TNFα-Induced LDL Cholesterol Accumulation Involve Elevated LDLR Cell Surface Levels and SR-B1 Downregulation in Human Arterial Endothelial Cells
Abstract
1. Introduction
2. Results
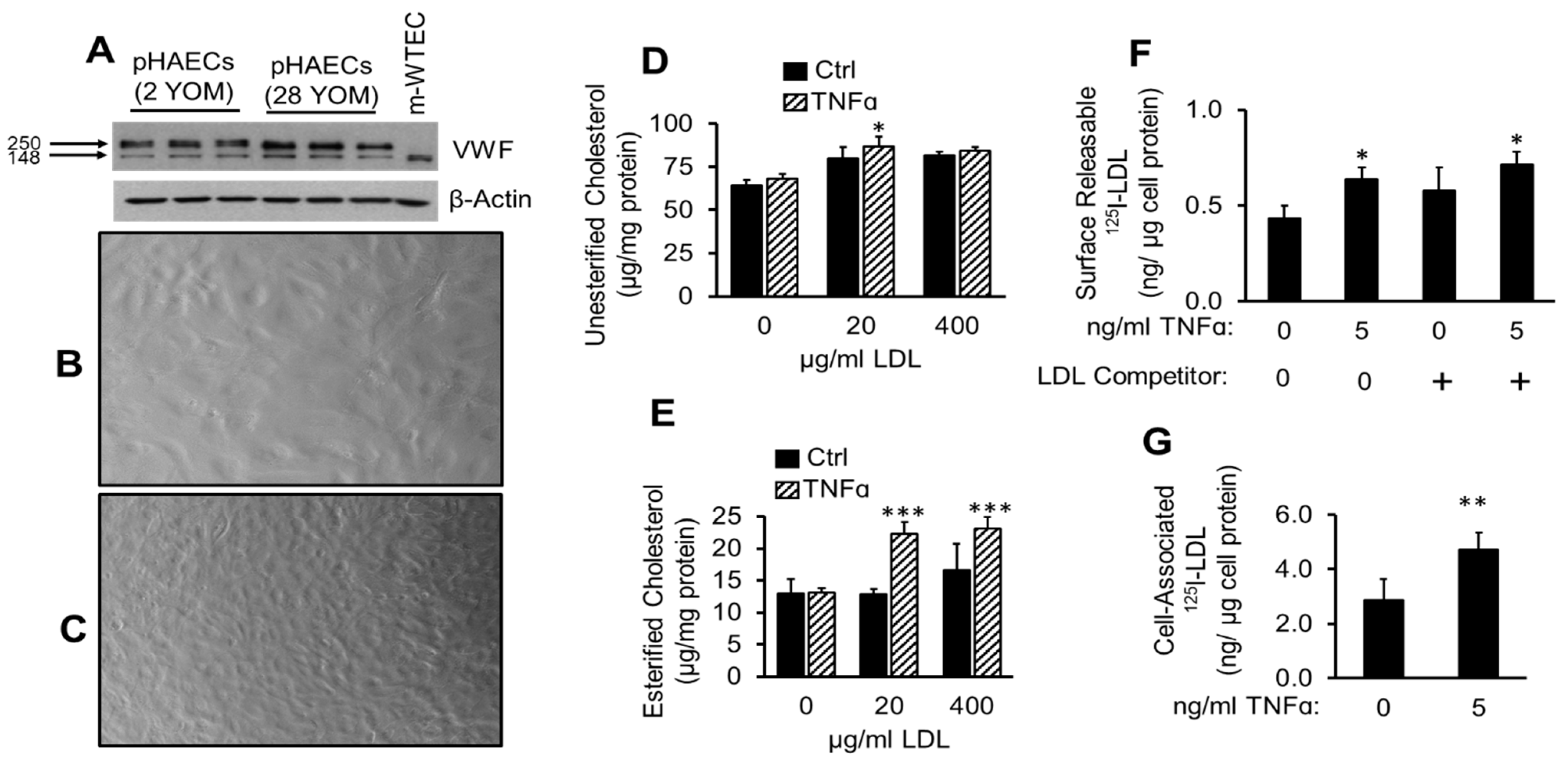
2.1. TNFα Enhances Cholesterol Accumulation and LDL Binding to pHAECs
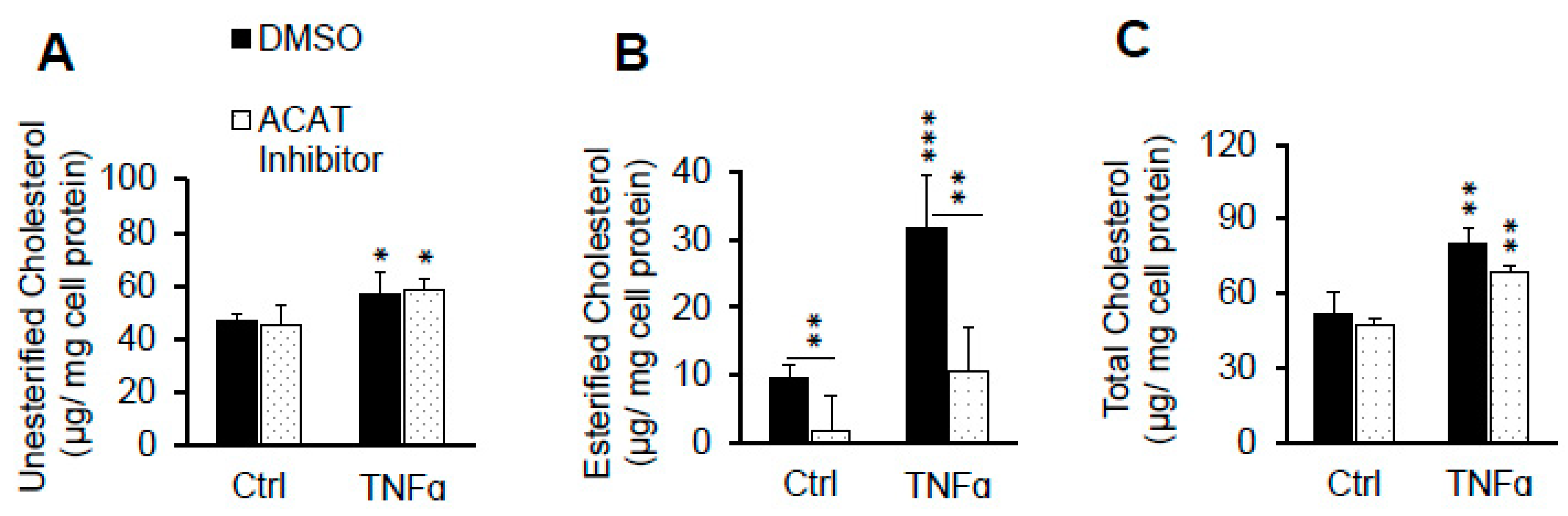
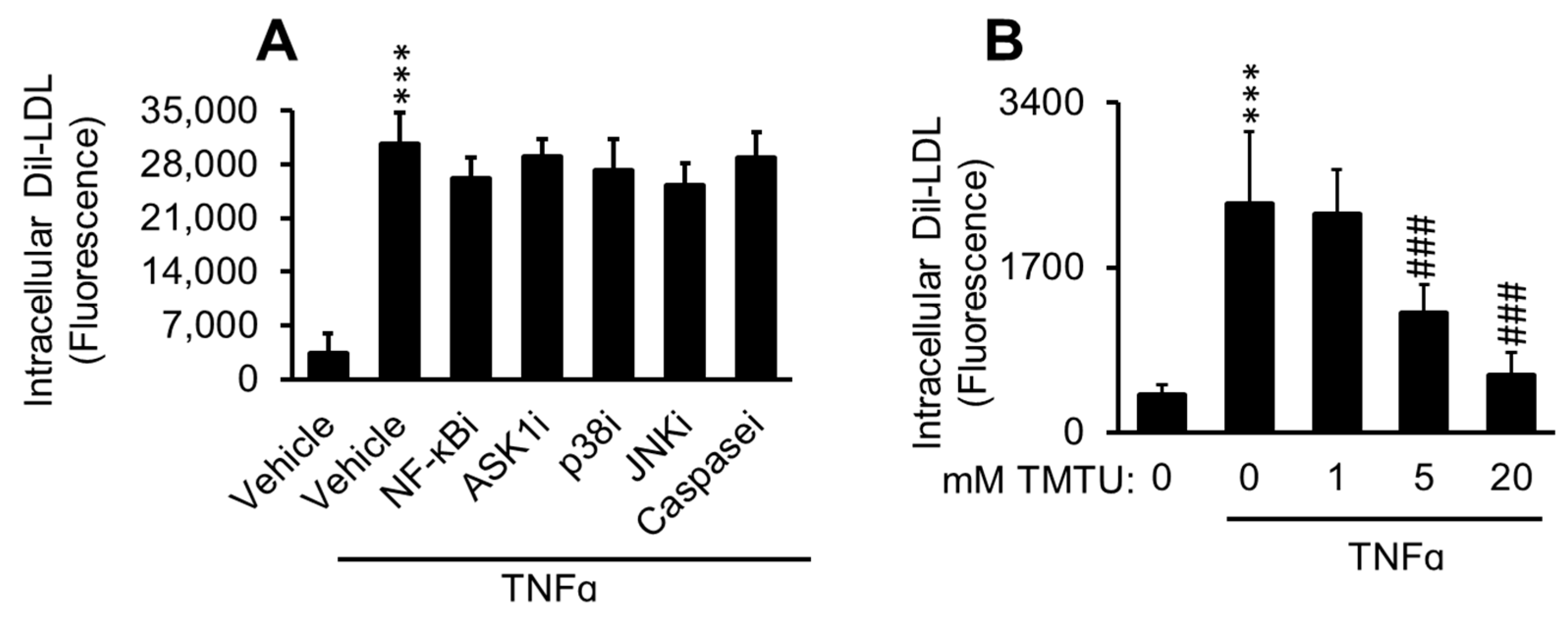
2.2. ACAT Inhibitor Does Not Prevent TNFα-Induced LDL Cholesterol Accumulation
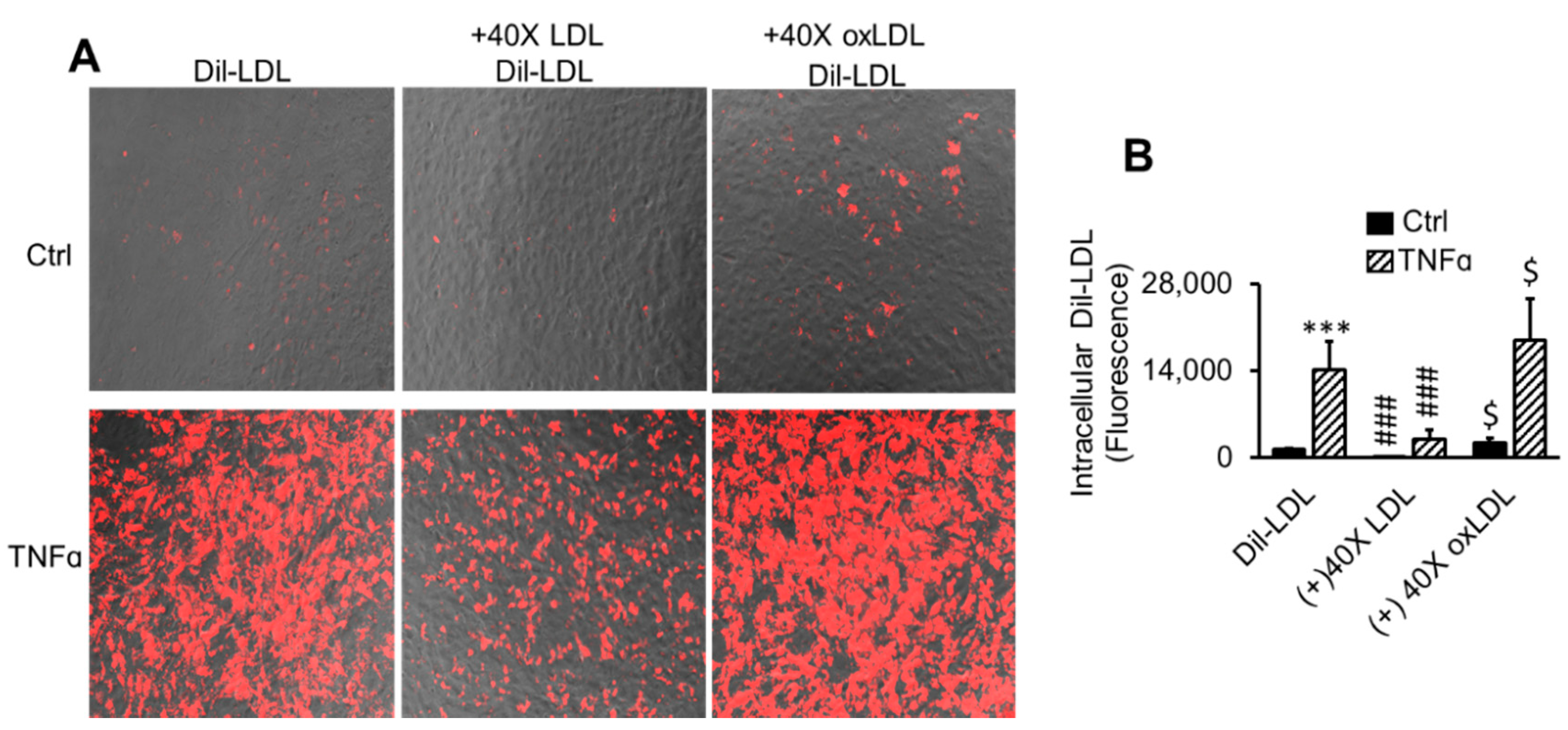
2.3. LDL Oxidation Is Not Required for TNFα-Induced LDL Accumulation
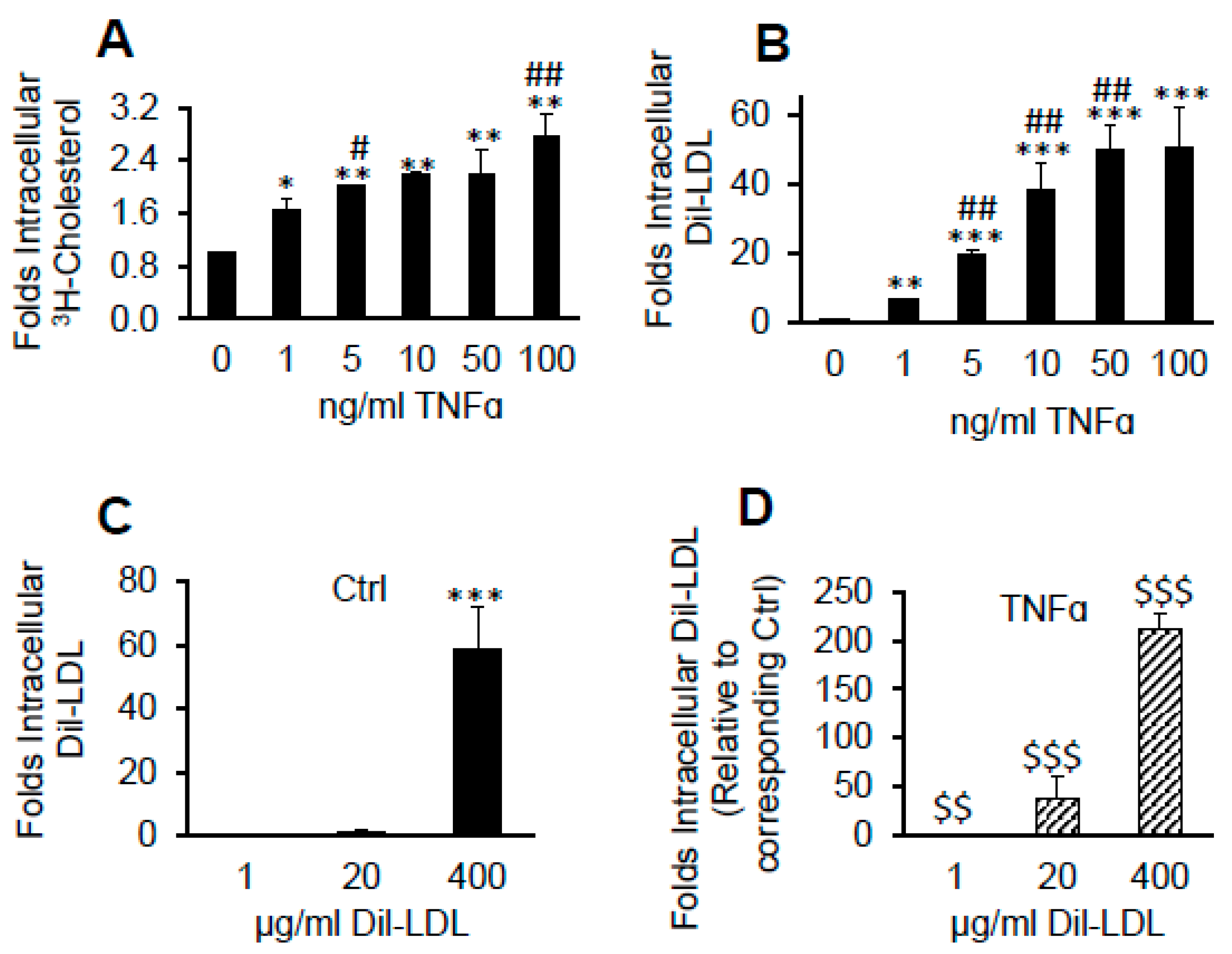
2.4. TNFα Induces Massive Dil over [3H]CE Lipid Accumulation from LDL
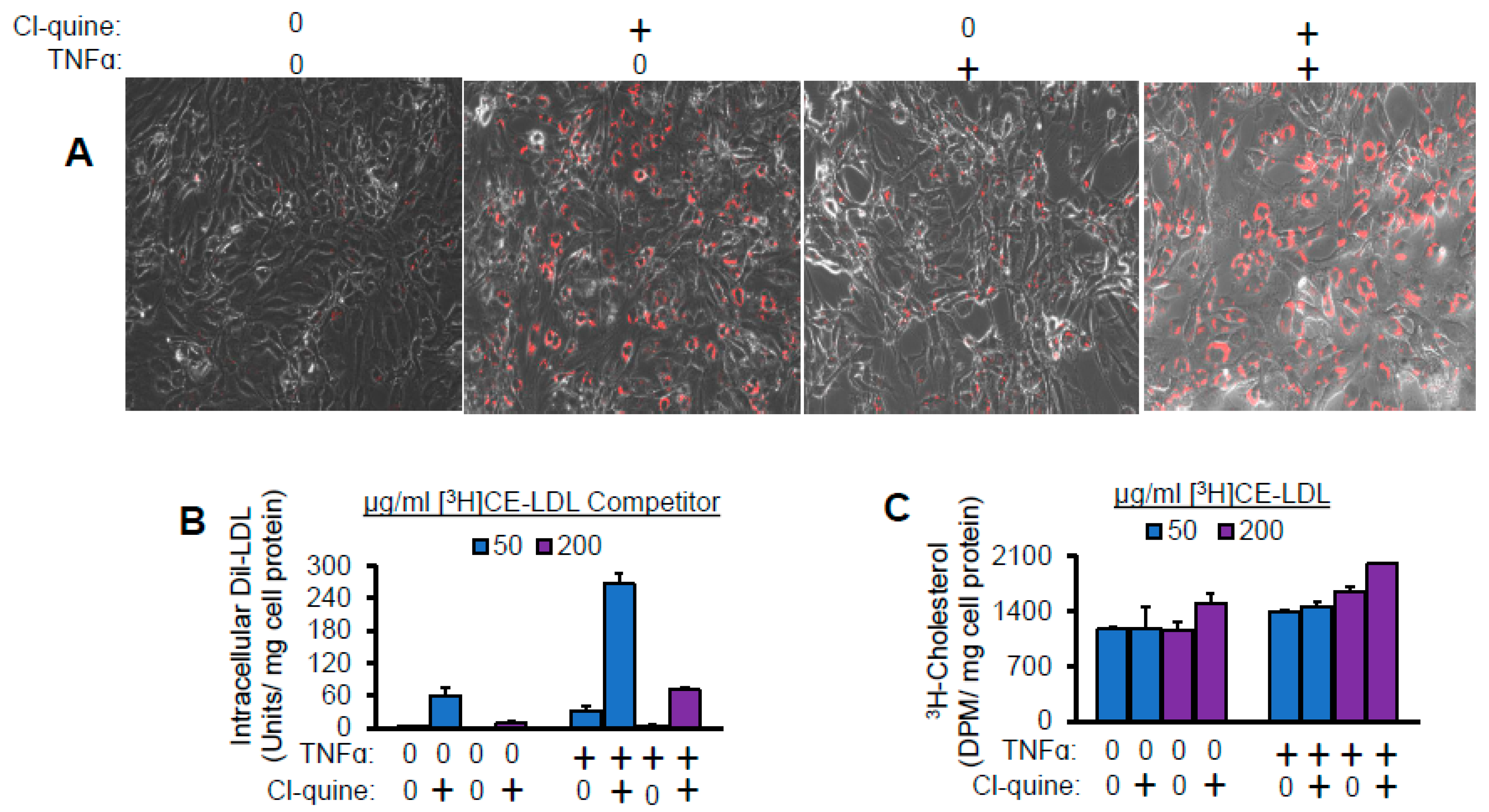
2.5. Lysosomal Inhibitor Enhances TNFα-Induced LDL Lipid Accumulation
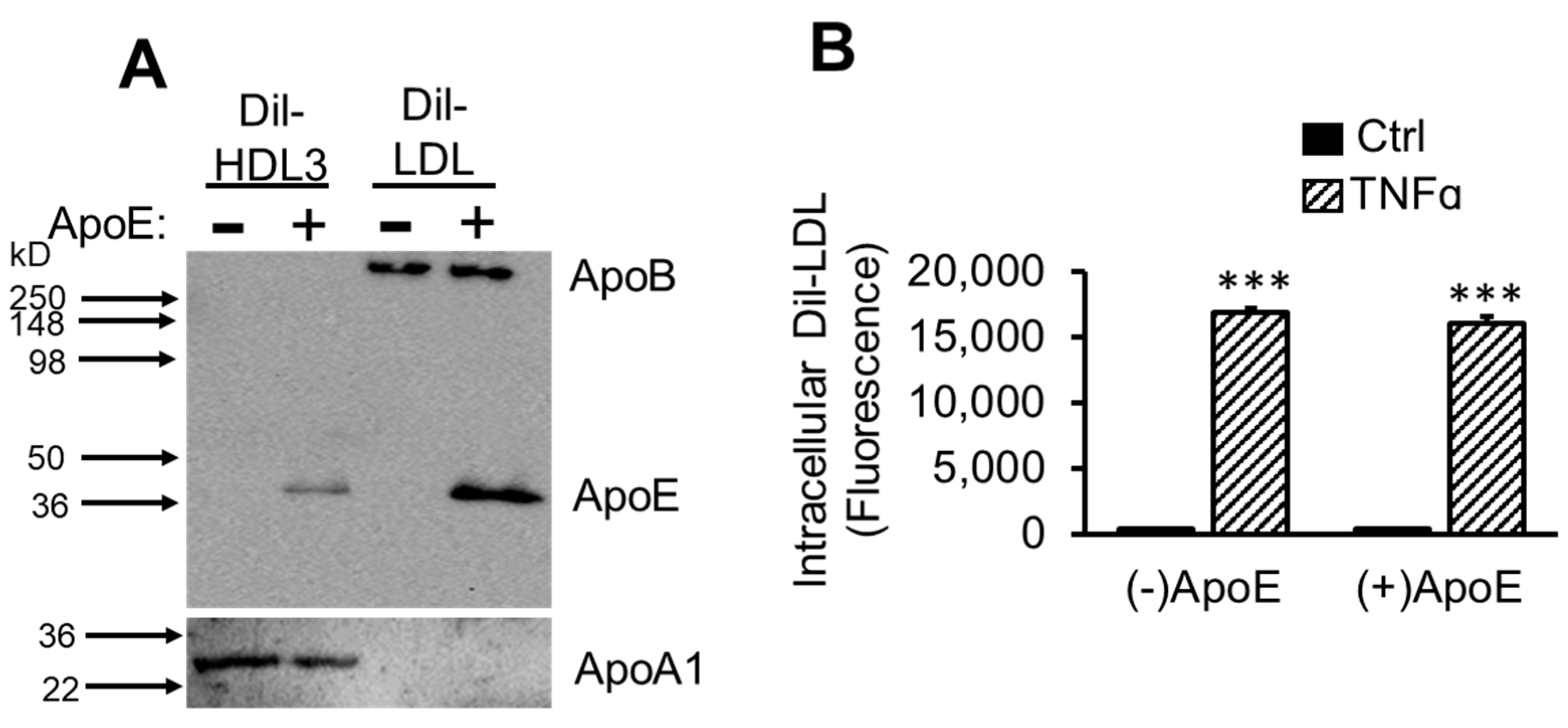
2.6. ApoE Is Not Required for TNFα-Induced Dil-LDL Accumulation
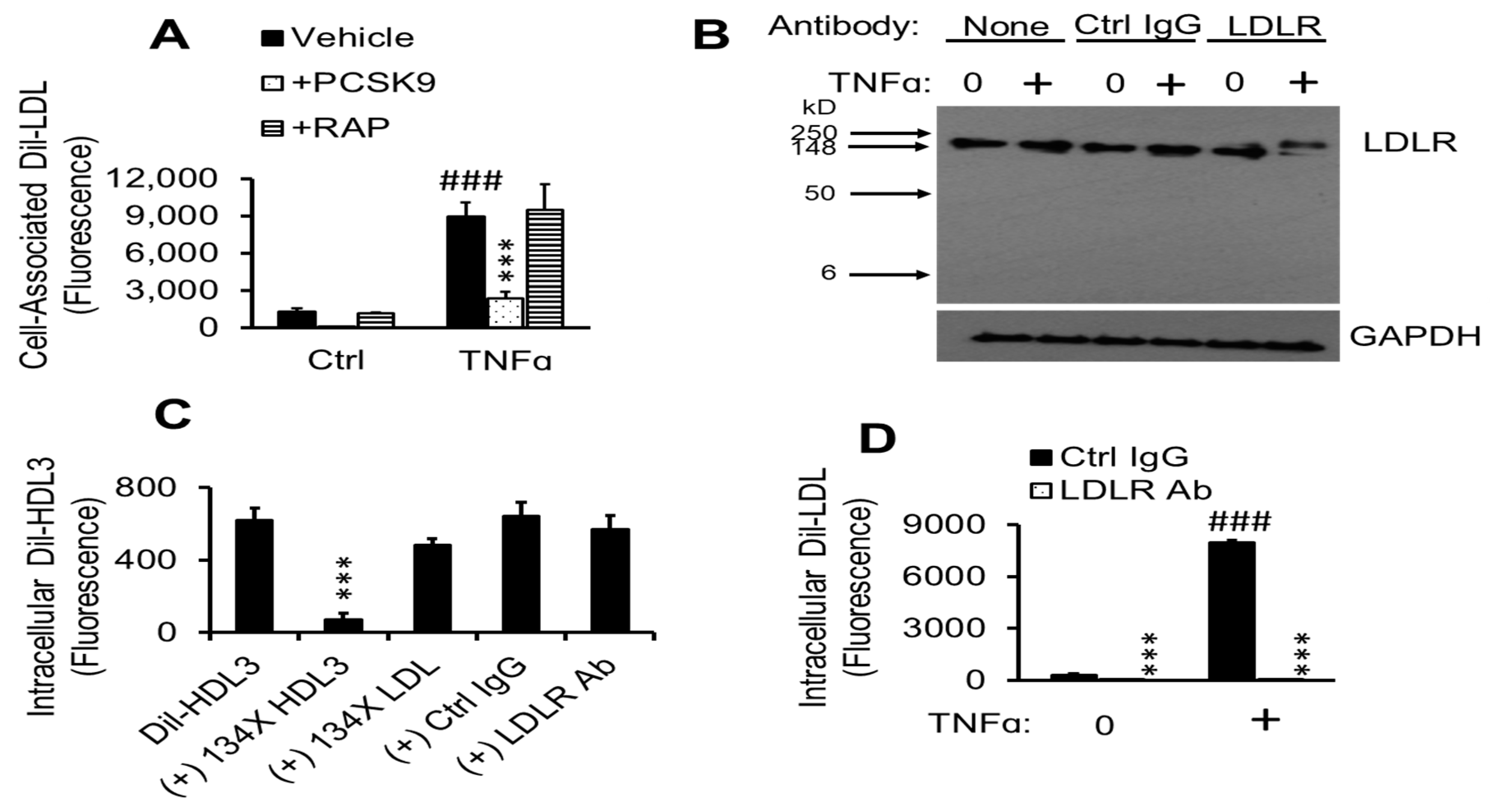
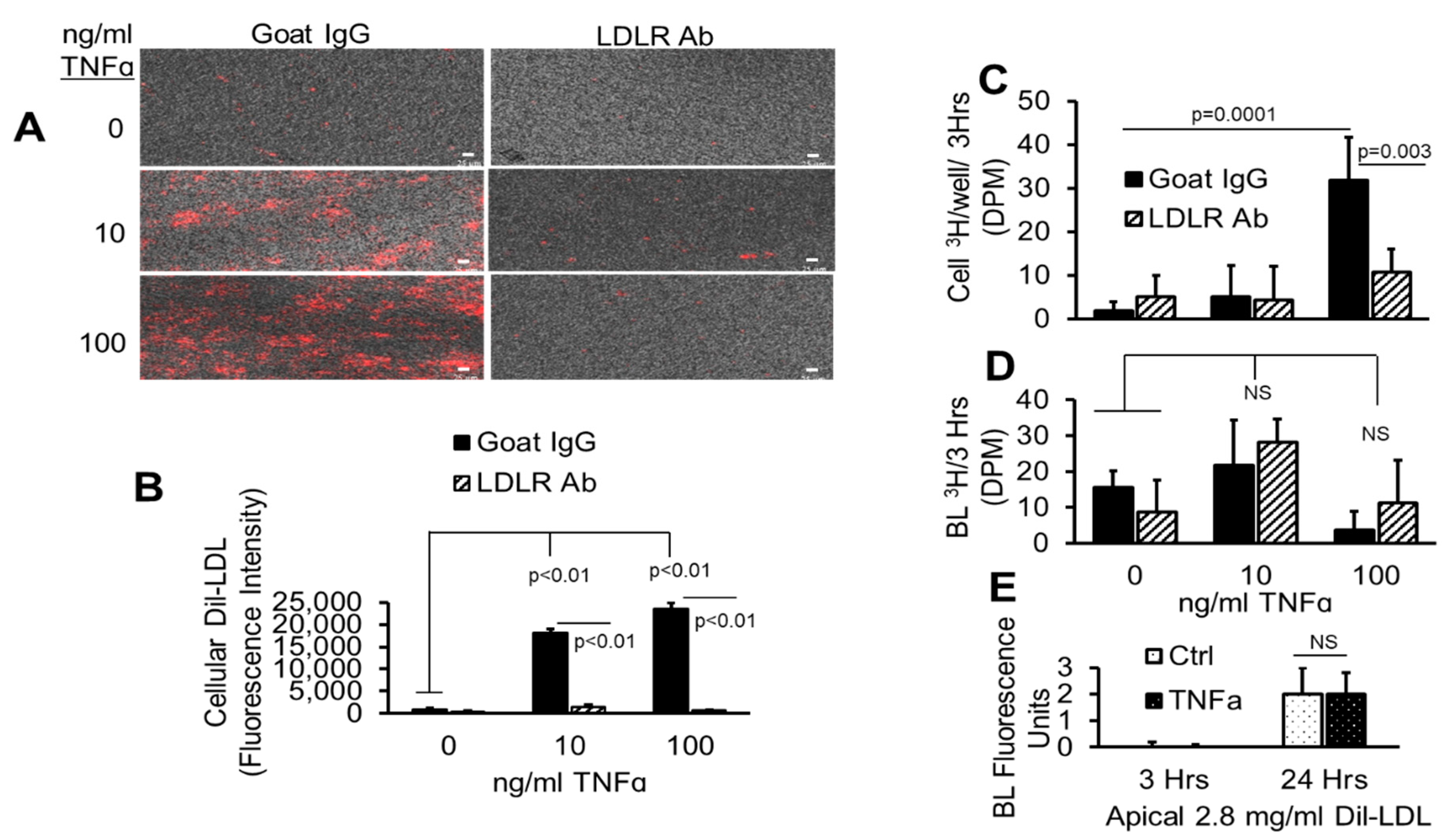
2.7. TNFα-Induced Dil-LDL Accumulation Is Blocked by Specific LDLR Antibody
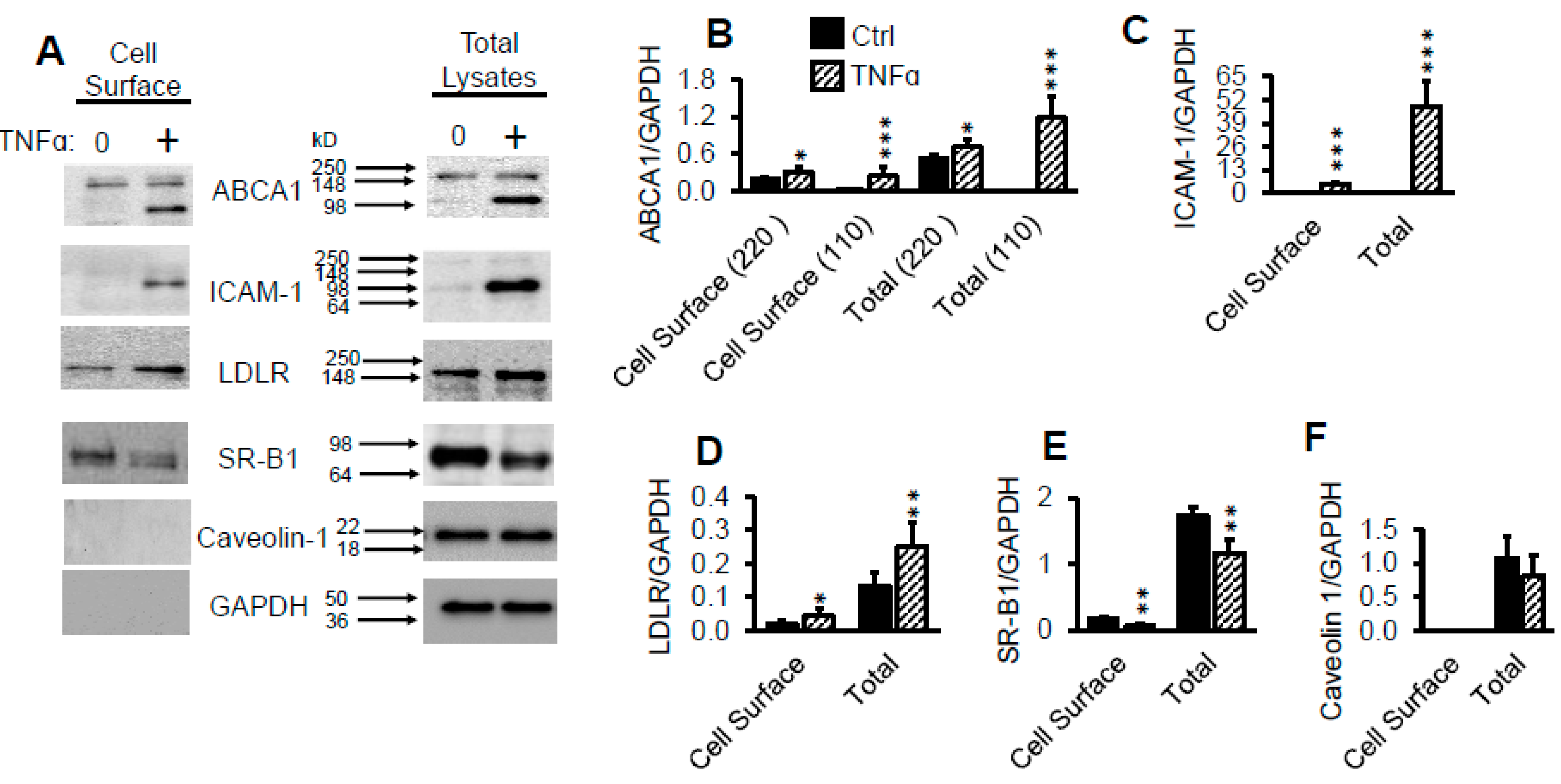
2.8. TNFα Upregulates Cell Surface LDLR Protein
2.9. TNFα Promotes Rapid Association of LDLR with Its Antibody
2.10. Antioxidant Suppresses TNFα-Induced Dil-LDL Accumulation
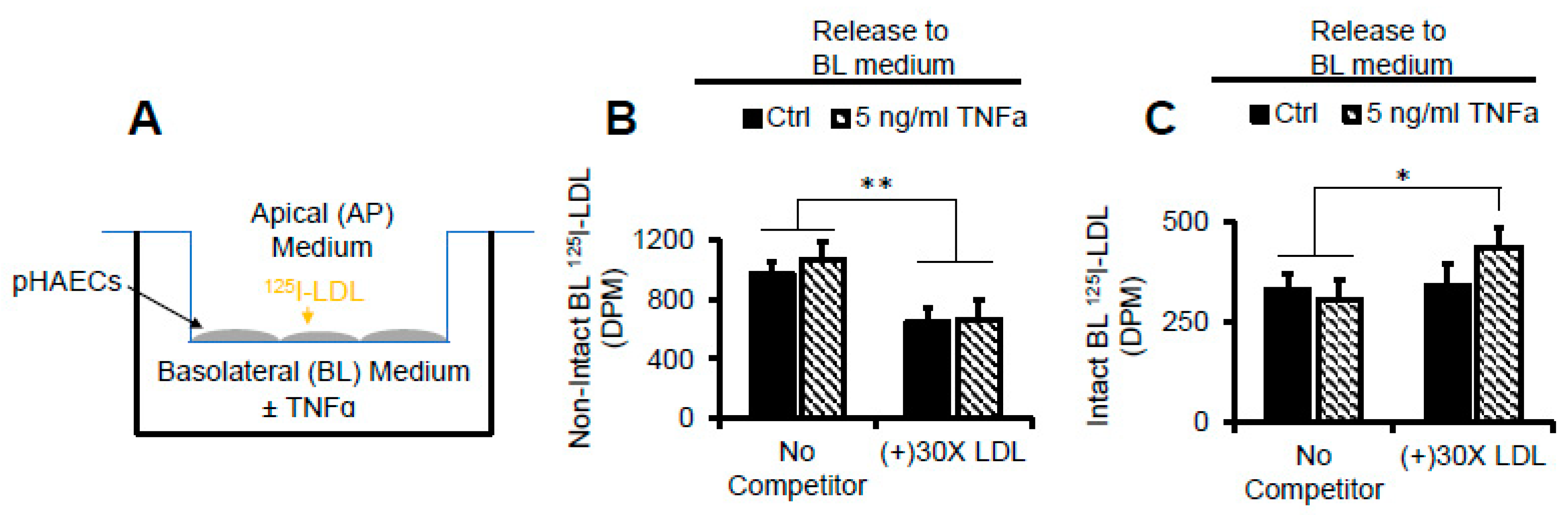
2.11. TNFα Does Not Affect AP to BL Release of Degraded LDL Protein
2.12. TNFα Does Not Affect AP to BL LDL Lipid Release
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Incubations
4.3. Lipoprotein Purification
4.4. Cellular Cholesterol Determination
4.5. Transwell Insert Experiments
4.6. 3H-Cholesteryl Ester ([3H]CE) Generation
4.7. Western Blotting
4.8. Dil- and/or [3H]CE-Lipoproteins
4.9. LDL Iodination with Na125I
4.10. 125I-LDL Cell Surface Binding
4.11. LDL Oxidation
4.12. TBARS Assay
4.13. Depletion of apoE from Dil-Lipoproteins
4.14. Cell Surface Biotinylation
4.15. Dextran-Mn Separation of Intact and Non-Intact 125I-LDL
4.16. Immunofluorescence
4.17. Fluorescence Microscopy
4.18. Statistical Analysis
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAT | Acyl-CoA cholesterol acyltransferase |
| AP | Apical |
| Apo (X) | Apolipoprotein (X) |
| BHT | Butylated hydroxytoluene |
| BL | Basolateral |
| CAB | Cholesterol assay buffer |
| DB | Dialysis buffer |
| Dil | 1,1′ Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate |
| DMSO | Dimethyl sulfoxide |
| FAF-BSA | Fatty acid free bovine serum albumin |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HDL3 | High density lipoprotein 3 |
| HRP | Horse radish peroxidase |
| LDL | Low density lipoprotein |
| LDLR | LDL receptor |
| LRP | LDLR-related protein |
| mHBSS | Modified d Hanks’ balanced salt solution |
| PES | Phosphate buffered saline |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| pHAECS | Primary human aortic endothelial cells; |
| RAP | Receptor associated protein |
| SDS | Sodium dodecyl sulfate |
| TMTU | Tetra methylthiourea |
| TNFa | Tumor necrosis factor alpha |
| VBM | Vascular basal medium |
| VEGF | Vascular endothelial growth factor kit |
References
