Characteristics of Clear Cell Papillary Renal Cell Carcinoma (ccpRCC)
Abstract
1. Introduction
2. Macroscopic Findings
3. Microscopic Findings
4. Immunohistochemistry
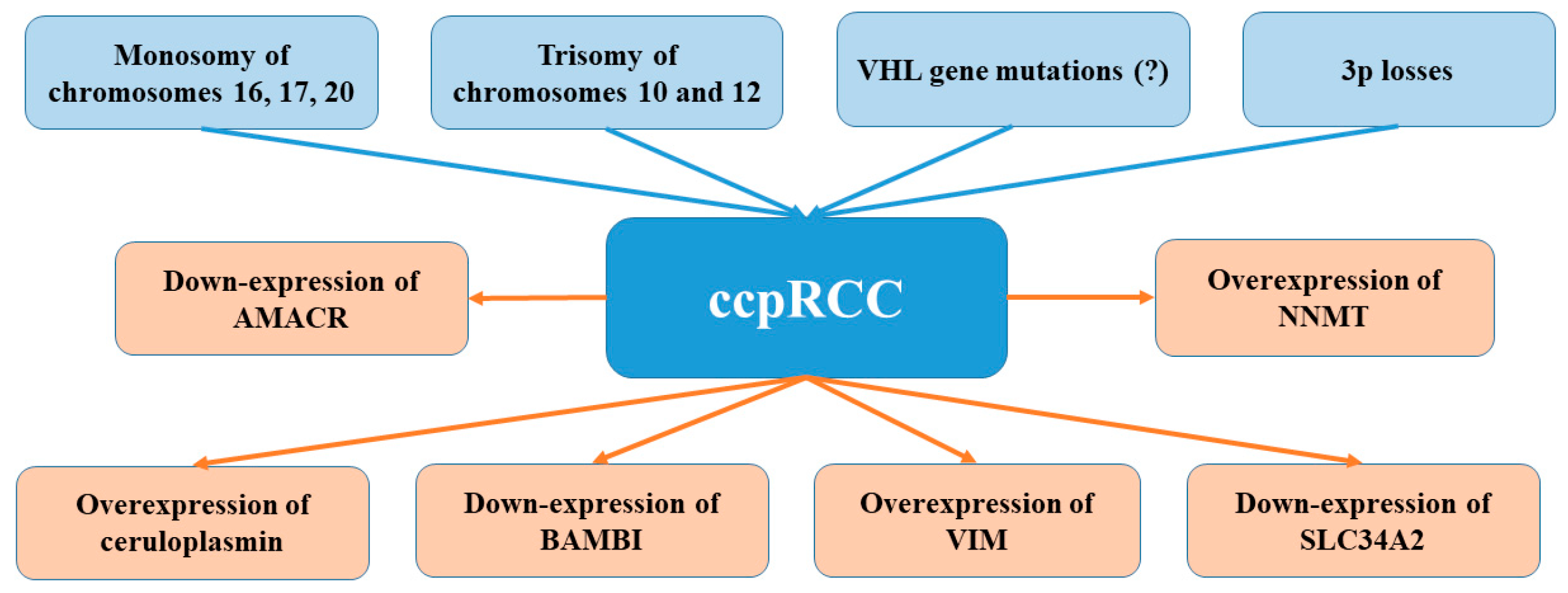
5. Molecular Pathways
6. Treatment and Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morlote, D.M.; Harada, S.; Batista, D.; Gordetsky, J.; Rais-Bahrami, S. Clear cell papillary renal cell carcinoma: Molecular profile and virtual karyotype. Hum. Pathol. 2019, 91, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Shuch, B.; Psutka, S.P. Advances in the characterization of clear cell papillary renal cell carcinoma: Identifying the sheep in wolf’s clothing. Eur. Urol. 2021, 79, 478–479. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Srigley, J.R.; Delahunt, B.; Eble, J.N.; Egevad, L.; Epstein, J.I.; Grignon, D.; Hes, O.; Moch, H.; Montironi, R.; Tickoo, S.K.; et al. The international society of urological pathology (ISUP) vancouver classification of renal neoplasia. Am. J. Surg. Pathol. 2013, 37, 1469–1489. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Ohe, C.; Kawakami, F.; Mikami, S.; Furuya, M.; Matsuura, K.; Moriyama, M.; Nagashima, Y.; Zhou, M.; Petersson, F.; et al. Clear cell papillary renal cell carcinoma: A review. Int. J. Clin. Exp. Pathol. 2014, 7, 7312–7318. [Google Scholar]
- Alexiev, B.A.; Drachenberg, C.B. Clear cell papillary renal cell carcinoma: Incidence, morphological features, immunohistochemical profile, and biologic behavior: A single institution study. Pathol. Res. Pract. 2014, 210, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zheng, S.; Truong, L.D.; Ro, J.Y.; Ayala, A.G.; Shen, S.S. Clear cell papillary renal cell carcinoma is the fourth most common histologic type of renal cell carcinoma in 290 consecutive nephrectomies for renal cell carcinoma. Hum. Pathol. 2014, 45, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R. Clear cell papillary renal cell carcinoma: An update after 15 years. Pathology 2021, 53, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Deml, K.-F.; Schildhaus, H.-U.; Compérat, E.; von Teichman, A.; Storz, M.; Schraml, P.; Bonventre, J.V.; Fend, F.; Fleige, B.; Nerlich, A.; et al. Clear cell papillary renal cell carcinoma and renal angiomyoadenomatous tumor: Two variants of a morphologic, immunohistochemical, and genetic distinct entity of renal cell carcinoma. Am. J. Surg. Pathol. 2015, 39, 889–901. [Google Scholar] [CrossRef]
- Herrera, L.; Hes, O.; Hirsch, M.; Comperat, E.; Camparo, P.; Rao, P.; Picken, M.; Montironi, R.; Annaiah, C.; Arora, K. Clear cell-papillary renal cell carcinoma (CP-RCC) not associated with end stage renal disease: Clinicopathologic analysis of 50 tumors confirming a novel subtype of renal cell carcinoma (RCC) occurring in a sporadic setting. In Laboratory Investigation; Nature Publishing Group: New York, NY, USA, 2011; p. 197A. [Google Scholar]
- Wang, K.; Zarzour, J.; Rais-Bahrami, S.; Gordetsky, J. Clear cell papillary renal cell carcinoma: New clinical and imaging characteristics. Urology 2017, 103, 136–141. [Google Scholar] [CrossRef]
