The Molecular Characteristics of Non-Clear Cell Renal Cell Carcinoma: What’s the Story Morning Glory?
Abstract
1. Introduction
2. Main Histological Subtypes
2.1. Papillary RCC
2.2. Chromophobe RCC
2.3. RCC with Sarcomatoid/Rhabdoid Features
2.4. Collecting Duct RCC
2.5. MiT Family Translocation RCC
2.6. Renal Medullary Carcinoma
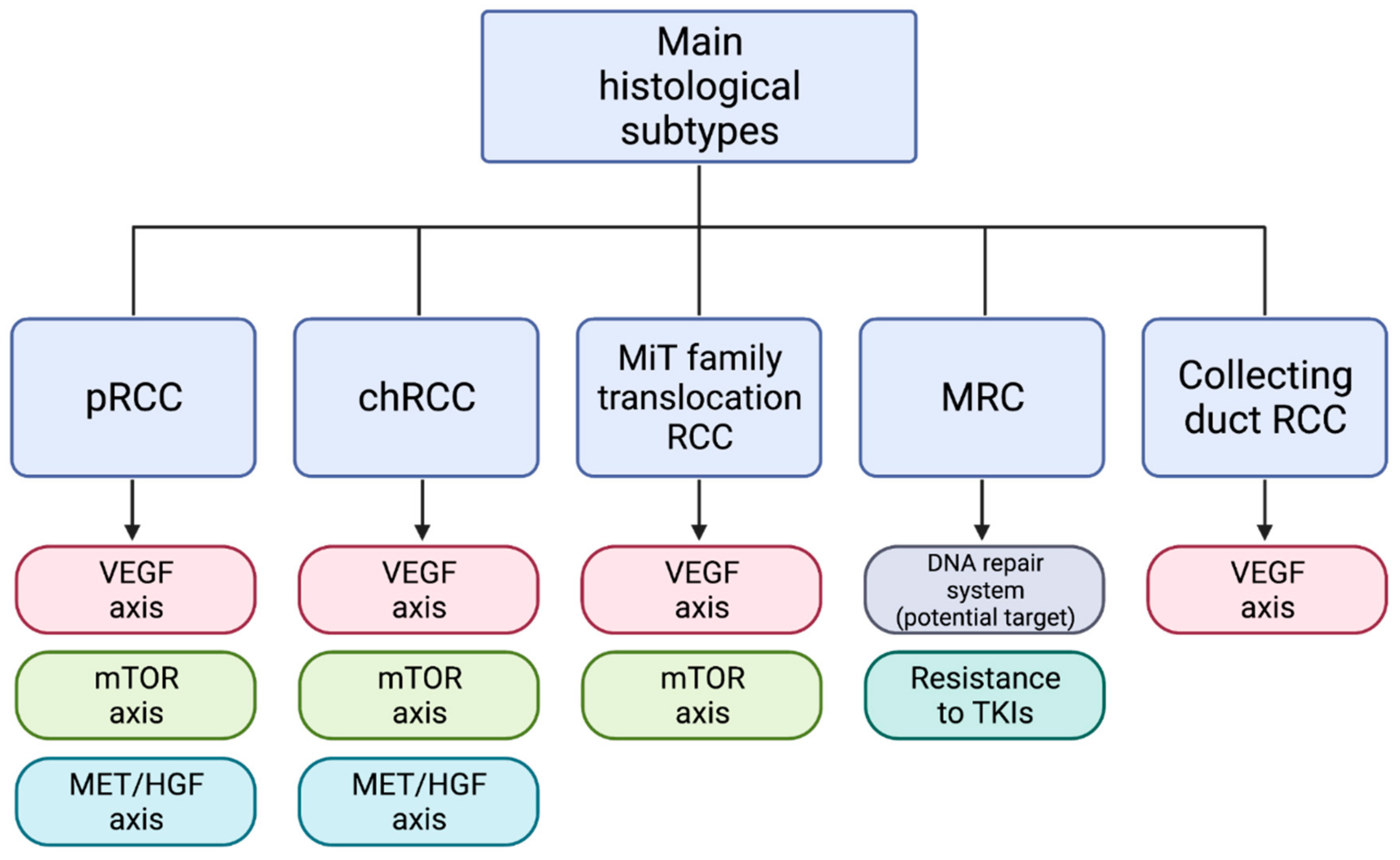
3. Molecular Targets
3.1. VEGF Axis Pathway
3.2. mTOR Pathway
3.3. MET Pathway
| Trials | Histologies | Drug | Setting | N. of Patients | ORR (%) | mOS (Months) | mPFS (Months) |
|---|---|---|---|---|---|---|---|
| SWOG 1500/PAPMET (phase II) [92] | pRCC | -cabozantinib -sunitinib -savolitinib -crizotinib | First or second line | −44 −46 −29 −28 | −23 −4 −3 −0 | −20.4 −16.4 −11.7 −19.9 | 9.0 −5.6 −3.0 −2.8 |
| SAVOIR (phase III) [96] | pRCC | -savolitinib -sunitinib | First or later line | −33 −27 | −27 −7 | -NR −13.2 | −7.0 −5.6 |
| SUPAP (phase II) [65] | pRCC: -type 1 -type 2 | -sunitinib | First line | −15 −46 | −13 −11 | −17.8 −12.4 | −6.6 −5.5 |
| CREATE (phase II) [98] | pRCC: -type 1 -MET-driven type1 -MET-independent type1 | -crizotinib | First or later line | −23 −4 −19 | −17 −50 −11 | −30.5 -NA −14.5 | −5.8 -NA −3.0 |
| SWOG S1107 (phase II) [100] | pRCC | -tivantinib -tivantinib + erlotinib | First or second line | −25 −25 | −0 −0 | −10.3 −11.3 | −2.0 −3.9 |
| NCT00726323 (phase II) [99] | pRCC | -foretinib | First or second line | 74 | 13.5 | NA | 9.3 |
| NCT02127710 (phase II) | -pRCC -MET-driven pRCC -MET-independent pRCC | -savolitinib | First or later line | −109 −44 −65 | −7 −18 −0 | -NA -NA -NA | -NA −6.2 −1.4 |
4. Immunotherapy and New Therapeutic Perspectives in nccRCC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Ordonez, M.A.; Iasonos, A.; Secin, F.P.; Guillonneau, B.; Russo, P.; Touijer, K. Renal Cell Carcinoma in Young and Old Patients—Is There a Difference? J. Urol. 2008, 180, 1262–1266. [Google Scholar] [CrossRef]
- Marchioni, M.; Rivas, J.G.; Autran, A.; Socarras, M.; Albisinni, S.; Ferro, M.; Schips, L.; Scarpa, R.M.; Papalia, R.; Esperto, F. Biomarkers for Renal Cell Carcinoma Recurrence: State of the Art. Curr. Urol. Rep. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Ahrens, M.; Scheich, S.; Hartmann, A.; Bergmann, L. IAG-N Interdisciplinary Working Group Kidney Cancer of the German Cancer Society Non-Clear Cell Renal Cell Carcinoma—Pathology and Treatment Options. Oncol. Res. Treat. 2019, 42, 128–135. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Flippot, R.; Rioux-Leclercq, N.; Choueiri, T.K. Non–Clear Cell Renal Cell Carcinomas: From Shadow to Light. J. Clin. Oncol. 2018, 36, 3624–3631. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Scarpelli, M.; Montironi, R.; Kirkali, Z. 2004 WHO Classification of the Renal Tumors of the Adults. Eur. Urol. 2006, 49, 798–805. [Google Scholar] [CrossRef]
- Rizzo, A.; Rosellini, M.; Marchetti, A.; Mollica, V.; Massari, F. Determinants of treatment for first-line immune-based combinations in metastatic renal cell carcinoma: A critical overview of recent evidence. Immunotherapy 2021, 13, 685–692. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, J.; Maia, M.C.; Pal, S.K. Systemic Therapy for Non–Clear Cell Renal Cell Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of renal cell carcinoma: Findings and clinical implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef]
- Ciccarese, C.; Massari, F.; Santoni, M.; Heng, D.Y.; Sotte, V.; Brunelli, M.; Conti, A.; Cheng, L.; Lopez-Beltran, A.; Scarpelli, M.; et al. New molecular targets in non clear renal cell carcinoma: An overview of ongoing clinical trials. Cancer Treat. Rev. 2015, 41, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Said, J.W.; Seligson, D.B.; Rao, P.N.; De Martino, M.; Shuch, B.; Zomorodian, N.; Kabbinavar, F.F.; Belldegrun, A.S.; Pantuck, A.J. Pathological, Immunohistochemical and Cytogenetic Features of Papillary Renal Cell Carcinoma With Clear Cell Features. J. Urol. 2011, 185, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Linehan, W.M.; Spellman, P.T.; Ricketts, C.J.; Creighton, C.J.; Fei, S.S.; Davis, C.; Wheeler, D.A.; Murray, B.A.; Schmidt, L.; et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 135–145. [Google Scholar] [CrossRef]
- Yamashita, S.; Ioritani, N.; Oikawa, K.; Aizawa, M.; Endoh, M.; Arai, Y. Morphological subtyping of papillary renal cell carcinoma: Clinicopathological characteristics and prognosis. Int. J. Urol. 2007, 14, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Di Lena, R.; Breau, R.H.; Pouliot, F.; Finelli, A.; Lavallée, L.T.; So, A.; Tanguay, S.; Fairey, A.; Rendon, R.; et al. Morphologic subtyping as a prognostic predictor for survival in papillary renal cell carcinoma: Type 1 vs. type 2. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Pignot, G.; Elie, C.; Conquy, S.; Vieillefond, A.; Flam, T.; Zerbib, M.; Debré, B.; Amsellem-Ouazana, D. Survival Analysis of 130 Patients with Papillary Renal Cell Carcinoma: Prognostic Utility of Type 1 and Type 2 Subclassification. Urology 2007, 69, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Eyzaguirre, E. Clear Cell Papillary Renal Cell Carcinoma. Arch. Pathol. Lab. Med. 2019, 143, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Duh, F.-M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Cimadamore, A.; Massari, F.; Santoni, M.; Mollica, V.; Di Nunno, V.; Cheng, L.; López-Beltrán, A.; Scarpelli, M.; Montironi, R.; Moch, H. Molecular characterization and diagnostic criteria of renal cell carcinoma with emphasis on liquid biopsies. Expert Rev. Mol. Diagn. 2019, 20, 141–150. [Google Scholar] [CrossRef]