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor alpha Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S.D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: Ramifications for the complex physiology of TNF. Cell 1988, 53, 45–53. [Google Scholar] [CrossRef]
- Jue, D.M.; Sherry, B.; Luedke, C.; Manogue, K.R.; Cerami, A. Processing of newly synthesized cachectin/tumor necrosis factor in endotoxin-stimulated macrophages. Biochemistry 1990, 29, 8371–8377. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef]
- Hiscott, J.; Marois, J.; Garoufalis, J.; D’Addario, M.; Roulston, A.; Kwan, I.; Pepin, N.; Lacoste, J.; Nguyen, H.; Bensi, G.; et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: Evidence for a positive autoregulatory loop. Mol. Cell. Biol. 1993, 13, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Paliogianni, F.; Raptis, A.; Ahuja, S.S.; Najjar, S.M.; Boumpas, D.T. Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J. Clin. Investig. 1993, 91, 1481–1489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laster, S.M.; Wood, J.G.; Gooding, L.R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 1988, 141, 2629–2634. [Google Scholar]
- Sugarman, B.J.; Aggarwal, B.B.; Hass, P.E.; Figari, I.S.; Palladino, M.A., Jr.; Shepard, H.M. Recombinant human tumor necrosis factor-alpha: Effects on proliferation of normal and transformed cells in vitro. Science 1985, 230, 943–945. [Google Scholar] [CrossRef]
- Frater-Schroder, M.; Risau, W.; Hallmann, R.; Gautschi, P.; Bohlen, P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc. Natl. Acad. Sci. USA 1987, 84, 5277–5281. [Google Scholar] [CrossRef]
- Verheij, M.; Bose, R.; Lin, X.H.; Yao, B.; Jarvis, W.D.; Grant, S.; Birrer, M.J.; Szabo, E.; Zon, L.I.; Kyriakis, J.M.; et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 1996, 380, 75–79. [Google Scholar] [CrossRef]
- Sluss, H.K.; Barrett, T.; Derijard, B.; Davis, R.J. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol. Cell. Biol. 1994, 14, 8376–8384. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Kang, P.M.; Hampe, J.; Yoshimura, K.; Noma, T.; Matsuzaki, M.; Izumo, S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J. Biol. Chem. 2002, 277, 10244–10250. [Google Scholar] [CrossRef]
- Scheurich, P.; Thoma, B.; Ucer, U.; Pfizenmaier, K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: Induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J. Immunol. 1987, 138, 1786–1790. [Google Scholar]
- Vilcek, J.; Palombella, V.J.; Henriksen-DeStefano, D.; Swenson, C.; Feinman, R.; Hirai, M.; Tsujimoto, M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J. Exp. Med. 1986, 163, 632–643. [Google Scholar] [CrossRef]
- Bruggeman, L.A.; Drawz, P.E.; Kahoud, N.; Lin, K.; Barisoni, L.; Nelson, P.J. TNFR2 interposes the proliferative and NF-kappaB-mediated inflammatory response by podocytes to TNF-alpha. Lab. Investig. 2011, 91, 413–425. [Google Scholar] [CrossRef]
- Ligresti, G.; Aplin, A.C.; Zorzi, P.; Morishita, A.; Nicosia, R.F. Macrophage-derived tumor necrosis factor-alpha is an early component of the molecular cascade leading to angiogenesis in response to aortic injury. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1151–1159. [Google Scholar] [CrossRef]