- Williamson, S.R.; Cheng, L. Clear cell renal cell tumors: Not all that is “clear” is cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 292.e17–292.e22. [Google Scholar] [CrossRef]
- Massari, F.; Ciccarese, C.; Hes, O.; Michal, M.; Caliò, A.; Fiorentino, M.; Giunchi, F.; D’Amuri, A.; Sanguedolce, F.; Sabbatini, R.; et al. The tumor entity denominated “clear cell-papillary renal cell carcinoma” according to the WHO 2016 new classification, have the clinical characters of a renal cell adenoma as does harbor a benign outcome. Pathol. Oncol. Res. 2018, 24, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Eble, J.N.; Cheng, L.; Grignon, D.J. Clear cell papillary renal cell carcinoma: Differential diagnosis and extended immunohistochemical profile. Mod. Pathol. 2013, 26, 697–708. [Google Scholar] [CrossRef]
- Gobbo, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; MacLennan, G.T.; Shah, R.B.; Zhang, S.; Brunelli, M.; Cheng, L. Clear cell papillary renal cell carcinoma: A distinct histopathologic and molecular genetic entity. Am. J. Surg. Pathol. 2008, 32, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Rohan, S.M.; Xiao, Y.; Liang, Y.; Dudas, M.E.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Reuter, V.E.; Rosenblum, M.K.; Russo, P.; et al. Clear-cell papillary renal cell carcinoma: Molecular and immunohistochemical analysis with emphasis on the von Hippel–Lindau gene and hypoxia-inducible factor pathway-related proteins. Mod. Pathol. 2011, 24, 1207–1220. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Alexiev, B.A. Renal-cell carcinomas in end-stage kidneys: A clinicopathological study with emphasis on clear-cell papillary renal-cell carcinoma and acquired cystic kidney disease-associated carcinoma. Int. J. Surg. Pathol. 2012, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.; Dobin, S.M.; Grossmann, P.; Michal, M.; Donner, L.R. Clonal trisomies 7,10 and 12, normal 3p and absence of VHL gene mutation in a clear cell tubulopapillary carcinoma of the kidney. Virchows Arch. 2011, 459, 457–463. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Thomas, C.; Zou, Y.S. Clear cell papillary renal cell carcinoma with angiomyomatous stroma: A histological, immunohistochemical, and fluorescence in situ hybridization study. Virchows Arch. 2014, 464, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Crumley, S.M.; Divatia, M.; Truong, L.; Shen, S.; Ayala, A.G.; Ro, J.Y. Renal cell carcinoma: Evolving and emerging subtypes. World J. Clin. Cases 2013, 1, 262–275. [Google Scholar] [CrossRef]
- Adam, J.; Couturier, J.; Molinié, V.; Vieillefond, A.; Sibony, M. Clear-cell papillary renal cell carcinoma: 24 cases of a distinct low-grade renal tumour and a comparative genomic hybridization array study of seven cases. Histopathology 2011, 58, 1064–1071. [Google Scholar] [CrossRef]
- Aydin, H.; Chen, L.; Cheng, L.; Vaziri, S.; He, H.; Ganapathi, R.; Delahunt, B.; Magi-Galluzzi, C.; Zhou, M. Clear cell tubulopapillary renal cell carcinoma: A study of 36 distinctive low-grade epithelial tumors of the kidney. Am. J. Surg. Pathol. 2010, 34, 1608–1621. [Google Scholar] [CrossRef]
