granado, P.; Aragón, M.; Calvo, D.; et al. Endothelial Dysfunction Is an Early Indicator of Sepsis and Neutrophil Degranulation of Septic Shock in Surgical Patients. BJS Open 2020. [Google Scholar] [CrossRef]
- Saeed, K.; Wilson, D.C.; Bloos, F.; Schuetz, P.; van der Does, Y.; Melander, O.; Hausfater, P.; Legramante, J.M.; Claessens, Y.-E.; Amin, D.; et al. The Early Identification of Disease Progression in Patients with Suspected Infection Presenting to the Emergency Department: A Multi-Centre Derivation and Validation Study. Crit. Care 2019, 23, 40. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Almansa, R.; Ortega, A.; Alonso, R.; Suescun, M.; Ferrando, A.; Feced, L.; Bermejo-Martin, J.F. Simultaneous Depression of Immunological Synapse and Endothelial Injury Is Associated with Organ Dysfunction in Community-Acquired Pneumonia. J. Clin. Med. 2019, 8, 1404. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [CrossRef] [PubMed]
- Schuetz, P.; Christ-Crain, M.; Müller, B. Biomarkers to Improve Diagnostic and Prognostic Accuracy in Systemic Infections. Curr. Opin. Crit. Care 2007, 13, 578–585. [Google Scholar] [CrossRef]
- Xing, K.; Murthy, S.; Liles, W.C.; Singh, J.M. Clinical Utility of Biomarkers of Endothelial Activation in Sepsis—A Systematic Review. Crit. Care 2012, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Paulus, P.; Jennewein, C.; Zacharowski, K. Biomarkers of Endothelial Dysfunction: Can They Help Us Deciphering Systemic Inflammation and Sepsis? Biomarkers 2011, 1 (Suppl. 16), S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Suraj, J.; Kus, K.; Kij, A.; Zakrzewska, A.; Chlopicki, S. Towards a Comprehensive Endothelial Biomarkers Profiling and Endothelium-Guided Pharmacotherapy. Pharmacol. Rep. 2015, 67, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; van der Poll, T. Endothelial Barrier Dysfunction in Septic Shock. J. Intern. Med. 2015, 277, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-Y.; Kung, C.-T.; Tsai, N.-W.; Su, C.-M.; Huang, C.-C.; Lai, Y.-R.; Wang, H.-C.; Cheng, B.-C.; Su, Y.-J.; Lin, W.-C.; et al. Concentration and Value of Endocan on Outcome in Adult Patients after Severe Sepsis. Clin. Chim. Acta 2018, 483, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2016, 194, 439–449. [Google Scholar] [CrossRef]
- Andaluz-Ojeda, D.; Nguyen, H.B.; Meunier-Beillard, N.; Cicuéndez, R.; Quenot, J.-P.; Calvo, D.; Dargent, A.; Zarca, E.; Andrés, C.; Nogales, L.; et al. Superior Accuracy of Mid-Regional Proadrenomedullin for Mortality Prediction in Sepsis with Varying Levels of Illness Severity. Ann. Intensive Care 2017, 7, 15. [Google Scholar] [CrossRef]
- Shen, Z.-G.; Guo, J.-L.; Li, D.-S. Screening of Differentially Expressed Genes Related to Severe Sepsis Induced by Multiple Trauma with DNA Microarray. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 734–739. [Google Scholar]
- Yazdan-Ashoori, P.; Liaw, P.; Toltl, L.; Webb, B.; Kilmer, G.; Carter, D.E.; Fraser, D.D. Elevated Plasma Matrix Metalloproteinases and Their Tissue Inhibitors in Patients with Severe Sepsis. J. Crit. Care 2011, 26, 556–565. [Google Scholar] [CrossRef]