- Jelinek, D.F.; Lipsky, P.E. Enhancement of human B cell proliferation and differentiation by tumor necrosis factor-alpha and interleukin 1. J. Immunol. 1987, 139, 2970–2976. [Google Scholar]
- Barath, P.; Fishbein, M.C.; Cao, J.; Berenson, J.; Helfant, R.H.; Forrester, J.S. Detection and localization of tumor necrosis factor in human atheroma. Am. J. Cardiol. 1990, 65, 297–302. [Google Scholar] [CrossRef]
- Barath, P.; Fishbein, M.C.; Cao, J.; Berenson, J.; Helfant, R.H.; Forrester, J.S. Tumor necrosis factor gene expression in human vascular intimal smooth muscle cells detected by in situ hybridization. Am. J. Pathol. 1990, 137, 503–509. [Google Scholar]
- Zhang, X.; Bishawi, M.; Zhang, G.; Prasad, V.; Salmon, E.; Breithaupt, J.J.; Zhang, Q.; Truskey, G.A. Modeling early stage atherosclerosis in a primary human vascular microphysiological system. Nat. Commun. 2020, 11, 5426. [Google Scholar] [CrossRef]
- Zeiher, A.M.; Fisslthaler, B.; Schray-Utz, B.; Busse, R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ. Res. 1995, 76, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Yuan, S.; Carter, P.; Bruzelius, M.; Vithayathil, M.; Kar, S.; Mason, A.M.; Lin, A.; Burgess, S.; Larsson, S.C. Effects of tumour necrosis factor on cardiovascular disease and cancer: A two-sample Mendelian randomization study. EBioMedicine 2020, 59, 102956. [Google Scholar] [CrossRef]
- Rus, H.G.; Niculescu, F.; Vlaicu, R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis 1991, 89, 247–254. [Google Scholar] [CrossRef]
- Paolisso, G.; Rizzo, M.R.; Mazziotti, G.; Tagliamonte, M.R.; Gambardella, A.; Rotondi, M.; Carella, C.; Giugliano, D.; Varricchio, M.; D’Onofrio, F. Advancing age and insulin resistance: Role of plasma tumor necrosis factor-alpha. Am. J. Physiol. 1998, 275, E294–E299. [Google Scholar] [CrossRef]
- Byl, B.; Roucloux, I.; Crusiaux, A.; Dupont, E.; Deviere, J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology 1993, 104, 1492–1497. [Google Scholar] [CrossRef]
- Nagashima, S.; Mendes, M.C.; Camargo Martins, A.P.; Borges, N.H.; Godoy, T.M.; Miggiolaro, A.; da Silva Deziderio, F.; Machado-Souza, C.; de Noronha, L. Endothelial Dysfunction and Thrombosis in Patients With COVID-19-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2404–2407. [Google Scholar] [CrossRef]
- Liu, Y.N.; Peng, Y.L.; Liu, L.; Wu, T.Y.; Zhang, Y.; Lian, Y.J.; Yang, Y.Y.; Kelley, K.W.; Jiang, C.L.; Wang, Y.X. TNFalpha mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur. Cytokine Netw. 2015, 26, 15–25. [Google Scholar] [CrossRef]
- Schreyer, S.A.; Peschon, J.J.; LeBoeuf, R.C. Accelerated atherosclerosis in mice lacking tumor necrosis factor receptor p55. J. Biol. Chem. 1996, 271, 26174–26178. [Google Scholar] [CrossRef]
- Schreyer, S.A.; Vick, C.M.; LeBoeuf, R.C. Loss of lymphotoxin-alpha but not tumor necrosis factor-alpha reduces atherosclerosis in mice. J. Biol. Chem. 2002, 277, 12364–12368. [Google Scholar] [CrossRef]
- Branen, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Jovinge, S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar] [CrossRef]
- Zhang, L.; Peppel, K.; Sivashanmugam, P.; Orman, E.S.; Brian, L.; Exum, S.T.; Freedman, N.J. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1087–1094. [Google Scholar] [CrossRef]