- Martignoni, G.; Brunelli, M.; Segala, D.; Munari, E.; Gobbo, S.; Cima, L.; Borze, I.; Wirtanen, T.; Sarhadi, V.K.; Atanesyan, L.; et al. Validation of 34βE12 immunoexpression in clear cell papillary renal cell carcinoma as a sensitive biomarker. Pathology 2017, 49, 10–18. [Google Scholar] [CrossRef][Green Version]
- Shi, S.S.; Shen, Q.; Xia, Q.Y.; Tu, P.; Shi, Q.L.; Zhou, X.J.; Rao, Q. Clear cell papillary renal cell carcinoma: A clinicopathological study emphasizing ultrastructural features and cytogenetic heterogeneity. Int. J. Clin. Exp. Pathol. 2013, 6, 2936–2942. [Google Scholar] [PubMed]
- Xu, J.; Reznik, E.; Lee, H.J.; Gundem, G.; Jonsson, P.; Sarungbam, J.; Bialik, A.; Sanchez-Vega, F.; Creighton, C.J.; Hoekstra, J.; et al. Abnormal oxidative metabolism in a quiet genomic background underlies clear cell papillary renal cell carcinoma. eLife 2019, 8, e38986. [Google Scholar] [CrossRef]
- Diolombi, M.L.; Cheng, L.; Argani, P.; Epstein, J.I. Do clear cell papillary renal cell carcinomas have malignant potential? Am. J. Surg. Pathol. 2015, 39, 1621–1634. [Google Scholar] [CrossRef]
- Weng, S.; DiNatale, R.G.; Silagy, A.; Mano, R.; Attalla, K.; Kashani, M.; Weiss, K.; Benfante, N.E.; Winer, A.G.; Coleman, J.A.; et al. The clinicopathologic and molecular landscape of clear cell papillary renal cell carcinoma: Implications in diagnosis and management. Eur. Urol. 2021, 79, 468–477. [Google Scholar] [CrossRef]
- Simhan, J.; Canter, D.J.; Sterious, S.N.; Smaldone, M.C.; Tsai, K.J.; Li, T.; Viterbo, R.; Chen, D.Y.; Greenberg, R.E.; Kutikov, A.; et al. Pathological concordance and surgical outcomes of sporadic synchronous unilateral multifocal renal masses treated with partial nephrectomy. J. Urol. 2013, 189, 43–47. [Google Scholar] [CrossRef]
- Alshenawy, H.A. Immunohistochemical panel for differentiating renal cell carcinoma with clear and papillary features. J. Microsc. Ultrastruct. 2015, 3, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-H.; Morrison, C.; Wang, P.; Yang, X.; Haven, C.J.; Zhang, C.; Zhao, P.; Tretiakova, M.S.; Korpi-Hyovalti, E.; Burgess, J.R.; et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin. Cancer Res. 2004, 10, 6629–6637. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ziober, A.; Bing, Z. Expression of parafibromin in clear cell papillary renal cell carcinoma. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Leroy, X.; Camparo, P.; Gnemmi, V.; Aubert, S.; Flamand, V.; Roupret, M.; Fantoni, J.C.; Comperat, E. Clear cell papillary renal cell carcinoma is an indolent and low-grade neoplasm with overexpression of cyclin-D1. Histopathology 2014, 64, 1032–1036. [Google Scholar] [CrossRef]
- Brunelli, M.; Erdini, F.; Cima, L.; Eccher, A.; Fioravanzo, A.; Gobbo, S.; Segala, D.; Ghimenton, C.; Mazzoleni, G.; Munari, E. Proximal CD13 versus distal GATA-3 Expression in renal neoplasia according to WHO 2016 classification. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 316–323. [Google Scholar] [CrossRef]
