4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer
Abstract
1. Introduction
2. 4-1BB/4-1BBL
2.1. Receptor 4-1BB
2.2. 4-1BB Ligand
2.3. 4-1BB/41BBL Complex
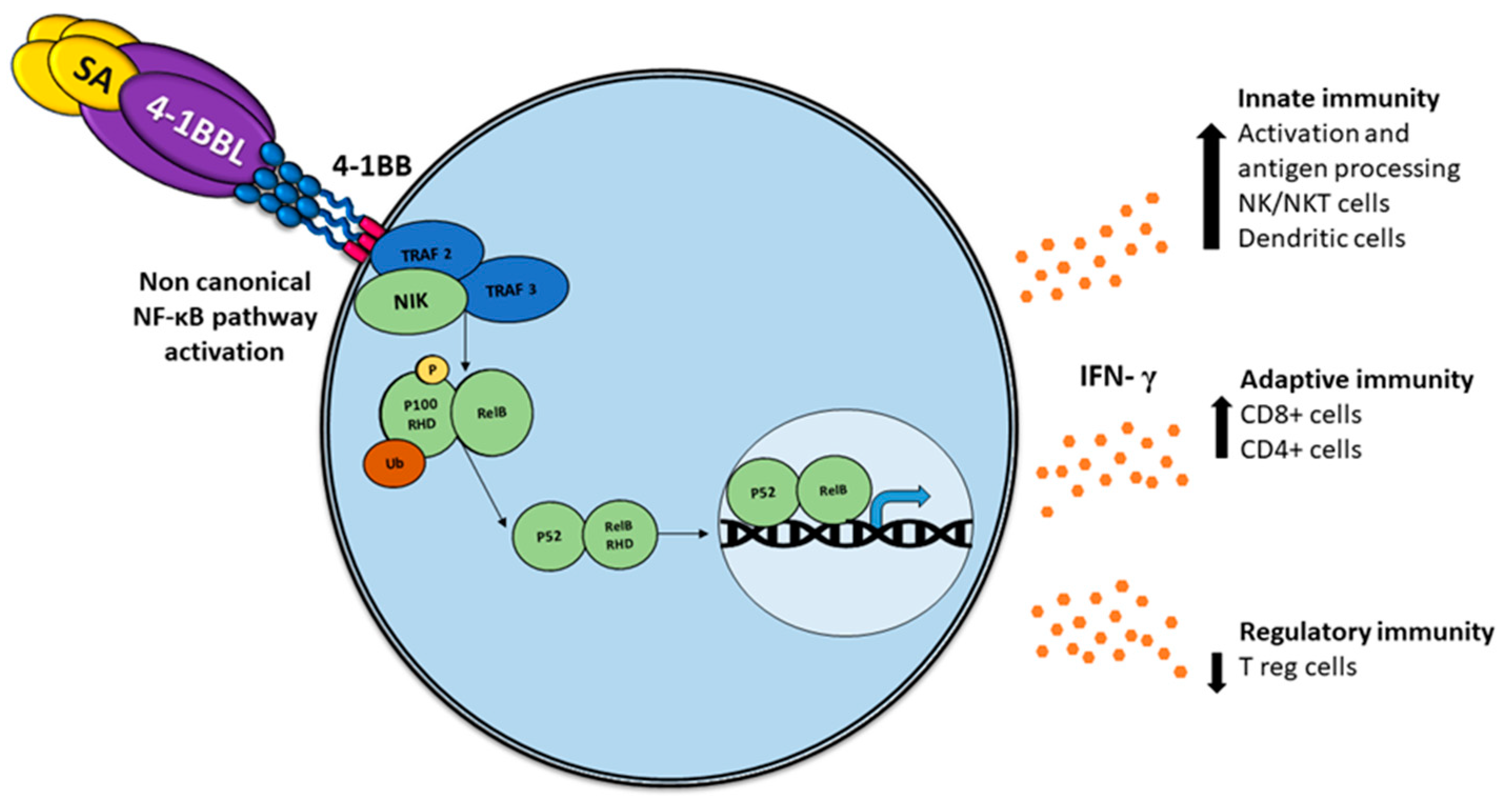
2.4. SA-4-1BBL
3. Tumor-Associated Antigens (TAAs)
SA-4-1BBL and TAAs
4. Tumor Microenvironment
5. Delivery Technologies for 4-1BBL
5.1. Fusion Proteins
5.2. DNA Vaccines
5.3. Oncolytic Virus
5.4. Antibodies
5.5. Cellular Therapy
6. 4-1BBL Current Clinical and Preclinical Applications
6.1. DSP107
6.2. LOAd703
6.3. RO7227166
6.4. EGFRt/19-28z/4-1BBL CAR-T Cells
6.5. HLA A2/4-1BB Ligand
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Available online: http://gco.iarc.fr (accessed on 14 January 2020).