- Wong, H.R.; Weiss, S.L.; Giuliano, J.S.; Wainwright, M.S.; Cvijanovich, N.Z.; Thomas, N.J.; Allen, G.L.; Anas, N.; Bigham, M.T.; Hall, M.; et al. Testing the Prognostic Accuracy of the Updated Pediatric Sepsis Biomarker Risk Model. PLoS ONE 2014, 9, e86242. [Google Scholar] [CrossRef]
- Solan, P.D.; Dunsmore, K.E.; Denenberg, A.G.; Odoms, K.; Zingarelli, B.; Wong, H.R. A Novel Role for Matrix Metalloproteinase-8 in Sepsis. Crit. Care Med. 2012, 40, 379–387. [Google Scholar] [CrossRef]
- Alder, M.N.; Opoka, A.M.; Lahni, P.; Hildeman, D.A.; Wong, H.R. Olfactomedin-4 Is a Candidate Marker for a Pathogenic Neutrophil Subset in Septic Shock. Crit. Care Med. 2017, 45, e426–e432. [Google Scholar] [CrossRef]
- Kangelaris, K.N.; Prakash, A.; Liu, K.D.; Aouizerat, B.; Woodruff, P.G.; Erle, D.J.; Rogers, A.; Seeley, E.J.; Chu, J.; Liu, T.; et al. Increased Expression of Neutrophil-Related Genes in Patients with Early Sepsis-Induced ARDS. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1102–L1113. [Google Scholar] [CrossRef]
- Patterson, E.K.; Cepinskas, G.K.; Inoue, K.; Fraser, D.D. Sepsis-Associated Elastase and Proteinase 3 Induce Endothelial Permeability. FASEB J. 2017, 31, 978.11. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Zhao, X.; Dong, G.; Li, C. Diagnostic and Prognostic Value of Neutrophil Gelatinase-Associated Lipocalin, Matrix Metalloproteinase-9, and Tissue Inhibitor of Matrix Metalloproteinases-1 for Sepsis in the Emergency Department: An Observational Study. Crit. Care 2014, 18, 634. [Google Scholar] [CrossRef]
- Macdonald, S.P.J.; Stone, S.F.; Neil, C.L.; van Eeden, P.E.; Fatovich, D.M.; Arendts, G.; Brown, S.G.A. Sustained Elevation of Resistin, NGAL and IL-8 Are Associated with Severe Sepsis/Septic Shock in the Emergency Department. PLoS ONE 2014, 9, e110678. [Google Scholar] [CrossRef]
- Wang, B.; Chen, G.; Zhang, J.; Xue, J.; Cao, Y.; Wu, Y. Increased Neutrophil Gelatinase-Associated Lipocalin Is Associated with Mortality and Multiple Organ Dysfunction Syndrome in Severe Sepsis and Septic Shock. Shock 2015, 44, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhu, S.; Pan, C.; Xie, J.-F.; Liu, S.-Q.; Qiu, H.-B.; Yang, Y. Predictive Utilities of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Severe Sepsis. Clin. Chim. Acta 2018, 481, 200–206. [Google Scholar] [CrossRef]
- Parnell, G.P.; Tang, B.M.; Nalos, M.; Armstrong, N.J.; Huang, S.J.; Booth, D.R.; McLean, A.S. Identifying Key Regulatory Genes in the Whole Blood of Septic Patients to Monitor Underlying Immune Dysfunctions. Shock 2013, 40, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Peltan, I.D.; Mitchell, K.H.; Rudd, K.E.; Mann, B.A.; Carlbom, D.J.; Hough, C.L.; Rea, T.D.; Brown, S.M. Physician Variation in Time to Antimicrobial Treatment for Septic Patients Presenting to the Emergency Department. Crit. Care Med. 2017, 45, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Peltan, I.D.; Brown, S.M.; Bledsoe, J.R.; Sorensen, J.; Samore, M.H.; Allen, T.L.; Hough, C.L. ED Door-to-Antibiotic Time and Long-Term Mortality in Sepsis. Chest 2019, 155, 938–946. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting Vascular (Endothelial) Dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Darwish, I.; Liles, W.C. Emerging Therapeutic Strategies to Prevent Infection-Related Microvascular Endothelial Activation and Dysfunction. Virulence 2013, 4, 572–582. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Cancel, L.M. The Glycocalyx and Its Significance in Human Medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Barabutis, N.; Khangoora, V.; Marik, P.E.; Catravas, J.D. Hydrocortisone and Ascorbic Acid Synergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest 2017, 152, 954–962. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L.; Weber, C. Mechanisms Underlying Neutrophil-Mediated Monocyte Recruitment. Blood 2009, 114, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Fan, P.; Huang, B.; Deng, H.-B.; Cheung, B.M.Y.; Félétou, M.; Vilaine, J.-P.; Villeneuve, N.; Xu, A.; Vanhoutte, P.M.; et al. Deamidated Lipocalin-2 Induces Endothelial Dysfunction and Hypertension in Dietary Obese Mice. J. Am. Heart Assoc. 2014, 3, e000837. [Google Scholar] [CrossRef]
- Manchanda, K.; Kolarova, H.; Kerkenpaß, C.; Mollenhauer, M.; Vitecek, J.; Rudolph, V.; Kubala, L.; Baldus, S.; Adam, M.; Klinke, A. MPO (Myeloperoxidase) Reduces Endothelial Glycocalyx Thickness Dependent on Its Cationic Charge. Arter. Thromb. Vasc. Biol. 2018, 38, 1859–1867. [Google Scholar] [CrossRef]
- Safaeian, L.; Javanmard, S.H.; Mollanoori, Y.; Dana, N. Cytoprotective and Antioxidant Effects of Human Lactoferrin against H2O2-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Adv. Biomed. Res. 2015, 4, 188. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef]
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaëlsson, E.; Lindstedt, E.-L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Chen, J.; Xu, H.; Van den Steen, P.E.; Opdenakker, G.; Wang, M.; Hu, J. Inhibition of Neutrophil Collagenase/MMP-8 and Gelatinase B/MMP-9 and Protection against Endotoxin Shock. J. Immunol. Res. 2014, 2014, 747426. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Kalita, J.; Weldon, S.; Taggart, C.C. Proteases and Their Inhibitors in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2018, 7, 244. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Fernández, M.; Tamayo-Velasco, Á.; Aller, R.; Gonzalo-Benito, H.; Martínez-Paz, P.; Tamayo, E. Endothelial Dysfunction and Neutrophil Degranulation as Central Events in Sepsis Physiopathology. Int. J. Mol. Sci. 2021, 22, 6272. https://doi.org/10.3390/ijms22126272
Martín-Fernández M, Tamayo-Velasco Á, Aller R, Gonzalo-Benito H, Martínez-Paz P, Tamayo E. Endothelial Dysfunction and Neutrophil Degranulation as Central Events in Sepsis Physiopathology. International Journal of Molecular Sciences. 2021; 22(12):6272. https://doi.org/10.3390/ijms22126272
Chicago/Turabian StyleMartín-Fernández, Marta, Álvaro Tamayo-Velasco, Rocío Aller, Hugo Gonzalo-Benito, Pedro Martínez-Paz, and Eduardo Tamayo. 2021. "Endothelial Dysfunction and Neutrophil Degranulation as Central Events in Sepsis Physiopathology" International Journal of Molecular Sciences 22, no. 12: 6272. https://doi.org/10.3390/ijms22126272
APA StyleMartín-Fernández, M., Tamayo-Velasco, Á., Aller, R., Gonzalo-Benito, H., Martínez-Paz, P., & Tamayo, E. (2021). Endothelial Dysfunction and Neutrophil Degranulation as Central Events in Sepsis Physiopathology. International Journal of Molecular Sciences, 22(12), 6272. https://doi.org/10.3390/ijms22126272





