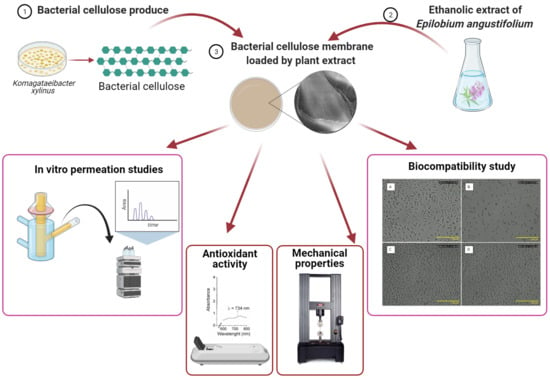
Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin
Abstract
1. Introduction
2. Results
2.1. Chemical Composition and Antioxidant Activity of the FEEs
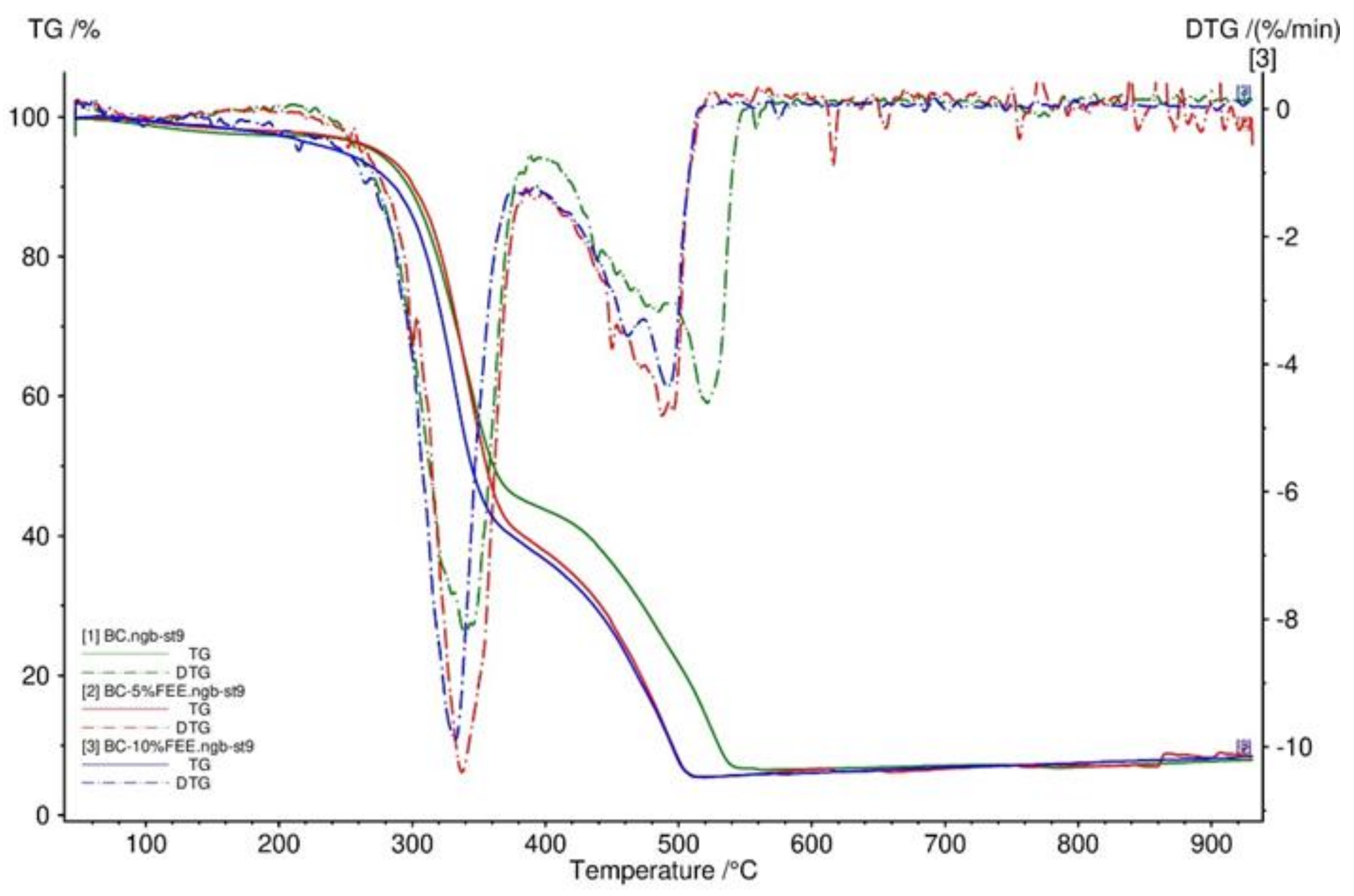
2.2. The TG, DTG, FTIR SEM, and Mechanical Properties of BC and BC-FEEs
2.3. Antioxidant Properties of Phenolic Acids Contain in BC and BC-FEEs
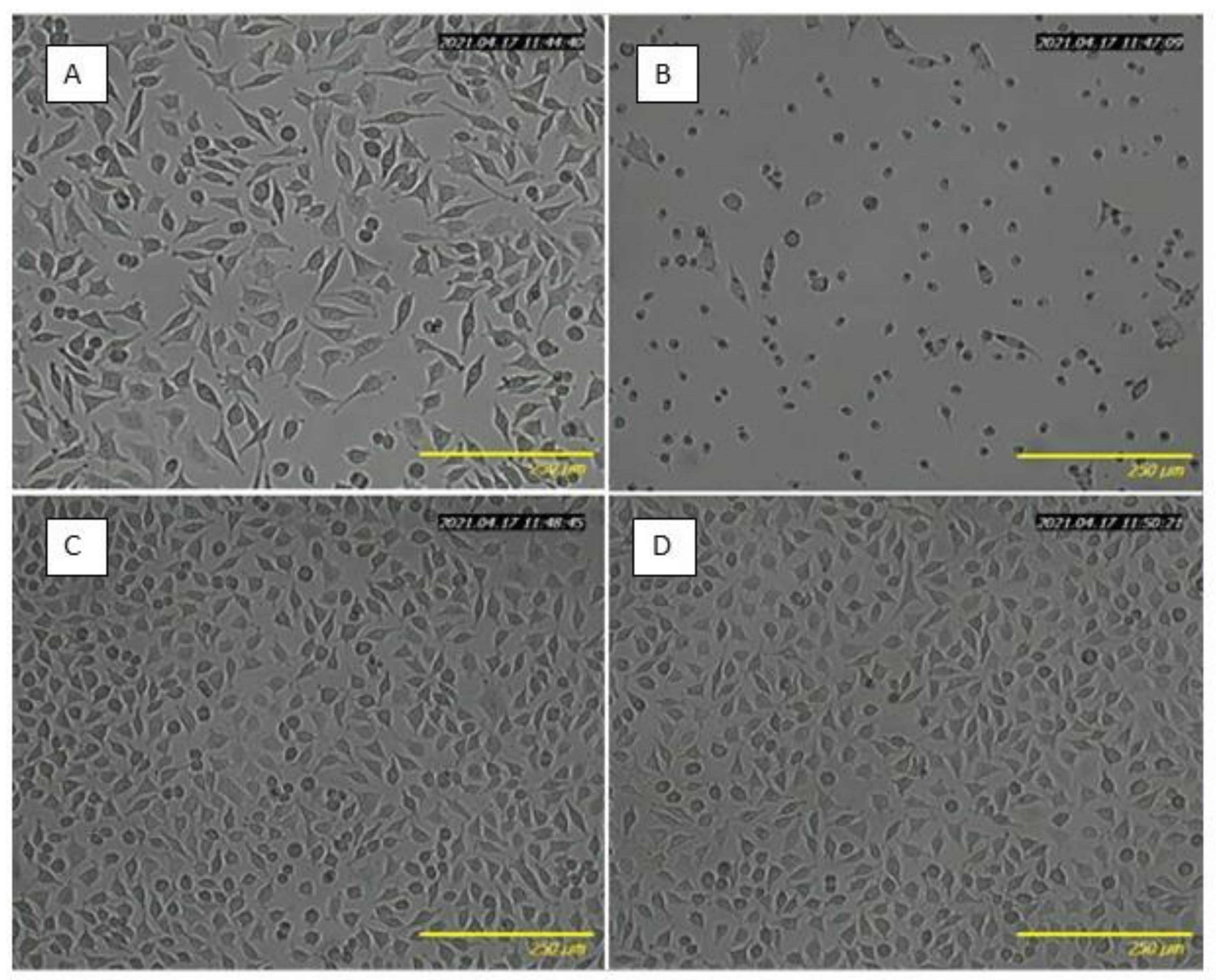
2.4. Biocompatibility Study
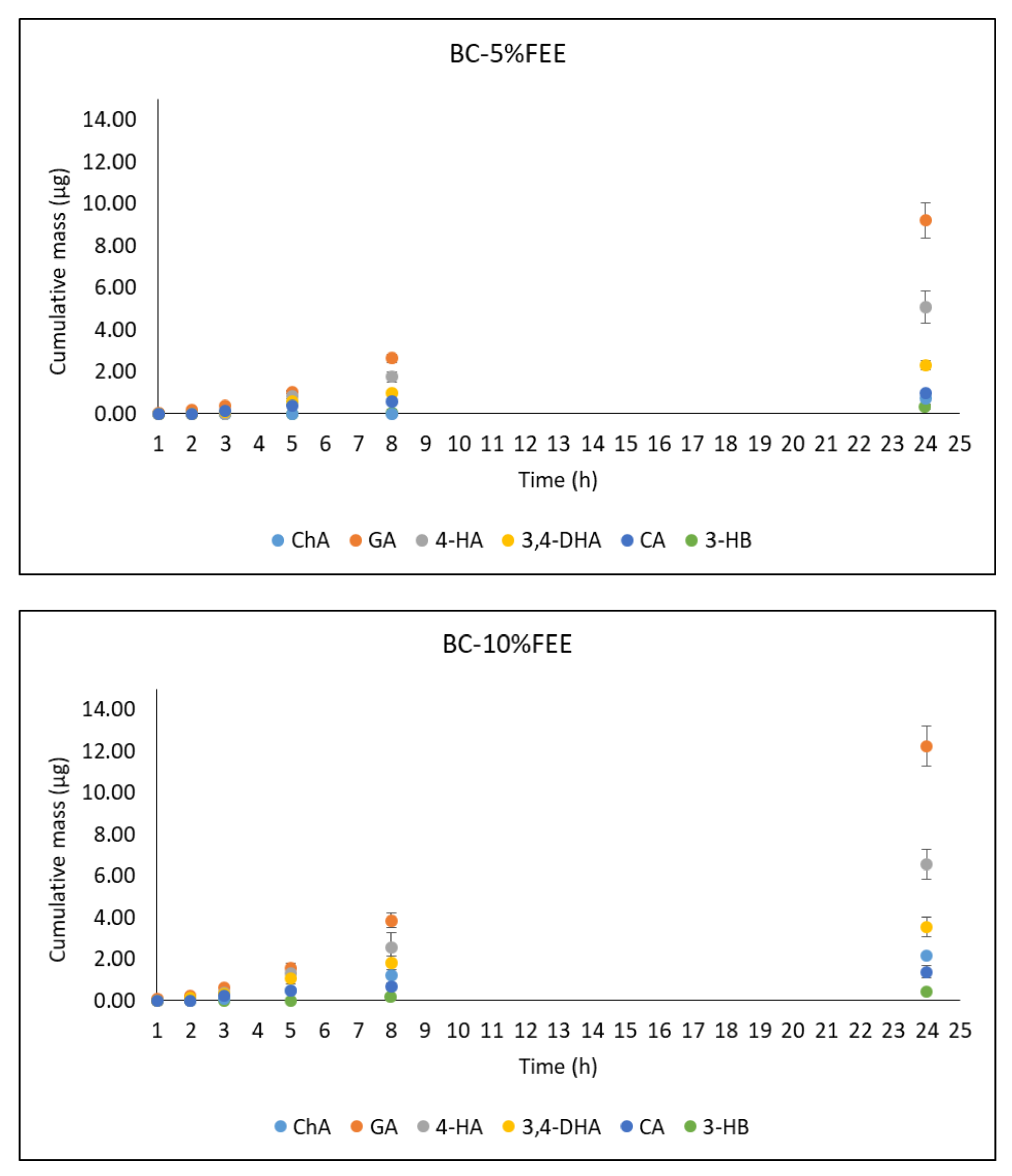
2.5. In Vitro Penetration Studies
3. Discussion
4. Materials and Methods
- Atest—cells with medium containing the extracts,
- Ablank—medium with the respective extract (without cells),
- Acontrol—cells with a free medium.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raiszadeh-Jahromi, Y.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization of Bacterial Cellulose Production by Komagataeibacter Xylinus PTCC 1734 in a Low-Cost Medium Using Optimal Combined Design. J. Food Sci. Technol. 2020, 57, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Hur, D.H.; Rhee, H.-S.; Lee, J.H.; Shim, W.Y.; Kim, T.Y.; Lee, S.Y.; Park, J.H.; Jeong, K.J. Enhanced Production of Cellulose in Komagataeibacter Xylinus by Preventing Insertion of IS Element into Cellulose Synthesis Gene. Biochem. Eng. J. 2020, 156, 107527. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Sousa Lobo, J.M.; Costa, P.C. Bacterial Cellulose Membranes as Drug Delivery Systems: An In Vivo Skin Compatibility Study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of Bacterial Cellulose and Its Composites in Biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of Bacterial Cellulose in Food, Cosmetics and Drug Delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent Advances in Composites Based on Cellulose Derivatives for Biomedical Applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, J.E.; Kim, H.R. Comparative Study on the Physical Entrapment of Soy and Mushroom Proteins on the Durability of Bacterial Cellulose Bio-Leather. Cellulose 2021, 28, 3183–3200. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Jiang, Q.; Yu, D.; Xu, Y.; Wang, B.; Xia, W. Development and Properties of Bacterial Cellulose, Curcumin, and Chitosan Composite Biodegradable Films for Active Packaging Materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial Cellulose Micro-Nano Fibres for Wound Healing Applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential Applications of Bacterial Cellulose and Its Composites for Cancer Treatment. Int. J. Biol. Macromol. 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Nicolai, M.; Mota, J.; Fernandes, A.S.; Pereira, F.; Pereira, P.; Reis, P.C.; Robles Velasco, M.V.; Baby, A.R.; Rosado, C.; Rijo, P. Assessment of the Potential Skin Application of Plectranthus Ecklonii Benth. Pharmaceuticals 2020, 13, 120. [Google Scholar] [CrossRef]