- Xanthoulea, S.; Thelen, M.; Pottgens, C.; Gijbels, M.J.; Lutgens, E.; de Winther, M.P. Absence of p55 TNF receptor reduces atherosclerosis, but has no major effect on angiotensin II induced aneurysms in LDL receptor deficient mice. PLoS ONE 2009, 4, e6113. [Google Scholar] [CrossRef]
- Blessing, E.; Bea, F.; Kuo, C.C.; Campbell, L.A.; Chesebro, B.; Rosenfeld, M.E. Lesion progression and plaque composition are not altered in older apoE-/- mice lacking tumor necrosis factor-alpha receptor p55. Atherosclerosis 2004, 176, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, U.M.; Mavrakis, L.; Bonfield, T.L.; Smith, J.D.; DiCorleto, P.E. Decreased atherosclerosis in mice deficient in tumor necrosis factor-alpha receptor-II (p75). Arterioscler. Thromb. Vasc. Biol. 2007, 27, e16–e17. [Google Scholar] [CrossRef]
- Canault, M.; Peiretti, F.; Mueller, C.; Kopp, F.; Morange, P.; Rihs, S.; Portugal, H.; Juhan-Vague, I.; Nalbone, G. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis 2004, 172, 211–218. [Google Scholar] [CrossRef]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Boesten, L.S.; Zadelaar, A.S.; van Nieuwkoop, A.; Gijbels, M.J.; de Winther, M.P.; Havekes, L.M.; van Vlijmen, B.J. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc. Res. 2005, 66, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, R.; Vlacil, A.K.; Schuett, J.; Schosser, F.; Schuett, H.; Tietge, U.J.F.; Schieffer, B.; Grote, K. Anti-tumor necrosis factor-alpha therapy increases plaque burden in a mouse model of experimental atherosclerosis. Atherosclerosis 2018, 277, 80–89. [Google Scholar] [CrossRef]
- Ross, R.; Wight, T.N.; Strandness, E.; Thiele, B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am. J. Pathol. 1984, 114, 79–93. [Google Scholar]
- Hoff, H.F.; Morton, R.E. Lipoproteins containing apo B extracted from human aortas. Structure and function. Ann. N. Y. Acad. Sci. 1985, 454, 183–194. [Google Scholar] [CrossRef]
- Mahley, R.W.; Innerarity, T.L.; Weisgraber, K.H.; Fry, D.L. Canine hyperlipoproteinemia and atherosclerosis. Accumulation of lipid by aortic medial cells in vivo and in vitro. Am. J. Pathol. 1977, 87, 205–226. [Google Scholar] [PubMed]
- Vlodavsky, I.; Fielding, P.E.; Fielding, C.J.; Gospodarowicz, D. Role of contact inhibition in the regulation of receptor-mediated uptake of low density lipoprotein in cultured vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1978, 75, 356–360. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Neyen, C.; Gordon, S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 2012, 217, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Freeman, M.W.; Libby, P. Regulation of smooth muscle cell scavenger receptor expression in vivo by atherogenic diets and in vitro by cytokines. J. Clin. Investig. 1995, 95, 122–133. [Google Scholar] [CrossRef]
- Poole, J.C.; Florey, H.W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J. Pathol. Bacteriol. 1958, 75, 245–251. [Google Scholar] [CrossRef]
- Simionescu, M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 266–274. [Google Scholar] [CrossRef]