- Beecher, S.M.; O’Leary, D.P.; McLaughlin, R.; Kerin, M.J. The impact of surgical complications on cancer recurrence rates: A literature review. Oncol. Res. Treat. 2018, 41, 478–482. [Google Scholar] [CrossRef]
- Butow, P.; Sharpe, L.; Thewes, B.; Turner, J.; Gilchrist, J.; Beith, J. Fear of cancer recurrence: A practical guide for clinicians. Oncol. Williston Park N 2018, 32, 32–38. [Google Scholar]
- Khan, K.H. DNA Vaccines: Roles against diseases. Germs 2013, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cannons, J.L.; Choi, Y.; Watts, T.H. Role of TNF receptor-associated factor 2 and P38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J. Immunol. 2000, 165, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Schabowsky, R.H.; Srivastava, A.K.; Elpek, K.G.; Madireddi, S.; Zhao, H.; Zhong, Z.; Miller, R.W.; MacLeod, K.J.; Yolcu, E.S.; et al. 4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res. 2010, 70, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Valilou, S.F.; Rezaei, N. Chapter 4—Tumor antigens. In Vaccines for Cancer Immunotherapy; Rezaei, N., Keshavarz-Fathi, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 61–74. ISBN 978-0-12-814039-0. [Google Scholar]
- Liu, C.-C.; Yang, H.; Zhang, R.; Zhao, J.-J.; Hao, D.-J. Tumour-associated antigens and their anti-cancer applications. Eur. J. Cancer Care 2017, 26, e12446. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; Plaen, E.D.; den Eynde, B.V.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic t lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Escors, D. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J. Sci. 2014. [Google Scholar] [CrossRef]
- Mousavi, T.; Sattari Saravi, S.; Valadan, R.; Haghshenas, M.R.; Rafiei, A.; Jafarpour, H.; Shamshirian, A. Different types of adjuvants in prophylactic and therapeutic human papillomavirus vaccines in laboratory animals: A systematic review. Arch. Virol. 2020, 165, 263–284. [Google Scholar] [CrossRef]
- Sharma, R.K.; Srivastava, A.K.; Yolcu, E.S.; MacLeod, K.J.; Schabowsky, R.-H.; Madireddi, S.; Shirwan, H. SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model. Vaccine 2010, 28, 5794–5802. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K. Basic overview of current immunotherapy approaches in cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 298–308. [Google Scholar] [CrossRef]
- Kwon, B.S.; Weissman, S.M. CDNA sequences of two inducible t-cell genes. Proc. Natl. Acad. Sci. USA 1989, 86, 1963–1967. [Google Scholar] [CrossRef]
- Smith, C.A.; Davis, T.; Anderson, D.; Solam, L.; Beckmann, M.P.; Jerzy, R.; Dower, S.K.; Cosman, D.; Goodwin, R.G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 1990, 248, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Tuckwell, J.; Lotz, M. A Receptor induced by lymphocyte activation (ILA): A new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene 1993, 134, 295–298. [Google Scholar] [CrossRef]
- Alderson, M.R.; Smith, C.A.; Tough, T.W.; Davis-Smith, T.; Armitage, R.J.; Falk, B.; Roux, E.; Baker, E.; Sutherland, G.R.; Din, W.S. Molecular and biological characterization of human 4-1BB and its ligand. Eur. J. Immunol. 1994, 24, 2219–2227. [Google Scholar] [CrossRef]
- Bitra, A.; Doukov, T.; Wang, J.; Picarda, G.; Benedict, C.A.; Croft, M.; Zajonc, D.M. Crystal structure of murine 4-1BB and its interaction with 4-1BBL support a role for galectin-9 in 4-1BB signaling. J. Biol. Chem. 2018, 293, 1317–1329. [Google Scholar] [CrossRef]
- Bitra, A.; Doukov, T.; Croft, M.; Zajonc, D.M. Crystal structures of the human 4-1BB receptor bound to its ligand 4-1BBL reveal covalent receptor dimerization as a potential signaling amplifier. J. Biol. Chem. 2018, 293, 9958–9969. [Google Scholar] [CrossRef]