- De Fernandes, I.A.A.; Maciel, G.M.; Oliveira, A.L.M.S.; Miorim, A.J.F.; Fontana, J.D.; Ribeiro, V.R.; Haminiuk, C.W.I. Hybrid Bacterial Cellulose-Collagen Membranes Production in Culture Media Enriched with Antioxidant Compounds from Plant Extracts. Polym. Eng. Sci. 2020, 60, 2814–2826. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan Mohd, S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant Extract-Loaded Bacterial Cellulose Composite Membrane for Potential Biomedical Applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Karl, B.; Alkhatib, Y.; Beekmann, U.; Bellmann, T.; Blume, G.; Steiniger, F.; Thamm, J.; Werz, O.; Kralisch, D.; Fischer, D. Development and Characterization of Bacterial Nanocellulose Loaded with Boswellia Serrata Extract Containing Nanoemulsions as Natural Dressing for Skin Diseases. Int. J. Pharm. 2020, 587, 119635. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Rosyida, V.T.; Apriyana, W.; Hayati, S.N.; Darsih, C.; Nisa, K.; Ratih, D. Antioxidant and Antibacterial Properties of Bacterial Cellulose—Indonesian Plant Extract Composites for Mask Sheet. J. Appl. Pharm. Sci. 2020, 37–42. [Google Scholar] [CrossRef]
- Moradian, S.; Almasi, H.; Moini, S. Development of Bacterial Cellulose-Based Active Membranes Containing Herbal Extracts for Shelf Life Extension of Button Mushrooms (Agaricus bisporus). Food Process. Preserv. 2017, 42, e13537. [Google Scholar] [CrossRef]
- Sukhtezari, S.; Almasi, H.; Pirsa, S.; Zandi, M.; Pirouzifard, M. Development of Bacterial Cellulose Based Slow-Release Active Films by Incorporation of Scrophularia striata Boiss. Extract. Carbohydr. Polym. 2017, 156, 340–350. [Google Scholar] [CrossRef]
- Pourali, P.; Yahyaei, B. The Healing Property of a Bioactive Wound Dressing Prepared by the Combination of Bacterial Cellulose (BC) and Zingiber Officinale Root Aqueous Extract in Rats. 3 Biotech 2019, 9, 59. [Google Scholar] [CrossRef]
- Asanarong, O.; Minh Quan, V.; Boonrungsiman, S.; Sukyai, P. Bioactive Wound Dressing Using Bacterial Cellulose Loaded with Papain Composite: Morphology, Loading/Release and Antibacterial Properties. Eur. Polym. J. 2021, 143, 110224. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Drumond, I.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Freire, C.S.R.; Silvestre, A.J.D. Topical Caffeine Delivery Using Biocellulose Membranes: A Potential Innovative System for Cellulite Treatment. Cellulose 2014, 21, 665–674. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial Cellulose Membranes Applied in Topical and Transdermal Delivery of Lidocaine Hydrochloride and Ibuprofen: In Vitro Diffusion Studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Mota, J.P.; Santos de Almeida, T.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Wu, J.; Zheng, Y.; Song, W.; Wang, G.; Guo, J.; Ding, X. Impregnation of Silver Sulfadiazine into Bacterial Cellulose for Antimicrobial and Biocompatible Wound Dressing. Biomed. Mater. 2012, 7, 065006. [Google Scholar] [CrossRef]