- Joris, I.; Zand, T.; Nunnari, J.J.; Krolikowski, F.J.; Majno, G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am. J. Pathol. 1983, 113, 341–358. [Google Scholar]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants (Basel) 2019, 8, 218. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, C. Oxidized LDL Causes Endothelial Apoptosis by Inhibiting Mitochondrial Fusion and Mitochondria Autophagy. Front Cell Dev Biol 2020, 8, 600950. [Google Scholar] [CrossRef]
- Mollace, V.; Gliozzi, M.; Musolino, V.; Carresi, C.; Muscoli, S.; Mollace, R.; Tavernese, A.; Gratteri, S.; Palma, E.; Morabito, C.; et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int. J. Cardiol. 2015, 184, 152–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Vittinghoff, E.; Pletcher, M.J.; Allen, N.B.; Zeki Al Hazzouri, A.; Yaffe, K.; Balte, P.P.; Alonso, A.; Newman, A.B.; Ives, D.G.; et al. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J. Am. Coll. Cardiol. 2019, 74, 330–341. [Google Scholar] [CrossRef]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Holthenrich, A.; Gerke, V. Regulation of von-Willebrand Factor Secretion from Endothelial Cells by the Annexin A2-S100A10 Complex. Int. J. Mol. Sci. 2018, 19, 1752. [Google Scholar] [CrossRef]
- Lei, L.; Xiong, Y.; Chen, J.; Yang, J.B.; Wang, Y.; Yang, X.Y.; Chang, C.C.; Song, B.L.; Chang, T.Y.; Li, B.L. TNF-alpha stimulates the ACAT1 expression in differentiating monocytes to promote the CE-laden cell formation. J. Lipid Res. 2009, 50, 1057–1067. [Google Scholar] [CrossRef]
- An, S.; Jang, Y.S.; Park, J.S.; Kwon, B.M.; Paik, Y.K.; Jeong, T.S. Inhibition of acyl-coenzyme A:cholesterol acyltransferase stimulates cholesterol efflux from macrophages and stimulates farnesoid X receptor in hepatocytes. Exp. Mol. Med. 2008, 40, 407–417. [Google Scholar] [CrossRef][Green Version]
- Satriano, J.A.; Shuldiner, M.; Hora, K.; Xing, Y.; Shan, Z.; Schlondorff, D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J. Clin. Investig. 1993, 92, 1564–1571. [Google Scholar] [CrossRef]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [PubMed]
- Trinh, M.N.; Brown, M.S.; Goldstein, J.L.; Han, J.; Vale, G.; McDonald, J.G.; Seemann, J.; Mendell, J.T.; Lu, F. Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proc. Natl. Acad. Sci. USA 2020, 117, 18521–18529. [Google Scholar] [CrossRef]
- Hevonoja, T.; Pentikainen, M.O.; Hyvonen, M.T.; Kovanen, P.T.; Ala-Korpela, M. Structure of low density lipoprotein (LDL) particles: Basis for understanding molecular changes in modified LDL. Biochim. Biophys. Acta 2000, 1488, 189–210. [Google Scholar] [CrossRef]
- Mendivil, C.O.; Rimm, E.B.; Furtado, J.; Sacks, F.M. Apolipoprotein E in VLDL and LDL with apolipoprotein C-III is associated with a lower risk of coronary heart disease. J. Am. Heart Assoc. 2013, 2, e000130. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, C.C.; Qiao, W.; Bu, G. Apolipoprotein E, Receptors, and Modulation of Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 347–357. [Google Scholar] [CrossRef]
- Shan, L.; Pang, L.; Zhang, R.; Murgolo, N.J.; Lan, H.; Hedrick, J.A. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem. Biophys. Res. Commun. 2008, 375, 69–73. [Google Scholar] [CrossRef]