- Kovac, M.; Navas, C.; Horswell, S.; Salm, M.; Bardella, C.; Rowan, A.; Stares, M.; Castro-Giner, F.; Fisher, R.; De Bruin, E.C.; et al. Recurrent chromosomal gains and heterogeneous driver mutations characterise papillary renal cancer evolution. Nat. Commun. 2015, 6, 6336. [Google Scholar] [CrossRef]
- Trpkov, K.; Hes, O.; Agaimy, A.; Bonert, M.; Martinek, P.; Magi-Galluzzi, C.; Kristiansen, G.; Lüders, C.; Nesi, G.; Compérat, E.; et al. Fumarate Hydratase–deficient Renal Cell Carcinoma Is Strongly Correlated With Fumarate Hydratase Mutation and Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Am. J. Surg. Pathol. 2016, 40, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Ali, S.M.; Yakirevich, E.; Geynisman, D.M.; Karam, J.A.; Elvin, J.A.; Frampton, G.M.; Huang, X.; Lin, D.I.; Rosenzweig, M.; et al. Characterization of Clinical Cases of Advanced Papillary Renal Cell Carcinoma via Comprehensive Genomic Profiling. Eur. Urol. 2018, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Campbell, S.C.; Escudier, B. Renal cell carcinoma. Lancet 2009, 28, 1119–1132. [Google Scholar] [CrossRef]
- Garje, R.; Elhag, D.; Yasin, H.A.; Acharya, L.; Vaena, D.; Dahmoush, L. Comprehensive review of chromophobe renal cell carcinoma. Crit. Rev. Oncol. 2021, 160, 103287. [Google Scholar] [CrossRef] [PubMed]
- Montironi, R.; Cimadamore, A.; Ohashi, R.; Cheng, L.; Scarpelli, M.; Lopez-Beltran, A.; Moch, H. Chromophobe Renal Cell Carcinoma Aggressiveness and Immuno-oncology Therapy: How to Distinguish the Good One from the Bad One. Eur. Urol. Oncol. 2021, 4, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Casuscelli, J.; Becerra, M.F.; Seier, K.; Manley, B.J.; Benfante, N.; Redzematovic, A.; Stief, C.G.; Hsieh, J.J.; Tickoo, S.K.; Reuter, V.E.; et al. Chromophobe Renal Cell Carcinoma: Results From a Large Single-Institution Series. Clin. Genitourin. Cancer 2019, 17, 373–379.e4. [Google Scholar] [CrossRef]
- Davis, C.F.; Ricketts, C.J.; Wang, M.; Yang, L.; Cherniack, A.D.; Shen, H.; Buhay, C.; Kang, H.; Kim, S.C.; Fahey, C.C.; et al. The Somatic Genomic Landscape of Chromophobe Renal Cell Carcinoma. Cancer Cell 2014, 26, 319–330. [Google Scholar] [CrossRef]
- Mollica, V.; Franceschini, T.; Gruppioni, E.; Rizzo, A.; Ricci, C.; Schiavina, R.; Brunocilla, E.; Ardizzoni, A.; Fiorentino, M.; Giunchi, F.; et al. Broad spectrum mutational analysis of chromophobe renal cell carcinoma using next-generation sequencing. Pathol.-Res. Pr. 2021, 219, 153350. [Google Scholar] [CrossRef]
- Yang, P.; Cornejo, K.M.; Sadow, P.; Cheng, L.; Wang, M.; Xiao, Y.; Jiang, Z.; Oliva, E.; Jozwiak, S.; Nussbaum, R.L.; et al. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Am. J. Surg. Pathol. 2014, 38, 895–909. [Google Scholar] [CrossRef]
- Ged, Y.; Chen, Y.-B.; Knezevic, A.; Casuscelli, J.; Redzematovic, A.; DiNatale, R.G.; Carlo, M.I.; Lee, C.-H.; Feldman, D.R.; Patil, S.; et al. Metastatic Chromophobe Renal Cell Carcinoma: Presence or Absence of Sarcomatoid Differentiation Determines Clinical Course and Treatment Outcomes. Clin. Genitourin. Cancer 2019, 17, e678–e688. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Chittoria, N.; Choueiri, T.K.; Kroeger, N.; Lee, J.-L.; Srinivas, S.; Knox, J.J.; Bjarnason, G.A.; Ernst, S.D.; Wood, L.A.; et al. Outcome of Patients With Metastatic Sarcomatoid Renal Cell Carcinoma: Results From the International Metastatic Renal Cell Carcinoma Database Consortium. Clin. Genitourin. Cancer 2015, 13, e79–e85. [Google Scholar] [CrossRef]
- Przybycin, C.G.; McKenney, J.K.; Reynolds, J.P.; Campbell, S.; Zhou, M.; Karafa, M.T.; Magi-Galluzzi, C. Rhabdoid Differentiation Is Associated With Aggressive Behavior in Renal Cell Carcinoma. Am. J. Surg. Pathol. 2014, 38, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.D.; Eble, J.N.; Wang, M.; MacLennan, G.T.; Jain, S.; Cheng, L. Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation. Cancer 2005, 104, 1195–1203. [Google Scholar] [CrossRef]
- Bakouny, Z.; Braun, D.A.; Shukla, S.A.; Pan, W.; Gao, X.; Hou, Y.; Flaifel, A.; Tang, S.; Bosma-Moody, A.; He, M.X.; et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Malouf, G.G.; Flippot, R.; Dong, Y.; DiNatale, R.G.; Chen, Y.-B.; Su, X.; Compérat, E.; Rouprêt, M.; Mano, R.; Blum, K.A.; et al. Molecular characterization of sarcomatoid clear cell renal cell carcinoma unveils new candidate oncogenic drivers. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, T.B.; Peng, B.; Karam, J.A.; Creighton, C.J.; Joon, A.; Kawakami, F.; Trevisan, P.; Jonasch, E.; Chow, C.-W.; et al. Sarcomatoid Renal Cell Carcinoma Has a Distinct Molecular Pathogenesis, Driver Mutation Profile, and Transcriptional Landscape. Clin. Cancer Res. 2017, 23, 6686–6696. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Murugan, P.; Patel, L.R.; Voicu, H.; Yoo, S.-Y.; Majewski, T.; Mehrotra, M.; Wani, K.; Tannir, N.M.; Karam, J.A.; et al. Intratumoral morphologic and molecular heterogeneity of rhabdoid renal cell carcinoma: Challenges for personalized therapy. Mod. Pathol. 2015, 28, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Philip, E.J.; Pal, S.K. Genomic profiling in renal cell carcinoma. Nat. Rev. Nephrol. 2020, 16, 1–17. [Google Scholar] [CrossRef]