- Pavaloiu, R.-D.; Stoica, A.; Stroescu, M.; Dobre, T. Controlled Release of Amoxicillin from Bacterial Cellulose Membranes. Open Chem. 2014, 12, 962–967. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and Anticancer Properties of Bacterial Cellulose Films Containing Ethanolic Extract of Mangosteen Peel. J. Biomater. Sci. Polym. Ed. 2014, 25, 907–922. [Google Scholar] [CrossRef]
- Kalle, R.; Belichenko, O.; Kuznetsova, N.; Kolosova, V.; Prakofjewa, J.; Stryamets, N.; Mattalia, G.; Šarka, P.; Simanova, A.; Prūse, B.; et al. Gaining Momentum: Popularization of Epilobium angustifolium as Food and Recreational Tea on the Eastern Edge of Europe. Appetite 2020, 150, 104638. [Google Scholar] [CrossRef]
- Shi, H.; Sun, S.; Liu, X.; Fan, J.; Wang, J.; Zhao, K.; Wang, W. Allelopathic Potential and Mechanism of Rosebay Willowherb [Chamaenerion angustifolium (L.) Scop.] Demonstrated on Model Plant Lettuce. Phyton 2021, 90, 159–170. [Google Scholar] [CrossRef]
- Güven, S.; Makbul, S.; Mertayak, F.; Coşkunçelebi, K. Anatomical Properties of Epilobium and Chamaenerion from a Taxonomical Perspective in Turkey. Protoplasma 2021. [Google Scholar] [CrossRef]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Flores, G.A.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial Activity of Epilobium Spp. Extracts. Il Farmaco 2001, 56, 345–348. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of Different Durations of Solid-Phase Fermentation for Fireweed (Chamerion angustifolium (L.) Holub) Leaves on the Content of Polyphenols and Antioxidant Activity In Vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef]
- Nowak, A.; Klimowicz, A.; Duchnik, W.; Kucharski, Ł.; Florkowska, K.; Muzykiewicz, A.; Wira, D.; Zielonkabrzezicka, J.; Siedłowska, A.; Nadarzewska, K. Application of Green-Extraction Technique to Evaluate of Antioxidative Capacity of Wild Population of Fireweed (Epilobium angustifolium). Herba Pol. 2019, 65, 18–30. [Google Scholar] [CrossRef]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In Vivo Bioactivity Assessment on Epilobium Species: A Particular Focus on Epilobium angustifolium and Its Components on Enzymes Connected with the Healing Process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Adamska-Szewczyk, A.; Zgórka, G. Plant Polyphenols in Cosmetics—A Review. Eur. J. Med. Technol. 