- Poirier, S.; Mayer, G.; Benjannet, S.; Bergeron, E.; Marcinkiewicz, J.; Nassoury, N.; Mayer, H.; Nimpf, J.; Prat, A.; Seidah, N.G. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 2008, 283, 2363–2372. [Google Scholar] [CrossRef]
- Canuel, M.; Sun, X.; Asselin, M.C.; Paramithiotis, E.; Prat, A.; Seidah, N.G. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS ONE 2013, 8, e64145. [Google Scholar] [CrossRef]
- Ruiz, J.; Kouiavskaia, D.; Migliorini, M.; Robinson, S.; Saenko, E.L.; Gorlatova, N.; Li, D.; Lawrence, D.; Hyman, B.T.; Weisgraber, K.H.; et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J. Lipid Res. 2005, 46, 1721–1731. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J.; Patel, S.; Osuga, J.; Ishibashi, S.; Azhar, S. The LDL receptor is not necessary for acute adrenal steroidogenesis in mouse adrenocortical cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E408–E412. [Google Scholar] [CrossRef]
- He, P.; Gelissen, I.C.; Ammit, A.J. Regulation of ATP binding cassette transporter A1 (ABCA1) expression: Cholesterol-dependent and - independent signaling pathways with relevance to inflammatory lung disease. Respir. Res. 2020, 21, 250. [Google Scholar] [CrossRef]
- Gerbod-Giannone, M.C.; Li, Y.; Holleboom, A.; Han, S.; Hsu, L.C.; Tabas, I.; Tall, A.R. TNFalpha induces ABCA1 through NF-kappaB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl. Acad. Sci. USA 2006, 103, 3112–3117. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Moser, A.H.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Regulation of scavenger receptor class B type I in hamster liver and Hep3B cells by endotoxin and cytokines. J. Lipid Res. 2001, 42, 1636–1644. [Google Scholar] [CrossRef]
- Simon, L.; Campos, A.; Leyton, L.; Quest, A.F.G. Caveolin-1 function at the plasma membrane and in intracellular compartments in cancer. Cancer Metastasis Rev. 2020, 39, 435–453. [Google Scholar] [CrossRef]
- Hubert, M.; Larsson, E.; Vegesna, N.V.G.; Ahnlund, M.; Johansson, A.I.; Moodie, L.W.; Lundmark, R. Lipid accumulation controls the balance between surface connection and scission of caveolae. Elife 2020, 9. [Google Scholar] [CrossRef]
- Ogier, J.M.; Nayagam, B.A.; Lockhart, P.J. ASK1 inhibition: A therapeutic strategy with multi-system benefits. J. Mol. Med. (Berl.) 2020, 98, 335–348. [Google Scholar] [CrossRef]
- Xia, X.; Xu, Q.; Liu, M.; Chen, X.; Liu, X.; He, J.; Hu, T.; Yu, C.; Huang, H.; Liu, S.; et al. Deubiquitination of CD36 by UCHL1 promotes foam cell formation. Cell Death Dis. 2020, 11, 636. [Google Scholar] [CrossRef]
- Ruuth, M.; Nguyen, S.D.; Vihervaara, T.; Hilvo, M.; Laajala, T.D.; Kondadi, P.K.; Gistera, A.; Lahteenmaki, H.; Kittila, T.; Huusko, J.; et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 2018, 39, 2562–2573. [Google Scholar] [CrossRef]
- Hurt, E.; Camejo, G. Effect of arterial proteoglycans on the interaction of LDL with human monocyte-derived macrophages. Atherosclerosis 1987, 67, 115–126. [Google Scholar] [CrossRef]
- Salisbury, B.G.; Falcone, D.J.; Minick, C.R. Insoluble low-density lipoprotein-proteoglycan complexes enhance cholesteryl ester accumulation in macrophages. Am. J. Pathol. 1985, 120, 6–11. [Google Scholar]