- Williamson, S.R.; Cheng, L.; Eble, J.N.; True, L.D.; Gupta, N.S.; Wang, M.; Zhang, S.; Grignon, D.J. Renal cell carcinoma with angioleiomyoma-like stroma: Clinicopathological, immunohistochemical, and molecular features supporting classification as a distinct entity. Mod. Pathol. 2015, 28, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Lal, P.; Master, S.; Ma, Y.; Baradet, T.; Bing, Z. Expression of parafibromin in major renal cell tumors. Eur. J. Histochem. 2012, 56, e39. [Google Scholar] [CrossRef]
- Schwartz, J.D.; Dumler, F.; Hafron, J.M.; Wilson, G.D.; Wolforth, S.C.; Rooney, M.T.; Li, W.; Zhang, P.L. CD133 staining detects acute kidney injury and differentiates clear cell papillary renal cell carcinoma from other renal tumors. ISRN Biomark. 2013, 2013, 353598. [Google Scholar] [CrossRef]
- Munari, E.; Brunelli, M.; Segala, D.; Gobbo, S.; Netto, G.; Cheng, L.; Eble, J.; Delahunt, B.; Raspollini, M.; Tardanico, R. GATA3 expression in clear cell papillary renal cell carcinoma and renal cell carcinoma with prominent leiomyomatous proliferation is a further evidence of the relationship between these two entities. Lab. Investig. 2014, 94, 250A. [Google Scholar]
- Williamson, S.R.; Zhang, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; Brunelli, M.; Wang, M.; Gobbo, S.; Baldridge, L.A.; Cheng, L. Clear cell papillary renal cell carcinoma-like tumors in patients with von Hippel-Lindau disease are unrelated to sporadic clear cell papillary renal cell carcinoma. Am. J. Surg. Pathol. 2013, 37, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Monzon, F.; Jonasch, E.; Matin, S.F.; Tamboli, P. Clear cell papillary renal cell carcinoma in patients with von Hippel-Lindau syndrome—Clinicopathological features and comparative genomic analysis of 3 cases. Hum. Pathol. 2014, 45, 1966–1972. [Google Scholar] [CrossRef]
- Behdad, A.; Monzon, F.; Hirsch, M.; Michal, M.; Comperat, E.; Camparo, P.; Rao, P.; Picken, M.; Montironi, R.; Annaiah, C. Relationship between sporadic clear cell-papillary renal cell carcinoma (CP-RCC) and renal angiomyoadenomatous tumor (RAT) of the kidney: Analysis by virtual-karyotyping, fluorescent in situ analysis and immunohistochemistry (IHC). Lab. Investig. 2011, 91, 179A. [Google Scholar]
- Martignoni, G.; Brunelli, M.; Segala, D.; Borze, I.; Atanesya, L.; Savola, S.; Barzon, L.; Masi, G.; Tardanico, R.; Eble, J. VHL mutation, VHL methylation, chromosome 3p and whole genomic status in clear cell papillary renal cell carcinoma. Lab. Investig. 2013, 93, 233A. [Google Scholar]
- Hes, O.; Compérat, E.M.; Rioux-Leclercq, N. Clear cell papillary renal cell carcinoma, renal angiomyoadenomatous tumor, and renal cell carcinoma with leiomyomatous stroma relationship of 3 types of renal tumors: A review. Ann. Diagn. Pathol. 2016, 21, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Tanaka, A. Recent classification of renal epithelial tumors. Med. Mol. Morphol. 2014, 47, 68–75. [Google Scholar] [CrossRef]