2019, 3, 1–10. [Google Scholar]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic Profiling, In Vitro Bioaccessibility and In Vivo Bioavailability of a Commercial Bioactive Epilobium angustifolium L. Extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure-Property Relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Barbi, S.; Taurino, C.; La China, S.; Anguluri, K.; Gullo, M.; Montorsi, M. Mechanical and Structural Properties of Environmental Green Composites Based on Functionalized Bacterial Cellulose. Cellulose 2021, 28, 1431–1442. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of Bacterial Cellulose in Skin and Bone Tissue Engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Dreger, M.; Adamczak, A.; Seidler-Łożykowska, K.; Wielgus, K. Pharmacological Properties of Fireweed (Epilobium angustifolium L.) and Bioavailability of Ellagitannins. A Review. Herba Pol. 2020, 66, 52–64. [Google Scholar] [CrossRef]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; et al. Evaluation of Phytochemical Composition of Fresh and Dried Raw Material of Introduced Chamerion angustifolium L. Using Chromatographic, Spectrophotometric and Chemometric Techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Wu, J.; Lin, P.C. Chemical Composition and Antimicrobial Activity of the Essential Oil from Epilobium angustifolium. Chem. Nat. Compd. 2016, 52, 1113–1115. [Google Scholar] [CrossRef]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and Antimicrobial Activity of the Essential Oil, Distilled Aromatic Water and Herbal Infusion from Epilobium Parviflorum Schreb. Ind. Crop. Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Canli, K.; Yetgin, A.; Akata, I.; Altuner, E.M. Antimicrobial Activity and Chemical Composition Screening of Epilobium Montanum Root. Indian J. Pharm. Educ. Res. 2017, 51, s239–s243. [Google Scholar] [CrossRef]
- Jariene, E.; Lasinskas, M.; Danilcenko, H.; Vaitkeviciene, N.; Slepetiene, A.; Najman, K.; Hallmann, E. Polyphenols, Antioxidant Activity and Volatile Compounds in Fermented Leaves of Medicinal Plant Rosebay Willowherb (Chamerion angustifolium (L.) Holub). Plants 2020, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for Skin and Brain Penetration of Major Components from Essential Oils Used in Aromatherapy for Dementia Patients. J. Biomol. Struct. Dyn. 2020, 38, 2402–2411. [Google Scholar] [CrossRef]
- Ruszová, E.; Cheel, J.; Pávek, S.; Moravcová, M.; Hermannová, M.; Matějková, I.; Spilková, J.; Velebný, V.; Kubala, L. Epilobium angustifolium Extract Demonstrates Multiple Effects on Dermal FIbroblasts In Vitro and Skin Photo-Protection In Vivo. Gen. Physiol. Biophys. 2013, 32, 347–359. [Google Scholar] [CrossRef]