- Hurt, E.; Bondjers, G.; Camejo, G. Interaction of LDL with human arterial proteoglycans stimulates its uptake by human monocyte-derived macrophages. J. Lipid Res. 1990, 31, 443–454. [Google Scholar] [CrossRef]
- Puig, N.; Montolio, L.; Camps-Renom, P.; Navarra, L.; Jimenez-Altayo, F.; Jimenez-Xarrie, E.; Sanchez-Quesada, J.L.; Benitez, S. Electronegative LDL Promotes Inflammation and Triglyceride Accumulation in Macrophages. Cells 2020, 9, 583. [Google Scholar] [CrossRef]
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. (Lausanne) 2020, 7, 467. [Google Scholar] [CrossRef]
- LaRosa, J.C.; Grundy, S.M.; Waters, D.D.; Shear, C.; Barter, P.; Fruchart, J.C.; Gotto, A.M.; Greten, H.; Kastelein, J.J.; Shepherd, J.; et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N. Engl. J. Med. 2005, 352, 1425–1435. [Google Scholar] [CrossRef]
- Silverstein, R.L. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Goes “DAMP”. Circulation 2021, 143, 62–64. [Google Scholar] [CrossRef]
- Adorni, M.P.; Zimetti, F.; Lupo, M.G.; Ruscica, M.; Ferri, N. Naturally Occurring PCSK9 Inhibitors. Nutrients 2020, 12, 1440. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Zhao, S.P.; Dong, S.Z. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clin. Chim. Acta 2008, 389, 67–71. [Google Scholar] [CrossRef]
- Ma, K.L.; Ruan, X.Z.; Powis, S.H.; Chen, Y.; Moorhead, J.F.; Varghese, Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology 2008, 48, 770–781. [Google Scholar] [CrossRef]
- Field, F.J.; Watt, K.; Mathur, S.N. TNF-alpha decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J. Lipid Res. 2010, 51, 1407–1415. [Google Scholar] [CrossRef]
- Wehmeier, K.R.; Kurban, W.; Chandrasekharan, C.; Onstead-Haas, L.; Mooradian, A.D.; Haas, M.J. Inhibition of ABCA1 Protein Expression and Cholesterol Efflux by TNF alpha in MLO-Y4 Osteocytes. Calcif. Tissue Int. 2016, 98, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jiang, J.; Jing, Y.; Liu, W.; Yang, X.; Hou, X.; Gao, L.; Wei, L. The concentration of tumor necrosis factor-alpha determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020, 11, 70. [Google Scholar] [CrossRef]
- Gareb, B.; Otten, A.T.; Frijlink, H.W.; Dijkstra, G.; Kosterink, J.G.W. Review: Local Tumor Necrosis Factor-alpha Inhibition in Inflammatory Bowel Disease. Pharmaceutics 2020, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Hsin-Hui, H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. medRxiv 2020. [Google Scholar] [CrossRef]
- Gerriets, V.; Bansal, P.; Goyal, A.; Khaddour, K. Tumor necrosis factor inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Vasile, E.; Simionescu, M.; Simionescu, N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J. Cell Biol. 1983, 96, 1677–1689. [Google Scholar] [CrossRef]
- Ghaffari, S.; Jang, E.; Naderinabi, F.; Sanwal, R.; Khosraviani, N.; Wang, C.; Steinberg, B.E.; Goldenberg, N.M.; Ikeda, J.; Lee, W.L. Endothelial HMGB1 Is a Critical Regulator of LDL Transcytosis via an SREBP2-SR-BI Axis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 200–216. [Google Scholar] [CrossRef]