- Williamson, S.R.; Cheng, L. Do clear cell papillary renal cell carcinomas occur in patients with von Hippel-Lindau disease? Hum. Pathol. 2015, 46, 340–341. [Google Scholar] [CrossRef]
- Xu, W.; Deng, F.; Melamed, J.; Zhou, M. Incidence and genetic characteristics of clear cell tububopapillary renal cell carcinoma. Lab. Investig. 2014, 94, 270A. [Google Scholar]
- Kim, W.Y.; Kaelin, W.G. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 2004, 22, 4991–5004. [Google Scholar] [CrossRef]
- Krieg, M.; Haas, R.; Brauch, H.; Acker, T.; Flamme, I.; Plate, K.H. Up-regulation of hypoxia-inducible factors HIF-1α and HIF-2α under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 2000, 19, 5435–5443. [Google Scholar] [CrossRef]
- Wiesener, M.S.; Münchenhagen, P.M.; Berger, I.; Morgan, N.V.; Roigas, J.; Schwiertz, A.; Jürgensen, J.S.; Gruber, G.; Maxwell, P.H.; Löning, S.A. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1α in clear cell renal carcinomas. Cancer Res. 2001, 61, 5215–5222. [Google Scholar] [PubMed]
- Grabmaier, K.; de Weijert, M.C.; Verhaegh, G.W.; Schalken, J.A.; Oosterwijk, E. Strict regulation of CAIX G250/MN by HIF-1 α in clear cell renal cell carcinoma. Oncogene 2004, 23, 5624–5631. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Tickoo, S.K.; Xu, J.; Lee, C.-H.; Mano, R.; Chen, Y.-B.; Stirdivant, S.; Neri, B.; Wolfert, R.; Fine, S.W. MP35-19 sorbitol as a novel mechanism of hypoxia-inducible factor (hif) pathway activation in clear cell papillary renal cell carcinoma (ccprcc). J. Urol. 2014, 191, e377–e378. [Google Scholar] [CrossRef]
- Fisher, K.E.; Yin-Goen, Q.; Alexis, D.; Sirintrapun, J.S.; Harrison, W.; Benjamin Isett, R.; Rossi, M.R.; Moreno, C.S.; Young, A.N.; Osunkoya, A.O. Gene expression profiling of clear cell papillary renal cell carcinoma: Comparison with clear cell renal cell carcinoma and papillary renal cell carcinoma. Mod. Pathol. 2014, 27, 222–230. [Google Scholar] [CrossRef]
- Osunkoya, A.O.; Yin-Goen, Q.; Phan, J.H.; Moffitt, R.A.; Stokes, T.H.; Wang, M.D.; Young, A.N. Diagnostic biomarkers for renal cell carcinoma: Selection using novel bioinformatics systems for microarray data analysis. Hum. Pathol. 2009, 40, 1671–1678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Seki, N.; Yamada, Y.; Yoshino, H.; Hidaka, H.; Chiyomaru, T.; Nohata, N.; Kinoshita, T.; Nakagawa, M.; Enokida, H. Tumor suppressive microRNA-138 contributes to cell migration and invasion through its targeting of vimentin in renal cell carcinoma. Int. J. Oncol. 2012, 41, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Larrea, E.; Larrinaga, G.; Goicoechea, I.; Arestin, M.; Fernandez-Mercado, M.; Hes, O.; Cáceres, F.; Manterola, L.; López, J.I. Targeted next-generation sequencing and non-coding RNA expression analysis of clear cell papillary renal cell carcinoma suggests distinct pathological mechanisms from other renal tumour subtypes. J. Pathol. 2014, 232, 32–42. [Google Scholar] [CrossRef]
- Schmidt, L.; Duh, F.-M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Castiglione, F.; Cheng, L.; Montironi, R.; Lopez-Beltran, A. Genetic mutations in accordance with a low malignant potential tumour are not demonstrated in clear cell papillary renal cell carcinoma. J. Clin. Pathol. 2016, 69, 547–550. [Google Scholar] [CrossRef]