- Shikov, A.N.; Poltanov, E.A.; Dorman, H.J.D.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical Composition and In Vitro Antioxidant Evaluation of Commercial Water-Soluble Willow Herb (Epilobium angustifolium L.) Extracts. J. Agric. Food Chem. 2006, 54, 3617–3624. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Falahati-Anbaran, M.; Rohloff, J. Comparative Analyses of Phytochemical Variation Within and Between Congeneric Species of Willow Herb, Epilobium hirsutum and E. parviflorum: Contribution of Environmental Factors. Front. Plant Sci. 2021, 11, 595190. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Avilés-Betanzos, K.A.; de Ramírez-Rivera, E.J.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Polyphenols Content in Capsicum Chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants 2020, 9, 1394. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium angustifolium (Fireweed): Polyphenols from Fireweed. Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium Spp: Pharmacology and Phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant Extracts Rich in Polyphenols: Antibacterial Agents and Natural Preservatives for Meat and Meat Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Romman, M.; Ishaq, M.; Islam, Y.; Khan, N. Antimicrobial and Antioxidant Activities of Methanolic Extract and Fractions of (Epilobium roseum) (Schreb.) against Bacterial Strains. Am. J. Plant Sci. 2021, 12, 275–284. [Google Scholar] [CrossRef]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Esposito, S.; De Simone, G.; Pan, A.; Brambilla, P.; Gattuso, G.; Mastroianni, C.; Kertusha, B.; Contini, C.; Massoli, L.; Francisci, D.; et al. Epidemiology and Microbiology of Skin and Soft Tissue Infections: Preliminary Results of a National Registry. J. Chemother. 2018, 31, 9–14. [Google Scholar] [CrossRef]
- Amparo, T.R.; Seibert, J.B.; de Vieira, P.M.A.; Teixeira, L.F.M.; dos Santos, O.D.H.; Souza, G.H.B. Herbal Medicines to the Treatment of Skin and Soft Tissue Infections: Advantages of the Multi-targets Action. Phytother. Res. 2019, 34, 94–103. [Google Scholar] [CrossRef]
- Onar, H.C.; Yusufoglu, A.; Turker, G.; Yanardag, R. Elastase, Tyrosinase and Lipoxygenase Inhibition and Antioxidant Activity of an Aqueous Extract from Epilobium angustifolium L. Leaves. J. Med. Plants Res. 2012, 6, 716–726. [Google Scholar] [CrossRef]
- Surma-Ślusarska, B.; Presler, S.; Danielewicz, D. Characteristics of Bacterial Cellulose Obtained from Acetobacter Xylinum Culture for Application in Papermaking. Fibres Text. East. Eur. 2008, 16, 108–111. [Google Scholar]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate Groups from Sulfuric Acid Hydrolysis on the Thermal Degradation Behavior of Bacterial Cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Kumbhar, J.V.; Rajwade, J.M.; Paknikar, K.M. Fruit Peels Support Higher Yield and Superior Quality Bacterial Cellulose Production. Appl. Microbiol. Biotechnol. 2015, 99, 6677–6691. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a Variety of Polyphenols Compounds and Antioxidant Properties of Rhubarb (Rheum rhabarbarum). LWT 2020, 118, 108775. [Google Scholar] [CrossRef]