- Bi, M.; Zhao, S.; Said, J.W.; Merino, M.J.; Adeniran, A.J.; Xie, Z.; Nawaf, C.B.; Choi, J.; Belldegrun, A.S.; Pantuck, A.J.; et al. Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma. Proc. Natl. Acad. Sci. USA 2016, 113, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Mattelaer, P.; Wolff, J.M.; Brauers, A.; Ijzerman, W.; Füzesi, L.; Jakse, G. Bellini duct carcinoma: A rare variant of renal cell carcinoma. Acta Urol. Belg. 1996, 64, 33–35. [Google Scholar] [PubMed]
- Orsola, A.; Trias, I.; Raventós, C.; Español, I.; Cecchini, L.; Orsola, I. Renal collecting (Bellini) duct carcinoma displays similar characteristics to upper tract urothelial cell carcinoma. Urology 2005, 65, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Milowsky, M.I.; Rosmarin, A.S.; Tickoo, S.K.; Papanicolaou, N.; Nanus, D.M. Active chemotherapy for collecting duct carcinoma of the kidney. Cancer 2001, 94, 111–116. [Google Scholar] [CrossRef]
- Pal, S.K.; Choueiri, T.K.; Wang, K.; Khaira, D.; Karam, J.A.; Van Allen, E.; Palma, N.A.; Stein, M.N.; Johnson, A.; Squillace, R.; et al. Characterization of Clinical Cases of Collecting Duct Carcinoma of the Kidney Assessed by Comprehensive Genomic Profiling. Eur. Urol. 2016, 70, 516–521. [Google Scholar] [CrossRef]
- Camparo, P.; Vasiliu, V.; Molinie, V.; Couturier, J.; Dykema, K.J.; Petillo, D.; Furge, K.A.; Comperat, E.M.; Lae, M.; Bouvier, R.; et al. Renal Translocation Carcinomas: Clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am. J. Surg. Pathol. 2008, 32, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.Y.; Harrison, D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef]
- Argani, P.; Olgac, S.; Tickoo, S.K.; Goldfischer, M.; Moch, H.; Chan, D.Y.; Eble, J.N.; Bonsib, S.M.; Jimeno, M.; Lloreta, J.; et al. Xp11 Translocation Renal Cell Carcinoma in Adults: Expanded Clinical, Pathologic, and Genetic Spectrum. Am. J. Surg. Pathol. 2007, 31, 1149–1160. [Google Scholar] [CrossRef]
- Mir, M.C.; Trilla, E.; De Torres, I.M.; Panizo, Á.; Zlotta, A.R.; Van Rhijn, B.; Morote, J. Altered transcription factor E3 expression in unclassified adult renal cell carcinoma indicates adverse pathological features and poor outcome. BJU Int. 2010, 108, E71–E76. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.I.; Argani, P.; Adeniran, A.; Hampton, E.; De Marzo, A.; Hicks, J.; Collins, M.H. Translocation renal cell carcinoma: Lack of negative impact due to lymph node spread. Cancer 2008, 112, 1607–1616. [Google Scholar] [CrossRef]
- Msaouel, P.; Hong, A.L.; Mullen, E.A.; Atkins, M.B.; Walker, C.L.; Lee, C.-H.; Carden, M.A.; Genovese, G.; Linehan, W.M.; Rao, P.; et al. Updated Recommendations on the Diagnosis, Management, and Clinical Trial Eligibility Criteria for Patients With Renal Medullary Carcinoma. Clin. Genitourin. Cancer 2019, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734.e13. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharmacal Res. 2021, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Bracarda, S.; Nabissi, M.; Massari, F.; Conti, A.; Bria, E.; Tortora, G.; Santoni, G.; Cascinu, S. CXC and CC Chemokines as Angiogenic Modulators in Nonhaematological Tumors. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Tumor Hypoxia: Causative Mechanisms, Microregional Heterogeneities, and the Role of Tissue-Based Hypoxia Markers. Adv. Exp. Med. Biol. 2016, 923, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol. Res. 2017, 120, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Jr, W.G.K. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat. Med. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Ljungberg, B.J.; Jacobsen, J.; Rudolfsson, S.H.; Lindh, G.; Grankvist, K.; Rasmuson, T. Different vascular endothelial growth factor (VEGF), VEGF-receptor 1 and -2 mRNA expression profiles between clear cell and papillary renal cell carcinoma. BJU Int. 2006, 98, 661–667. [Google Scholar] [CrossRef]
- Li, Z.-C.; Zhai, G.; Zhang, J.; Wang, Z.; Liu, G.; Wu, G.-Y.; Liang, D.; Zheng, H. Differentiation of clear cell and non-clear cell renal cell carcinomas by all-relevant radiomics features from multiphase CT: A VHL mutation perspective. Eur. Radiol. 2018, 29, 3996–4007. [Google Scholar] [CrossRef]
- Stenehjem, D.D.; Hahn, A.W.; Gill, D.M.; Albertson, D.; Gowrishankar, B.; Merriman, J.; Agarwal, A.M.; Thodima, V.; Harrington, E.B.; Au, T.H.; et al. Predictive genomic markers of response to VEGF targeted therapy in metastatic renal cell carcinoma. PLoS ONE 2019, 14, e0210415. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Eisen, T.; Broderick, S.; Stadler, W.M.; Jones, R.J.; Garcia, J.A.; Vaishampayan, U.N.; Picus, J.; Hawkins, R.E.; et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016, 17, 378–388. [Google Scholar] [CrossRef]
- Tannir, N.M.; Jonasch, E.; Albiges, L.; Altinmakas, E.; Ng, C.S.; Matin, S.F.; Wang, X.; Qiao, W.; Lim, Z.D.; Tamboli, P.; et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non–Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur. Urol. 2016, 69, 866–874. [Google Scholar] [CrossRef]