- Farooq, T.; Saeed, F.; Zhang, D.; Huang, W.; Yin, C.; Zhong, M. 334 whole exome sequencing of end stage renal disease associated papillary renal cell carcinoma (PRCCs). Am. J. Clin. Pathol. 2018, 149, S144. [Google Scholar] [CrossRef]
- Mertz, K.D.; Demichelis, F.; Sboner, A.; Hirsch, M.S.; Dal Cin, P.; Struckmann, K.; Storz, M.; Scherrer, S.; Schmid, D.M.; Strebel, R.T.; et al. Association of cytokeratin 7 and 19 expression with genomic stability and favorable prognosis in clear cell renal cell cancer. Int. J. Cancer 2008, 123, 569–576. [Google Scholar] [CrossRef]
- Dahinden, C.; Ingold, B.; Wild, P.; Boysen, G.; Luu, V.-D.; Montani, M.; Kristiansen, G.; Sulser, T.; Bühlmann, P.; Moch, H. Mining tissue microarray data to uncover combinations of biomarker expression patterns that improve intermediate staging and grading of clear cell renal cell cancer. Clin. Cancer Res. 2010, 16, 88–98. [Google Scholar] [CrossRef]
- Bielski, C.M.; Zehir, A.; Penson, A.V.; Donoghue, M.T.A.; Chatila, W.; Armenia, J.; Chang, M.T.; Schram, A.M.; Jonsson, P.; Bandlamudi, C.; et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 2018, 50, 1189–1195. [Google Scholar] [CrossRef]
- Munari, E.; Marchionni, L.; Chitre, A.; Hayashi, M.; Martignoni, G.; Brunelli, M.; Gobbo, S.; Argani, P.; Allaf, M.; Hoque, M.O.; et al. Clear cell papillary renal cell carcinoma: Micro-RNA expression profiling and comparison with clear cell renal cell carcinoma and papillary renal cell carcinoma. Hum. Pathol. 2014, 45, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Lee, C.Y.; Park, J.-H.; Park, M.-S.; Maeng, L.-S.; Yoon, C.S.; Lee, M.Y.; Hwang, K.-C.; Chung, Y.-A. Survival of hypoxic human mesenchymal stem cells is enhanced by a positive feedback loop involving miR-210 and hypoxia-inducible factor 1. J. Vet. Sci. 2013, 14, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wotschofsky, Z.; Liep, J.; Meyer, H.-A.; Jung, M.; Wagner, I.; Disch, A.C.; Schaser, K.D.; Melcher, I.; Kilic, E.; Busch, J. Identification of metastamirs as metastasis-associated microRNAs in clear cell renal cell carcinomas. Int. J. Biol. Sci. 2012, 8, 1363. [Google Scholar] [CrossRef] [PubMed]
- White, N.M.; Bao, T.T.; Grigull, J.; Youssef, Y.M.; Girgis, A.; Diamandis, M.; Fatoohi, E.; Metias, M.; Honey, R.J.; Stewart, R. miRNA profiling for clear cell renal cell carcinoma: Biomarker discovery and identification of potential controls and consequences of miRNA dysregulation. J. Urol. 2011, 186, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Saini, S.; Majid, S.; Hirata, H.; Ueno, K.; Chang, I.; Tanaka, Y.; Gupta, A.; Dahiya, R. MicroRNA-34a suppresses malignant transformation by targeting c-Myc transcriptional complexes in human renal cell carcinoma. Carcinogenesis 2012, 33, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Castellano, L.; Giamas, G.; Jacob, J.; Coombes, R.C.; Lucchesi, W.; Thiruchelvam, P.; Barton, G.; Jiao, L.R.; Wait, R.; Waxman, J. The estrogen receptor-α-induced microRNA signature regulates itself and its transcriptional response. Proc. Natl. Acad. Sci. USA 2009, 106, 15732–15737. [Google Scholar] [CrossRef]