- Wiegand, C.; Elsner, P.; Hipler, U.-C.; Klemm, D. Protease and ROS Activities Influenced by a Composite of Bacterial Cellulose and Collagen Type I In Vitro. Cellulose 2006, 13, 689–696. [Google Scholar] [CrossRef]
- Alonso, C.; Lucas, R.; Barba, C.; Marti, M.; Rubio, L.; Comelles, F.; Morales, J.C.; Coderch, L.; Parra, J.L. Skin Delivery of Antioxidant Surfactants Based on Gallic Acid and Hydroxytyrosol. J. Pharm. Pharmacol. 2015, 67, 900–908. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the Wound Healing Effect of Calendula Extracts Using the Scratch Assay with 3T3 Fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Da Pitz, H.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell. Longev. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An In Vitro Model for Human Skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of Ibuprofen Solubility and Skin Permeation by Conjugation with L-Valine Alkyl Esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef]
- Bertges, F.S.; da Penha Henriques do Amaral, M.; Rodarte, M.P.; Vieira Fonseca, M.J.; Sousa, O.V.; Pinto Vilela, F.M.; Alves, M.S. Assessment of Chemical Changes and Skin Penetration of Green Arabica Coffee Beans Biotransformed by Aspergillus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101512. [Google Scholar] [CrossRef]
- Pirvu, L.; Nicorescu, I.; Hlevca, C.; Albu, B.; Nicorescu, V. Epilobi Hirsuti Herba Extracts Influence the In Vitro Activity of Common Antibiotics on Standard Bacteria. Open Chem. 2016, 14, 65–75. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Shangguan, L.; Ma, J.; Mao, K.; Zhang, Q.; Wang, Y.; Liu, Z.; Mao, K. A Novel Bacterial Cellulose Membrane Immobilized with Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosome Prevents Epidural Fibrosis. Int. J. Nanomed. 2018, 13, 5257–5273. [Google Scholar] [CrossRef]
- Subtaweesin, C.; Woraharn, W.; Taokaew, S.; Chiaoprakobkij, N.; Sereemaspun, A.; Phisalaphong, M. Characteristics of Curcumin-Loaded Bacterial Cellulose Films and Anticancer Properties against Malignant Melanoma Skin Cancer Cells. Appl. Sci. 2018, 16, 1188. [Google Scholar] [CrossRef]