- Molino, Y.; David, M.; Varini, K.; Jabes, F.; Gaudin, N.; Fortoul, A.; Bakloul, K.; Masse, M.; Bernard, A.; Drobecq, L.; et al. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. 2017, 31, 1807–1827. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef]
- Havel, R.; Eder, H.; Bragdon, J. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Skipski, V.P.; Barclay, M.; Barclay, R.K.; Fetzer, V.A.; Good, J.J.; Archibald, F.M. Lipid composition of human serum lipoproteins. Biochem. J. 1967, 104, 340–352. [Google Scholar] [CrossRef]
- Kuchinskiene, Z.; Carlson, L.A. Composition, concentration, and size of low density lipoproteins and of subfractions of very low density lipoproteins from serum of normal men and women. J. Lipid Res. 1982, 23, 762–769. [Google Scholar] [CrossRef]
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C., Jr.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [CrossRef]
- Andreae, W.A. A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature 1955, 175, 859–860. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Saxena, U.; Ferguson, E.; Bisgaier, C.L. Apolipoprotein E modulates low density lipoprotein retention by lipoprotein lipase anchored to the subendothelial matrix. J. Biol. Chem. 1993, 268, 14812–14819. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Kaluzny, M.A.; Duncan, L.A.; Merritt, M.V.; Epps, D.E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid Res. 1985, 26, 135–140. [Google Scholar] [CrossRef]
- Pitas, R.E.; Innerarity, T.L.; Weinstein, J.N.; Mahley, R.W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis 1981, 1, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, B.J.; Bisgaier, C.L.; Wolle, J.; Saxena, U. Oxidation of low density lipoproteins greatly enhances their association with lipoprotein lipase anchored to endothelial cell matrix. J. Biol. Chem. 1996, 271, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Steinbrecher, U.P.; Barnett, J.; Witztum, J.L.; Steinberg, D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc. Natl. Acad. Sci. USA 1985, 82, 3000–3004. [Google Scholar] [CrossRef]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, E.U. TNFα-Induced LDL Cholesterol Accumulation Involve Elevated LDLR Cell Surface Levels and SR-B1 Downregulation in Human Arterial Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 6236. https://doi.org/10.3390/ijms22126236
Okoro EU. TNFα-Induced LDL Cholesterol Accumulation Involve Elevated LDLR Cell Surface Levels and SR-B1 Downregulation in Human Arterial Endothelial Cells. International Journal of Molecular Sciences. 2021; 22(12):6236. https://doi.org/10.3390/ijms22126236
Chicago/Turabian StyleOkoro, Emmanuel Ugochukwu. 2021. "TNFα-Induced LDL Cholesterol Accumulation Involve Elevated LDLR Cell Surface Levels and SR-B1 Downregulation in Human Arterial Endothelial Cells" International Journal of Molecular Sciences 22, no. 12: 6236. https://doi.org/10.3390/ijms22126236
APA StyleOkoro, E. U. (2021). TNFα-Induced LDL Cholesterol Accumulation Involve Elevated LDLR Cell Surface Levels and SR-B1 Downregulation in Human Arterial Endothelial Cells. International Journal of Molecular Sciences, 22(12), 6236. https://doi.org/10.3390/ijms22126236