- Jung, M.; Mollenkopf, H.J.; Grimm, C.; Wagner, I.; Albrecht, M.; Waller, T.; Pilarsky, C.; Johannsen, M.; Stephan, C.; Lehrach, H. MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J. Cell. Mol. Med. 2009, 13, 3918–3928. [Google Scholar] [CrossRef]
- Yamada, Y.; Hidaka, H.; Seki, N.; Yoshino, H.; Yamasaki, T.; Itesako, T.; Nakagawa, M.; Enokida, H. Tumor-suppressive micro RNA-135a inhibits cancer cell proliferation by targeting the c-MYC oncogene in renal cell carcinoma. Cancer Sci. 2013, 104, 304–312. [Google Scholar] [CrossRef]
- Mikhaylova, O.; Stratton, Y.; Hall, D.; Kellner, E.; Ehmer, B.; Drew, A.F.; Gallo, C.A.; Plas, D.R.; Biesiada, J.; Meller, J. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell 2012, 21, 532–546. [Google Scholar] [CrossRef]
- Aron, M.; Chang, E.; Herrera, L.; Hes, O.; Hirsch, M.S.; Comperat, E.; Camparo, P.; Rao, P.; Picken, M.; Michal, M.; et al. Clear cell-papillary renal cell carcinoma of the kidney not associated with end-stage renal disease: Clinicopathologic correlation with expanded immunophenotypic and molecular characterization of a large cohort with emphasis on relationship with renal angiomyoadenomatous tumor. Am. J. Surg. Pathol. 2015, 39, 873–888. [Google Scholar] [CrossRef]
- Collins, J.; Epstein, J.I. Prognostic significance of extensive necrosis in renal cell carcinoma. Hum. Pathol. 2017, 66, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Kauffman, E.C.; Kandel, S.; George, S.; Schwaab, T.; Xu, B. Incidence of clear cell papillary renal cell carcinoma in low-grade renal cell carcinoma cases: A 12-year retrospective clinicopathologic study from a single cancer center. Int. J. Surg. Pathol. 2015, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Steward, J.E.; Kern, S.Q.; Cheng, L.; Boris, R.S.; Tong, Y.; Bahler, C.D.; Masterson, T.A.; Cary, K.C.; Kaimakliotis, H.; Gardner, T.; et al. Clear cell papillary renal cell carcinoma: Characteristics and survival outcomes from a large single institutional series. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 370.e21–370.e25. [Google Scholar] [CrossRef]
- Griffin, B.B.; Lin, X. Cytomorphologic analysis of clear cell papillary renal cell carcinoma: Distinguishing diagnostic features. Cancer Cytopathol. 2021, 129, 192–203. [Google Scholar] [CrossRef] [PubMed]
| Macroscopic Findings | Microscopic Findings |
|---|---|
| Solid or cystic tumour, rarely with flattened peripheral cysts | Tightly packed secondary branching resembling a solid component |
| Small and well encapsulated (well-defined fibrous capsule) | Not always visible papillary architecture |
Mix of:
| If papillary architecture is visible—the fibrovascular cores are usually thin and lined by a single layer of cells with the characteristic nuclear arrangement |
| All components submerged in clear cytoplasm | Cystic changes are sometimes predominant |