- Čuříková, B.A.; Procházková, K.; Filková, B.; Diblíková, P.; Svoboda, J.; Kováčik, A.; Vávrová, K.; Zbytovská, J. Simplified Stratum Corneum Model Membranes for Studying the Effects of Permeation Enhancers. Int. J. Pharm. 2017, 534, 287–296. [Google Scholar] [CrossRef]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin Penetration Enhancement by a Microneedle Device (Dermaroller®) In Vitro: Dependency on Needle Size and Applied Formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with In Vivo-In Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of Solvents and Penetration Enhancers on Transdermal Delivery of Thymoquinone: Permeability and Skin Deposition Study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Galactosyl Pentadecene Reversibly Enhances Transdermal and Topical Drug Delivery. Pharm. Res. 2017, 34, 2097–2108. [Google Scholar] [CrossRef]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-Species Assessment of Electrical Resistance as a Skin Integrity Marker for In Vitro Percutaneous Absorption Studies. Toxicol. In Vitro 2004, 18, 351–358. [Google Scholar] [CrossRef]
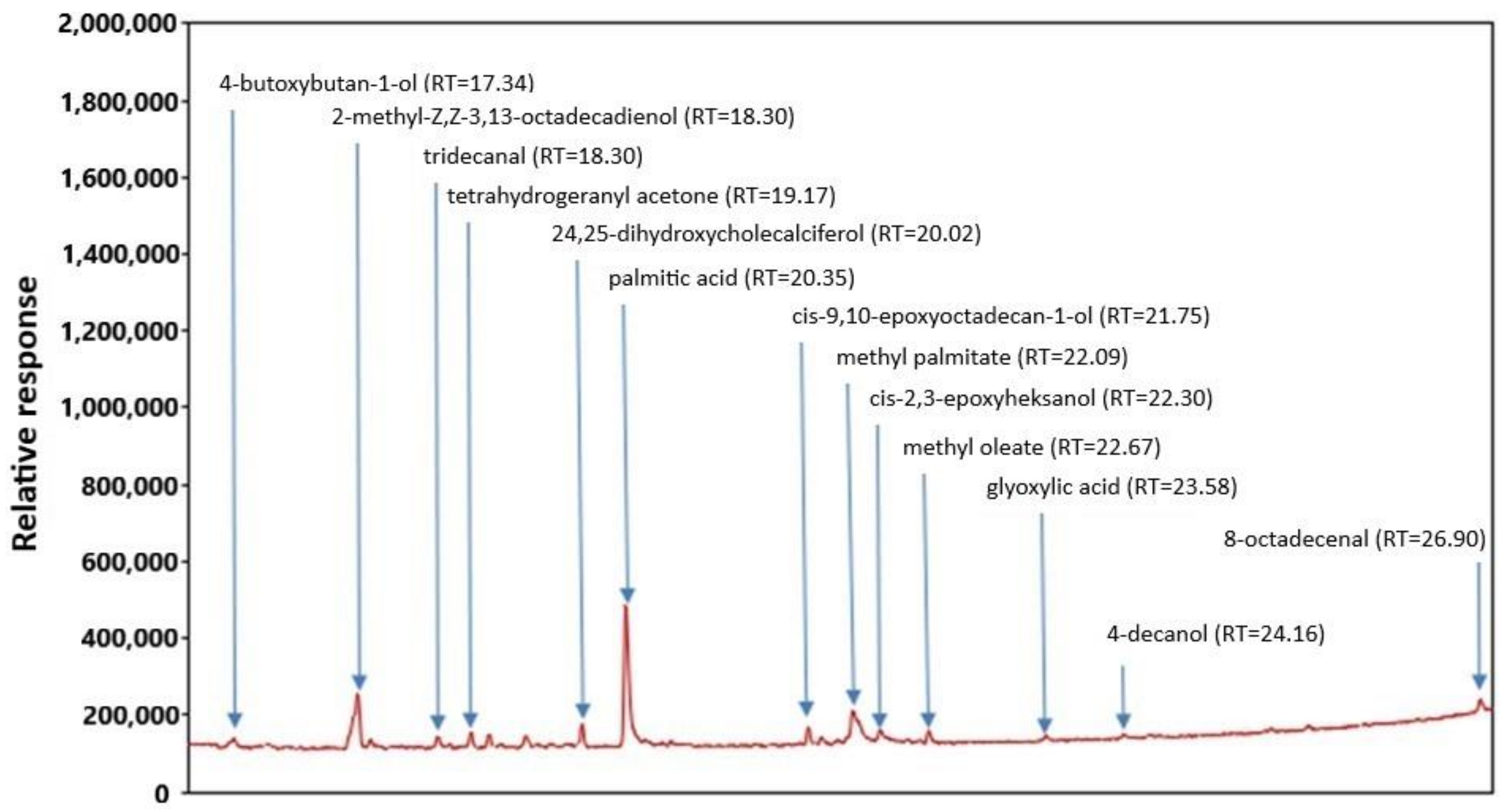
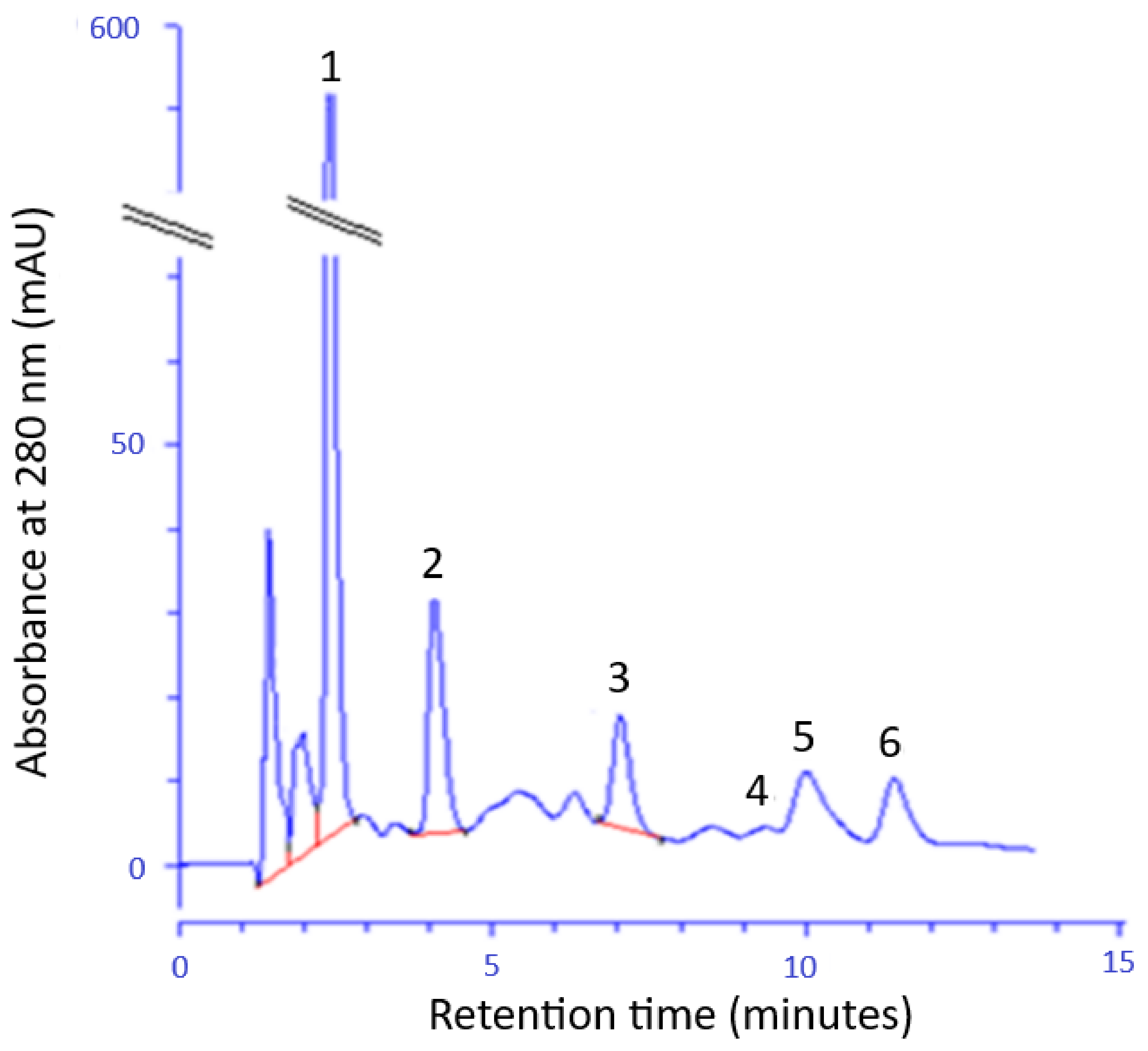



| Evaluated Compound/Parameter | (mg/100 mL) |
|---|---|
| Chlorogenic acid (ChA) | 26.78 ± 0.55 |
| Gallic acid (GA) | 78.02 ± 1.00 |
| 4- hydroxybenzoic acid (4-HA) | 34.97 ± 0.07 |
| 3- hydroxybenzoic acid (3-HB) | 12.64 ± 1.20 |
| 3,4-dihydroxybenzoic acid (3,4-DHA) | 15.55 ± 0.38 |
| Caffeic acid (CA) | 7.13 ± 0.33 |
| Total polyphenol content (mmol GA/l) | 41.04 ± 0.10 |
| DPPH (mmol Trolox/l) | 19.36 ± 0.24 |
| ABTS (mmol Trolox/l) | 21.51 ± 0.86 |
| Sample | Young Modulus [MPa] | Elongation at Break [%] | Tensile Strength [MPa] |
|---|---|---|---|
| BC | 13,807.88 ± 596.43 | 0.85 ± 0.34 | 115.53 ± 15.28 |
| BC-5%FEE | 20,974.64 ± 115.12 | 1.08 ± 0.16 | 137.38 ± 40.86 |
| BC-10%FEE | 11,327.83 ± 144.20 | 0.76 ± 0.14 | 76.48 ± 19.06 |
| BC-5%FEE | BC-10%FEE | BC (Control) | ||
|---|---|---|---|---|
| Phenolic acid (µg/g membrane) | ChA | 83.69 ± 2.57 | 140.52 ± 6.44 | nd |
| GA | 275.44 ± 56.44 | 453.66 ± 6.95 | nd | |
| 4-HB | 150. 31 ± 11.31 | 285.15 ± 21.28 | nd | |
| 3-HB | 43.74 ± 4.83 | 72.50 ± 6.48 | nd | |
| 3,4-DHA | 75.93 ± 1.13 | 116.17 ± 8.65 | nd | |
| CA | 31.83 ± 1.23 | 57.80 ± 4.12 | nd | |
| Total polyphenol content (mmol GA/l) | 0.45 ± 0.01 | 0.63 ± 0.02 | na | |
| DPPH (mmol Trolox/l) | 0.44 ± 0.05 | 0.55 ± 0.01 | na | |
| ABTS (mmol Trolox/l) | 1.59 ± 0.01 | 2.09 ± 0.01 | na | |
| BC + FEE5% | BC + FEE 10% | BC | |
|---|---|---|---|
| Total polyphenol content (mmol GA/dm3) | 0.29 ± 0.02 | 0.59 ± 0.09 | nd |
| Cell viability (% of the control medium) | 73.99 ± 7.14 | 14.16 ± 5.91 | 102.21 ± 3.73 |
| BC + FEE5% | BC + FEE 10% | BC (Control) | ||
|---|---|---|---|---|
| Phenolic acid (µg) | ChA | 1.28 ± 0.25 | 2.16 ± 0.430 | nd |
| GA | 9.22 ± 0.84 | 12.26 ± 1.96 | nd | |
| 4-HB | 5.07 ± 0.78 | 6.56 ± 0.09 | nd | |
| 3-HB | < 0.50 | < 0.50 | nd | |
| 3,4-DHA | 2.01 ± 0.23 | 3.56 ± 0.46 | nd | |
| CA | < 0.50 | 1.40 ± 0.31 | nd | |
| Total polyphenol content (mmol GA/l) | 0.016 ± 0.01 | 0.051 ± 0.01 | na | |