- Motzer, R.J.; Barrios, C.H.; Kim, T.M.; Falcon, S.; Cosgriff, T.; Harker, W.G.; Srimuninnimit, V.; Pittman, K.; Sabbatini, R.; Rha, S.Y.; et al. Phase II Randomized Trial Comparing Sequential First-Line Everolimus and Second-Line Sunitinib Versus First-Line Sunitinib and Second-Line Everolimus in Patients With Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2014, 32, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Chen, D.; Wang, P.I.; Marker, M.; Redzematovic, A.; Chen, Y.-B.; Selcuklu, S.D.; Weinhold, N.; Bouvier, N.; Huberman, K.H.; et al. Genomic Biomarkers of a Randomized Trial Comparing First-line Everolimus and Sunitinib in Patients with Metastatic Renal Cell Carcinoma. Eur. Urol. 2017, 71, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, L.; Grünwald, V.; Maute, L.; Grimm, M.-O.; Weikert, S.; Schleicher, J.; Klotz, T.; Greiner, J.; Flörcken, A.; Hartmann, A.; et al. A Randomized Phase IIa Trial with Temsirolimus versus Sunitinib in Advanced Non-Clear Cell Renal Cell Carcinoma: An Intergroup Study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society. Oncol. Res. Treat. 2020, 43, 333–339. [Google Scholar] [CrossRef]
- Ravaud, A.; Oudard, S.; De Fromont, M.; Chevreau, C.; Gravis, G.; Zanetta, S.; Theodore, C.; Jimenez, M.; Sevin, E.; Laguerre, B.; et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: A phase II study (SUPAP) by the French Genitourinary Group (GETUG). Ann. Oncol. 2015, 26, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Ged, Y.; Lee, C.; Ms, A.K.; Molina, A.M.; Chen, Y.; Chaim, J.; Ms, D.T.C.; Murray, S.; Tickoo, S.K.; et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: Final results from a phase II trial. Cancer 2020, 126, 5247–5255. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Wang, X.; Zhang, L.; Collins, M.P.; Signoretti, S.; Alsop, D.C.; Goldberg, S.N.; Atkins, M.B.; Mier, J.W. Renal Cancer Resistance to Antiangiogenic Therapy Is Delayed by Restoration of Angiostatic Signaling. Mol. Cancer Ther. 2010, 9, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Plantade, A.; Elson, P.; Negrier, S.; Ravaud, A.; Oudard, S.; Zhou, M.; Rini, B.I.; Bukowski, R.M.; Escudier, B. Efficacy of Sunitinib and Sorafenib in Metastatic Papillary and Chromophobe Renal Cell Carcinoma. J. Clin. Oncol. 2008, 26, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Brunelli, M.; Gnetti, L.; Maestroni, U.; Buti, S. Pazopanib as a possible option for the treatment of metastatic non-clear cell renal carcinoma patients: A systematic review. Ther. Adv. Med Oncol. 2020, 12. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Srinivasan, R.; Schmidt, L.S. The genetic basis of kidney cancer: A metabolic disease. Nat. Rev. Urol. 2010, 7, 277–285. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef]
- Ramkumar, R.R.; Murthy, P.B.; Nguyen, J.K.; McKenney, J.; Eng, C.; Campbell, S.C. PTEN Hamartoma Tumor Syndrome: A Case of Renal Cell Carcinoma in a Young Female. Urol. 2021, 148, 113–117. [Google Scholar] [CrossRef]
- Trnka, P.; Kennedy, S.E. Renal tumors in tuberous sclerosis complex. Pediatr. Nephrol. 2021, 36, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824. [Google Scholar] [CrossRef]
- Reyes, J.M.J.R.; Cuesta, R.; Pause, A. Folliculin: A Regulator of Transcription through AMPK and mTOR Signaling Pathways. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Molecular genetics and clinical features of Birt–Hogg–Dubé syndrome. Nat. Rev. Urol. 2015, 12, 558–569. [Google Scholar] [CrossRef]
- Argani, P.; Hicks, J.; De Marzo, A.M.; Albadine, R.; Illei, P.B.; Ladanyi, M.; Reuter, V.E.; Netto, G.J. Xp11 Translocation Renal Cell Carcinoma (RCC): Extended Immunohistochemical Profile Emphasizing Novel RCC Markers. Am. J. Surg. Pathol. 2010, 34, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, N.P.; Budka, J.A.; Khella, H.W.; Ferris, M.W.; Ku, S.Y.; Kauffman, E.; Wood, A.C.; Ahmed, K.; Chintala, V.N.; Adelaiye-Ogala, R.; et al. Therapeutic Targeting of TFE3/IRS-1/PI3K/mTOR Axis in Translocation Renal Cell Carcinoma. Clin. Cancer Res. 2018, 24, 5977–5989. [Google Scholar] [CrossRef]
- Morris, M.R.; Maina, E.; Morgan, N.V.; Gentle, D.; Astuti, D.; Moch, H.; Kishida, T.; Yao, M.; Schraml, P.; Richards, F.M.; et al. Molecular genetic analysis of FIH-1, FH, and SDHB candidate tumour suppressor genes in renal cell carcinoma. J. Clin. Pathol. 2004, 57, 706–711. [Google Scholar] [CrossRef]
- Aghamir, S.M.K.; Heshmat, R.; Ebrahimi, M.; Ketabchi, S.E.; Dizaji, S.P.; Khatami, F. The Impact of Succinate Dehydrogenase Gene (SDH) Mutations In Renal Cell Carcinoma (RCC): A Systematic Review. OncoTargets Ther. 2019, ume 12, 7929–7940. [Google Scholar] [CrossRef]
- Ciccarese, C.; Iacovelli, R.; Brunelli, M.; Massari, F.; Bimbatti, D.; Fantinel, E.; De Marco, V.; Porcaro, A.B.; Martignoni, G.; Artibani, W.; et al. Addressing the best treatment for non-clear cell renal cell carcinoma: A meta-analysis of randomised clinical trials comparing VEGFR-TKis versus mTORi-targeted therapies. Eur. J. Cancer 2017, 83, 237–246. [Google Scholar] [CrossRef]