| Usually (but not always) single and unilateral | Cuboidal cells covering the papillae are usually small to medium |
Tumour cut surface:
| Low-grade nuclei lie in the horizontal line apically distant from the basal membrane (except for the basement membrane) |
| The nuclear atypia was found to correspond to Fuhrman grade 1 or 2 | |
| Frequent proteinaceous secretion in lumina of the tubules or acini Seroanguinous fluid or colloid-like section in cystic spaces | |
| Fibrous stroma displaying smooth muscle metaplasia | |
| Lack of mitoses, pleomorphism, hyaline globules, foamy macrophages, hemosiderin depositions, psammoma bodies, lymphovascular invasion, renal sinus invasion and tumour necrosis | |
| Higher multifocality and bilaterality rates compared to ccRCC |
| Clear Cell Papillary Renal Cell Carcinoma | Clear Cell Renal Cell Carcinoma | Papillary Renal Cell Carcinomas | |
|---|---|---|---|
| Cytokeratin (CK) 7 | Robust, diffuse positivity immunoreactivity | Negative immunoreactivity | Positive immunoreactivity |
| Cytokeratin 34βE12 | Robust, diffuse positivity immunoreactivity | - | - |
| Carbonic anhydrase IX (CA9) | Cup-like staining pattern | Diffusely and intensively stained with a box pattern | Positive immunoreactivity (in tips of papillae) |
| Parafibromin | Diffuse and strong nuclear positivity immunoreactivity | Negative immunoreactivity | Negative immunoreactivity |
| RCC-Ma | Negative immunoreactivity | High positivity rate | Positive immunoreactivity |
| Vimentin | Positive immunoreactivity | Positive staining (more common in high-grade areas) | Diffuse cytoplasmic staining |
| Alpha-methylacyl-CoA racemase (AMACR) | Negative immunoreactivity | Variably positive immunoreactivity | Diffusely and strongly positive immunoreactivity |
| CD10 | Negative or focally positive in most cases | Sawtooth pattern along a scalloped luminal contour | Variably positive immunoreactivity |
| GLUT-1 | Positive immunoreactivity | Diffusely and strongly positive immunoreactivity | Positive immunoreactivity |
| HIF-1 | Positive immunoreactivity | Diffusely and strongly positive immunoreactivity | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Characteristics of Clear Cell Papillary Renal Cell Carcinoma (ccpRCC). Int. J. Mol. Sci. 2022, 23, 151. https://doi.org/10.3390/ijms23010151
Rysz J, Franczyk B, Ławiński J, Gluba-Brzózka A. Characteristics of Clear Cell Papillary Renal Cell Carcinoma (ccpRCC). International Journal of Molecular Sciences. 2022; 23(1):151. https://doi.org/10.3390/ijms23010151
Chicago/Turabian StyleRysz, Jacek, Beata Franczyk, Janusz Ławiński, and Anna Gluba-Brzózka. 2022. "Characteristics of Clear Cell Papillary Renal Cell Carcinoma (ccpRCC)" International Journal of Molecular Sciences 23, no. 1: 151. https://doi.org/10.3390/ijms23010151
APA StyleRysz, J., Franczyk, B., Ławiński, J., & Gluba-Brzózka, A. (2022). Characteristics of Clear Cell Papillary Renal Cell Carcinoma (ccpRCC). International Journal of Molecular Sciences, 23(1), 151. https://doi.org/10.3390/ijms23010151