| DPPH (mmol Trolox/l) | na | na | na | |
| ABTS (mmol Trolox/l) | 0.084 ± 0.02 | 0.15 ± 0.03 | na | |
| Phenolic Acid | BC-5%FEE | BC-10%FEE | ||||
|---|---|---|---|---|---|---|
| JSS, μg cm−2 h−1 | KP 10−5, cm h−1 | LT, h | JSS, μg cm−2 h−1 | KP 10−5, cm h−1 | LT, h | |
| ChA | -iv | -iv | ~5 | 0.267 ± 0.021 | 379.315 ± 36.493 | 2.379 |
| GA | 0.389 ± 0.096 | 309.168 ± 76.150 | 2.009 | 0.549 ± 0.079 | 242.032 ± 34.83 | 1.479 |
| 4-HB | 0.389 ± 0.043 | 517.582 ± 57.202 | 2.444 | 0.345 ± 0.008 | 241.976 ± 5.516 | 1.359 |
| 3-HB | -iv | -iv | ~24 | -iv | -iv | ~5 |
| 3,4-DHB | 0.195 ± 0.031 | 512.116 ± 81.140 | 1.820 | 0.345 ± 0.019 | 593.944 ± 33.447 | 1.359 |
| CA | -iv | -iv | 2.204 | 0.138 ± 0.022 | 477.516 ± 77.129 | 1.377 |
| BC + FEE5% | BC + FEE 10% | BC (Control) | ||
|---|---|---|---|---|
| Phenolic acid (µg/g skin) | ChA | 30.77 ± 0.95 | 41.05 ± 1.99 | nd |
| GA | 151.34 ± 13.85 | 222.94 ± 16.60 | nd | |
| 4-HB | 45.36 ± 1.33 | 127.67 ± 1.59 | nd | |
| 3-HB | 17.93 ± 0.93 | 30.62 ± 4.06 | nd | |
| 3,4-DHA | 31.91 ± 0.62 | 52.03 ± 5.11 | nd | |
| CA | 21.58 ±1.42 | 41.18 ± 3.16 | nd | |
| Total polyphenol content (mmol GA/l) | 0.33 ± 0.01 | 0.44 ± 0.01 | na | |
| DPPH (mmol Trolox/l) | 0.27 ± 0.005 | 0.39 ± 0.01 | na | |
| ABTS (mmol Trolox/l) | 1.02 ± 0.01 | 1.52 ± 0.06 | na | |
| Sample | mg FEE/g Membrane * |
|---|---|
| BC | - |
| BC-5%FEE | 465.0 |
| BC-10%FEE | 857.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Ossowicz-Rupniewska, P.; Rakoczy, R.; Konopacki, M.; Perużyńska, M.; Droździk, M.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Wenelska, K.; et al. Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. Int. J. Mol. Sci. 2021, 22, 6269. https://doi.org/10.3390/ijms22126269
Nowak A, Ossowicz-Rupniewska P, Rakoczy R, Konopacki M, Perużyńska M, Droździk M, Makuch E, Duchnik W, Kucharski Ł, Wenelska K, et al. Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. International Journal of Molecular Sciences. 2021; 22(12):6269. https://doi.org/10.3390/ijms22126269
Chicago/Turabian StyleNowak, Anna, Paula Ossowicz-Rupniewska, Rafał Rakoczy, Maciej Konopacki, Magdalena Perużyńska, Marek Droździk, Edyta Makuch, Wiktoria Duchnik, Łukasz Kucharski, Karolina Wenelska, and et al. 2021. "Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin" International Journal of Molecular Sciences 22, no. 12: 6269. https://doi.org/10.3390/ijms22126269
APA StyleNowak, A., Ossowicz-Rupniewska, P., Rakoczy, R., Konopacki, M., Perużyńska, M., Droździk, M., Makuch, E., Duchnik, W., Kucharski, Ł., Wenelska, K., & Klimowicz, A. (2021). Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. International Journal of Molecular Sciences, 22(12), 6269. https://doi.org/10.3390/ijms22126269