- Escudier, B.; Molinie, V.; Bracarda, S.; Maroto, P.; Szczylik, C.; Nathan, P.; Negrier, S.; Weiss, C.; Porta, C.; Grünwald, V.; et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur. J. Cancer 2016, 69, 226–235. [Google Scholar] [CrossRef]
- Blank, C.U.; Bono, P.; Larkin, J.M.G.; Gogov, S.; Panneerselvam, A.; Garay, C.A.; Grünwald, V. Safety and Efficacy of Everolimus in Patients with Non-Clear Cell Renal Cell Carcinoma Refractory to VEGF-Targeted Therapy: Subgroup Analysis of REACT. J. Clin. Oncol. 2012, 30 (Suppl. 5), 402. [Google Scholar] [CrossRef]
- Koh, Y.; Lim, H.Y.; Ahn, J.H.; Lee, J.-L.; Rha, S.Y.; Kim, Y.J.; Kim, T.M.; Lee, S.-H. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann. Oncol. 2013, 24, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, B.; Ansari, J.; Aitchison, M.; Tho, L.M.; Campbell, R.; Jones, R.J. Efficacy of temsirolimus in metastatic chromophobe renal cell carcinoma. BMC Urology 2013, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.P.; De Souza, P.; McDermott, D.; Figlin, R.A.; Berkenblit, A.; Thiele, A.; Krygowski, M.; Strahs, A.; Feingold, J.; Hudes, G. Effect of temsirolimus versus interferon-α on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med. Oncol. 2009, 26, 202–209. [Google Scholar] [CrossRef]
- Garajová, I.; Giovannetti, E.; Biasco, G.; Peters, G.J. c-Met as a Target for Personalized Therapy. Transl. Oncogenomics 2015, 7 (Suppl. 1), 13–31. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Di Nunno, V.; Santoni, M.; Gatto, L.; Caserta, C.; Morelli, F.; Zafarana, E.; Carrozza, F.; Mosca, A.; Mollica, V.; et al. Toward a genome-based treatment landscape for renal cell carcinoma. Crit. Rev. Oncol. Hematol. 2019, 142, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Di Nunno, V.; Gatto, L.; Santoni, M.; Scarpelli, M.; Cimadamore, A.; Lopez-Beltran, A.; Cheng, L.; Battelli, N.; Montironi, R.; et al. Resistance to Systemic Agents in Renal Cell Carcinoma Predict and Overcome Genomic Strategies Adopted by Tumor. Cancers 2019, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Guegan, J.; Le Formal, A.; Verkarre, V.; Rioux-Leclercq, N.; Sibony, M.; Bernhard, J.-C.; Camparo, P.; Merabet, Z.; Molinie, V.; et al. MET Is a Potential Target across All Papillary Renal Cell Carcinomas: Result from a Large Molecular Study of pRCC with CGH Array and Matching Gene Expression Array. Clin. Cancer Res. 2014, 20, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Tangen, C.; Thompson, I.M.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Juárez, V.M.O.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- McGregor, B.; Agarwal, N.; Suarez, C.; Tsao, C.-K.; Kelly, W.; Pagliaro, L.; Vaishampayan, U.; Castellano, D.; Loriot, Y.; Werneke, S.; et al. 709P Cabozantinib (C) in combination with atezolizumab (A) in non-clear cell renal cell carcinoma (nccRCC): Results from cohort 10 of the COSMIC-021 study. Ann. Oncol. 2020, 31, S558. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Plimack, E.; Arkenau, H.-T.; Jonasch, E.; Heng, D.Y.C.; Powles, T.; Frigault, M.M.; Clark, E.A.; Handzel, A.A.; Gardner, H.; et al. Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J. Clin. Oncol. 2017, 35, 2993–3001. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Heng, D.Y.C.; Lee, J.L.; Cancel, M.; Verheijen, R.B.; Mellemgaard, A.; Ottesen, L.H.; Frigault, M.M.; L’Hernault, A.; Szijgyarto, Z.; et al. Efficacy of Savolitinib vs Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Ran-domized Clinical Trial. JAMA Oncol. 2020, 6, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Rizzo, A.; Massari, F. Re: Toni K. Choueiri, Daniel Y.C. Heng, Jae Lyun Lee; et al. Efficacy of Savolitinib vs. Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol. In press. https://doi-org.ezproxy.unibo.it/10.1001/jamaoncol.2020.2218: SAVOIR: From Own Goal to Winning Goal? Eur. Urol. Oncol. 2020, 3, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P.; Wozniak, A.; Escudier, B.; Rutkowski, P.; Anthoney, A.; Bauer, S.; Sufliarsky, J.; van Herpen, C.; Lindner, L.H.; Grünwald, V.; et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur. J. Cancer 2017, 87, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Vaishampayan, U.; Rosenberg, J.E.; Logan, T.F.; Harzstark, A.L.; Bukowski, R.M.; Rini, B.I.; Srinivas, S.; Stein, M.N.; Adams, L.M.; et al. Phase II and Biomarker Study of the Dual MET/VEGFR2 Inhibitor Foretinib in Patients With Papillary Renal Cell Carcinoma. J. Clin. Oncol. 2013, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, P.W.; Tangen, C.M.; Wu, X.; Plets, M.R.; Plimack, E.R.; Agarwal, N.; Vogelzang, N.J.; Wang, J.; Tao, S.; Thompson, I.M.; et al. Parallel (Randomized) Phase II Evaluation of Tivantinib (ARQ197) and Tivantinib in Combination with Erlotinib in Papillary Renal Cell Carcinoma: SWOG S1107. Kidney Cancer 2017, 1, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Davis, I.J.; Argani, P.; Shukla, N.; McGill, G.G.; Nagai, M.; Saito, T.; Laé, M.; Fisher, D.E.; Ladanyi, M. TFE3 Fusions Activate MET Signaling by Transcriptional Up-regulation, Defining Another Class of Tumors as Candidates for Therapeutic MET Inhibition. Cancer Res. 2007, 67, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Goldberg, J.M.; Dubois, S.G.; Choy, E.; Rosen, L.; Pappo, A.; Geller, J.; Judson, I.; Hogg, D.; Senzer, N.; et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: Results of a multicenter phase 2 trial. Cancer 2012, 118, 5894–5902. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nat. Cell Biol. 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Massari, F.; Di Nunno, V.; Mollica, V.; Montironi, R.; Cheng, L.; Cimadamore, A.; Blanca, A.; Lopez-Beltran, A. Immunotherapy in renal cell carcinoma from poverty to the spoiled of choice. Immunotherapy 2019, 11, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Buti, S.; Conti, A.; Porta, C.; Procopio, G.; Sternberg, C.N.; Bracarda, S.; Basso, U.; De Giorgi, U.; Rizzo, M.; et al. Prognostic significance of host immune status in patients with late relapsing renal cell carcinoma treated with targeted therapy. Target. Oncol. 2015, 10, 517–522. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef]
- Braun, D.A.; Hou, Y.; Bakouny, Z.; Ficial, M.; Angelo, M.S.; Forman, J.; Ross-Macdonald, P.; Berger, A.C.; Jegede, O.A.; Elagina, L.; et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat. Med. 2020, 26, 909–918. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- McDermott, D.F.; Lee, J.-L.; Ziobro, M.; Suarez, C.; Langiewicz, P.; Matveev, V.B.; Wiechno, P.; Gafanov, R.A.; Tomczak, P.; Pouliot, F.; et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non–Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Voss, M.H.; Carlo, M.I.; Chen, Y.-B.; Reznik, E.; Knezevic, A.; Lefkowitz, R.A.; Shapnik, N.; Tassone, D.; Dadoun, C.; et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: Results of a phase 2 trial. J. Clin. Oncol. 2021, 39, 4509. [Google Scholar] [CrossRef]
- McKay, R.R.; McGregor, B.A.; Gray, K.; Steinharter, J.A.; Walsh, M.K.; Braun, D.A.; Flaifel, A.; VanAllen, E.; Wei, X.X.; Signoretti, S.; et al. Results of a phase II study of atezolizumab and bevacizumab in non-clear cell renal cell carcinoma (nccRCC) and clear cell renal cell carcinoma with sarcomatoid differentiation (sccRCC). J. Clin. Oncol. 2019, 37, 548. [Google Scholar] [CrossRef]
- Powles, T.; Larkin, J.M.G.; Patel, P.; Pérez-Valderrama, B.; Rodriguez-Vida, A.; Glen, H.; Thistlethwaite, F.; Ralph, C.; Srinivasan, G.; Mendez-Vidal, M.J.; et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J. Clin. Oncol. 2019, 37, 545. [Google Scholar] [CrossRef]
- Pal, S.K.; Tsao, C.K.; Suarez, C.; Kelly, W.; Pagliaro, L.; Vaishampayan, U.N.; Loriot, Y.; Srinivas, S.; McGregor, B.A.; Panneerselvam, A.; et al. 702O—Cabozantinib (C) in combination with atezolizumab (A) as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Results from the COSMIC-021 study. Ann. Oncol. 2020, 31 (Suppl. 4), S550. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Albiges, L.; Powles, T.; Scheffold, C.; Wang, F.; Motzer, R.J. A phase III study (COSMIC-313) of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in patients (pts) with previously untreated advanced renal cell carcinoma (aRCC) of intermediate or poor risk. J. Clin. Oncol. 2020, 38, TPS767. [Google Scholar] [CrossRef]
- Lee, C.-H.; Li, C.; Perini, R.F.; Hoehn, D.; Albiges, L. KEYNOTE-B61: Open-label phase 2 study of pembrolizumab in combination with lenvatinib as first-line treatment for non-clear cell renal cell carcinoma (nccRCC). J. Clin. Oncol. 2021, 39, TPS4595. [Google Scholar] [CrossRef]
- Hanif, A.; Pandey, M.; Khan, S.; Attwood, K.; George, S. Metastatic sarcomatoid renal cell carcinoma treated with immune checkpoint inhibitors. OncoImmunology 2019, 8, 1606639. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.M.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; Flaifel, A.; Pignon, J.-C.; Ficial, M.; Frontera, O.A.; George, S.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab versus Sunitinib in First-line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin. Cancer Res. 2021, 27, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Larkin, J.M.G.; Pal, S.K.; Motzer, R.J.; Venugopal, B.; Alekseev, B.Y.; Miyake, H.; Gravis, G.; Bilen, M.A.; Chudnovsky, A.; et al. DEfficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (sRCC): Subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A + Ax) vs. sunitinib (S). Ann. Oncol. 2019, 30 (Suppl. 5), v356–v402. [Google Scholar] [CrossRef]
- Rini, B.I.; Motzer, R.J.; Powles, T.; McDermott, D.F.; Escudier, B.; Donskov, F.; Hawkins, R.E.; Bracarda, S.; Bedke, J.; De Giorgi, U.; et al. Atezolizumab (atezo) + bevacizumab (bev) versus sunitinib (sun) in pts with untreated metastatic renal cell carcinoma (mRCC) and sarcomatoid (sarc) histology: IMmotion151 subgroup analysis. J. Clin. Oncol. 2019, 37, 4512. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Soulieres, D.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J. Clin. Oncol. 2019, 37, 4500. [Google Scholar] [CrossRef]
- Motzer, R.J.; Choueiri, T.K.; Powles, T.; Burotto, M.; Bourlon, M.T.; Hsieh, J.; Maruzzo, M.; Shah, A.Y.; Suarez, C.; Barrios, C.H.; et al. Nivolumab + cabozantinib (NIVO+CABO) vs. sunitinib (SUN) for advanced renal cell carcinoma (aRCC): Outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate-9ER. Abstract presented at: ASCO Genitourinary Cancers Symposium; 13 February 2021. Available online: https://meetinglibrary.asco.org/record/195192/abstract (accessed on 13 February 2021).
- Santoni, M.; Massari, F.; Aurilio, G.; Mollica, V.; Cimadamore, A.; Lopez-Beltran, A.; Cheng, L.; Battelli, N.; Nolé, F.; Montironi, R. Designing novel immunocombinations in metastatic renal cell carcinoma. Immunotherapy 2020, 12, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.; Saraiva, N.; Batinic-Haberle, I.; Castro, M.; Oliveira, N.G.; Fernandes, A.S. The SOD Mimic MnTnHex-2-PyP5+ Reduces the Viability and Migration of 786-O Human Renal Cancer Cells. Antioxidants 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Philip, E.; Salgia, S.; Pal, S.K. Evolving treatment paradigm in metastatic non clear cell renal cell carcinoma. Cancer Treat Res. Commun. 2020, 23, 100172. [Google Scholar] [CrossRef] [PubMed]
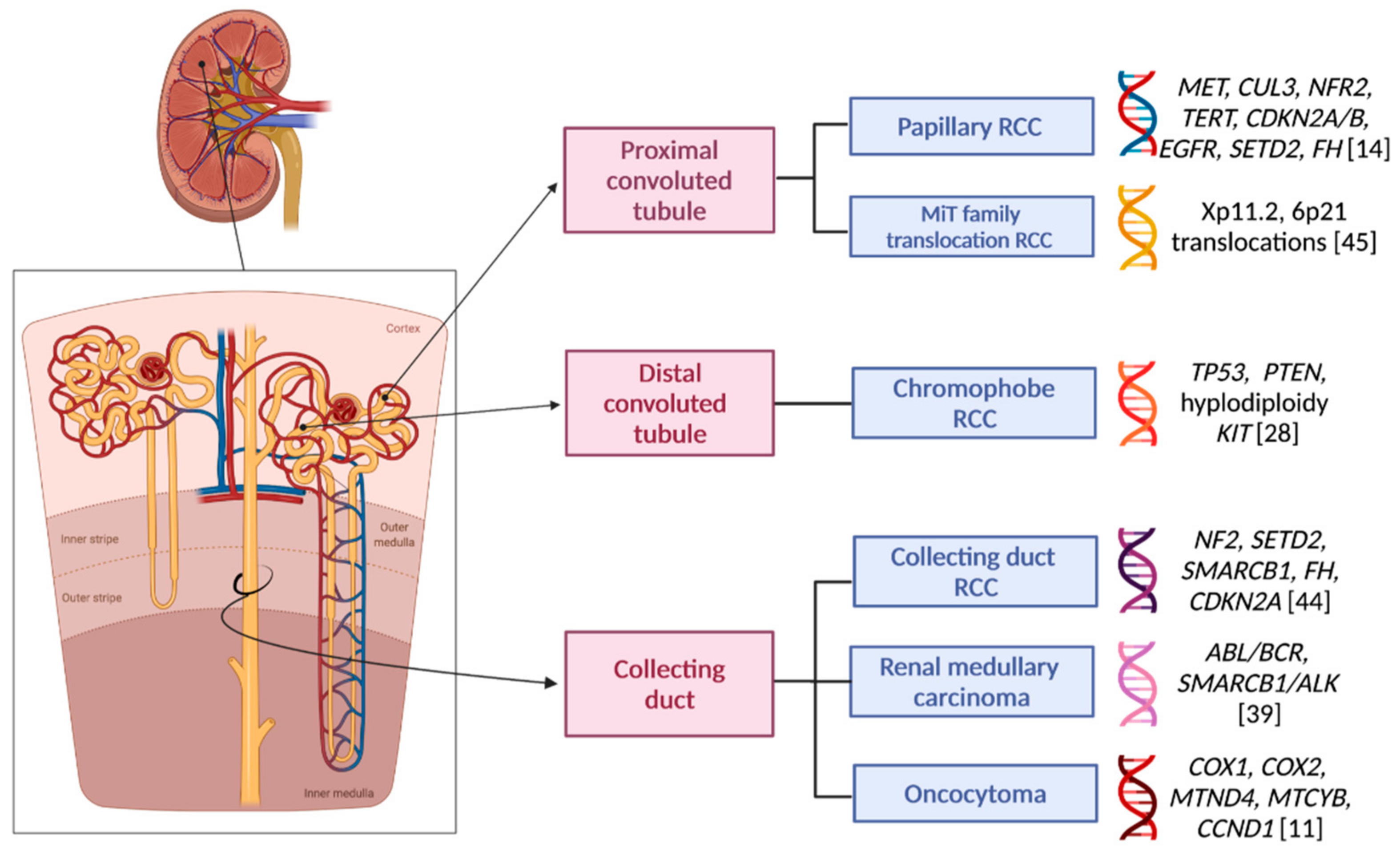
| Histotype | Frequency (% of All RCCs) | Cytogenetic Mutations | Genes Altered |
|---|---|---|---|
| Type 1 papillary RCC | 10–15% (considering all pRCCs) | +3q, +7, +8q, +12q, + 16p, +17, +20, −9p, −Y | MET, CUL3, NFR2, TERT, CDKN2A/B, EGFR |
| Type 2 papillary RCC | 10–15% (considering all pRCCs) | +7, +8q, +12, +16, +17, −1p, −9p, CpG island methylator phenotype, chromothripsis | CDKN2A silencing, SETD2, NF2, CUL3, TERT promoter, fumarate hydratase (FH) |
| Chromophobe RCC | 5% | −1, −2, −6, −7, −10, −13, −17, −21 | TP53, PTEN, hypodiploidy KIT |
| Oncocytoma | 3–7% | Diploid karyotype, loss of chromosome 1 or Y, or rearrangement of 11q13. | Mitochondrial genes (COX1, COX2, MTND4, MTCYB). The 11q13 rearrangement may affect the CCND1 gene. |
| Collecting duct carcinoma (also known as Bellini’s carcinoma) | 1% | −1q, −8p, −9p, −16p, +13q | NF2, SETD2, SMARCB1, FH, CDKN2A |
| Medullary RCC | 1% | ABL/BCR (rare), SMARCB1/ALK (rare), monosomy 11 | Not defined |
| MiT family translocation RCC | 1% | Recurrent translocations involving Xp11.2 (TFE3) or 6p21 (TFEB) | Not defined |
| Multilocular cystic renal neoplasm of low malignant potential | <1% | Not defined | Not defined |
| Hereditary leiomyomatosis with RCC | <1% | Not defined | FH |
| Succinate dehydrogenase-deficient RCC | <1% | Not defined | SDH (double hit inactivation) |
| Acquired cystic kidney disease-associated RCC | <1% | +3, +7, +17, −Y | Not defined |
| Unclassified RCC | about 5% | Not defined | Not defined |
| Clinical Trial (phase) | Experimental Arm | Histology | Setting | Primary Endpoint |
|---|---|---|---|---|
| KEYNOTE 427 (phase II) [110] | Pembrolizumab | pRCC type 1 and 2, chRCC, unclassified nccRCC | Previously untreated metastatic ccRCC (cohort A) and nccRCC (cohort B) | ORR (26.7% in the overall nccRCC population) |
| NCT03635892 (phase II) [111] | Nivolumab + cabozantinib | pRCC type 1 and 2, chRCC, MiT family translocation RCC, unclassified nccRCC | Previously untreated or treated with a prior VEGF-R TKI/mTORi metastatic pRCC, MiT family translocation RCC, unclassified RCC (cohort 1) and chRCC (cohort 2) | ORR (48% in cohort 1, 0% in cohort 2) |
| CONTACT-03 (phase III) | Atezolizumab + cabozantinib | All non-clear cell subtypes | Locally advanced or metastatic RCC in PD during or after one ICI-based regimen | PFS and OS (no results posted, recruiting underway) |
| COSMIC-021 (phase Ib/II) [114] | Atezolizumab + cabozantinib | All non-clear cell subtypes | Previously untreated locally advanced, metastatic, or recurrent solid tumors (including ccRCC and nccRCC) | ORR (no results posted) |
| COSMIC-313 (phase III) [115] | Nivolumab + ipilimumab + cabozantinib | All non-clear cell subtypes | Previously untreated IMDC intermediate-/poor-risk metastatic RCC (including ccRCC and nccRCC) | PFS (no results posted) |
| UNISoN (phase II) | Nivolumab (for a maximum of 12 months), then nivolumab + ipilimumab (4 cycles) and lastly maintenance with nivolumab single agent | pRCC type 1 and type 2, chRCC, S RCC, Xp11 translocation RCC, unclassified nccRCC | Metastatic nccRCC previously untreated or treated with a VEGF-R TKI or another systemic therapy | ORR (no results posted) |
| SUNIFORECAST (phase II) | Nivolumab + ipilimumab | All non-clear cell subtypes | Previously untreated locally advanced or metastatic nccRCC | OS (no results posted) |
| CALYPSO (phase Ib/II) [113] | Savolitinib + durvalumab | pRCC type 1 and type 2 | Previously untreated metastatic ccRCC (cohort A) and nccRCC (cohort B) | DLT, ORR (no results posted) |
| NCT02724878 (phase II) [112] | Atezolizumab + bevacizumab | All non-clear cell subtypes | Previously untreated locally advanced or metastatic nccRCC | ORR (33% in the experimental group) |
| KEYNOTE-B61 (phase II) [116] | Pembrolizumab + lenvatinib | All non-clear cell subtypes | Previously untreated locally advanced or metastatic nccRCC | ORR (no results posted, recruiting underway) |
| CheckMate 9ER [122] | KEYNOTE 426 [121] | Javelin RENAL 101 [119] | CheckMate 214 [118] | |
|---|---|---|---|---|
| Experimental arm | Nivolumab + cabozantinib | Pembrolizumab + axitinib | Avelumab + axitinib | Nivolumab + ipilimumab |
| mOS (months) | NR (95% CI, 22.8–NE) HR 0.36 (95% CI, 0.17–0.79) | NR HR 0.58 (95% CI, 0.21–1.59) | Data unavailable | NR (95% CI, 25.2–NE) HR 0.45 (95% CI, 0.30–0.70) |
| mPFS (months) | 10.3 (95% CI, 5.6–19.4) HR 0.42 (95% CI, 0.23–0.74) | NR HR 0.54 (95% CI, 0.29–1.00) | 7.0 (95% CI, 5.3–13.8) HR 0.57 (95% CI, 0.325–1.003) | 26.5 HR 0.54 (95% CI, 0.33–0.86) |
| ORR (CR) | 55.9% (CR unavailable) | 58.8% (13%) | 46.8% (4.3%) | 60.8% (18.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, A.; Rosellini, M.; Mollica, V.; Rizzo, A.; Tassinari, E.; Nuvola, G.; Cimadamore, A.; Santoni, M.; Fiorentino, M.; Montironi, R.; et al. The Molecular Characteristics of Non-Clear Cell Renal Cell Carcinoma: What’s the Story Morning Glory? Int. J. Mol. Sci. 2021, 22, 6237. https://doi.org/10.3390/ijms22126237
Marchetti A, Rosellini M, Mollica V, Rizzo A, Tassinari E, Nuvola G, Cimadamore A, Santoni M, Fiorentino M, Montironi R, et al. The Molecular Characteristics of Non-Clear Cell Renal Cell Carcinoma: What’s the Story Morning Glory? International Journal of Molecular Sciences. 2021; 22(12):6237. https://doi.org/10.3390/ijms22126237
Chicago/Turabian StyleMarchetti, Andrea, Matteo Rosellini, Veronica Mollica, Alessandro Rizzo, Elisa Tassinari, Giacomo Nuvola, Alessia Cimadamore, Matteo Santoni, Michelangelo Fiorentino, Rodolfo Montironi, and et al. 2021. "The Molecular Characteristics of Non-Clear Cell Renal Cell Carcinoma: What’s the Story Morning Glory?" International Journal of Molecular Sciences 22, no. 12: 6237. https://doi.org/10.3390/ijms22126237
APA StyleMarchetti, A., Rosellini, M., Mollica, V., Rizzo, A., Tassinari, E., Nuvola, G., Cimadamore, A., Santoni, M., Fiorentino, M., Montironi, R., & Massari, F. (2021). The Molecular Characteristics of Non-Clear Cell Renal Cell Carcinoma: What’s the Story Morning Glory? International Journal of Molecular Sciences, 22(12), 6237. https://doi.org/10.3390/ijms22126237