- Vinay, D.S.; Kwon, B.S. Immunotherapy of cancer with 4-1BB. Mol. Cancer Ther. 2012, 11, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, A.T.C.; Mufti, G.J.; Guinn, B. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004, 11, 215–226. [Google Scholar] [CrossRef]
- Li, S.-Y.; Liu, Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin. Pharm. Adv. Appl. 2013, 5, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bitra, A.; Doukov, T.; Destito, G.; Croft, M.; Zajonc, D.M. Crystal structure of the M4-1BB/4-1BBL complex reveals an unusual dimeric ligand that undergoes structural changes upon 4-1BB receptor binding. J. Biol. Chem. 2019, 294, 1831–1845. [Google Scholar] [CrossRef]
- Gilbreth, R.N.; Oganesyan, V.Y.; Amdouni, H.; Novarra, S.; Grinberg, L.; Barnes, A.; Baca, M. Crystal structure of the human 4-1BB/4-1BBL complex. J. Biol. Chem. 2018, 293, 9880–9891. [Google Scholar] [CrossRef]
- Bitra, A.; Zajonc, D.M. Evolution of differential 4-1BB signaling in human and murine immune system. FASEB J. 2019, 33, 461.3. [Google Scholar] [CrossRef]
- Zapata, J.M.; Perez-Chacon, G.; Carr-Baena, P.; Martinez-Forero, I.; Azpilikueta, A.; Otano, I.; Melero, I. CD137 (4-1BB) signalosome: Complexity is a matter of TRAFs. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Rabu, C.; Quéméner, A.; Jacques, Y.; Echasserieau, K.; Vusio, P.; Lang, F. Production of recombinant human trimeric CD137L (4-1BBL). Cross-linking is essential to its t cell co-stimulation activity. J. Biol. Chem. 2005, 280, 41472–41481. [Google Scholar] [CrossRef]
- Schabowsky, R.-H.; Sharma, R.K.; Madireddi, S.; Srivastava, A.; Yolcu, E.S.; Shirwan, H. ProtEx™ technology for the generation of novel therapeutic cancer vaccines. Exp. Mol. Pathol. 2009, 86, 198–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schabowsky, R.-H.; Elpek, K.G.; Madireddi, S.; Sharma, R.K.; Yolcu, E.S.; Bandura-Morgan, L.; Miller, R.; MacLeod, K.J.; Mittler, R.S.; Shirwan, H. A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4-1BB Ab without Ab associated severe toxicity. Vaccine 2009, 28, 512–522. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Batra, L.; Shrestha, P.; Bowen, W.S.; Zhao, H.; Egilmez, N.K.; Gomez-Gutierrez, J.G.; Yolcu, E.S.; Shirwan, H. A novel form of 4-1BBL prevents cancer development via nonspecific activation of CD4+ T and natural killer cells. Cancer Res. 2019, 79, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Tiriveedhi, V. The five immune forces impacting DNA-based cancer immunotherapeutic strategy. Int. J. Mol. Sci. 2017, 18, 650. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Ngiow, S.F.; Ribas, A.; Teng, M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016, 13, 143–158. [Google Scholar] [CrossRef]
- McPherson, A.J.; Snell, L.M.; Mak, T.W.; Watts, T.H. Opposing roles for TRAF1 in the alternative versus classical NF-ΚB pathway in t cells. J. Biol. Chem. 2012, 287, 23010–23019. [Google Scholar] [CrossRef]
- Dejardin, E. The alternative NF-KappaB pathway from biochemistry to biology: Pitfalls and promises for future drug development. Biochem. Pharm. 2006, 72, 1161–1179. [Google Scholar] [CrossRef]
- Sun, S.-C. The noncanonical NF-ΚB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef]
- Gerondakis, S.; Grumont, R.; Gugasyan, R.; Wong, L.; Isomura, I.; Ho, W.; Banerjee, A. Unravelling the complexities of the NF-KappaB signalling pathway using mouse knockout and transgenic models. Oncogene 2006, 25, 6781–6799. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yamamoto, M.; Taguchi, Y.; Miyauchi, M.; Akiyama, N.; Yamaguchi, N.; Gohda, J.; Akiyama, T.; Inoue, J. Visualization of RelB expression and activation at the single-cell level during dendritic cell maturation in relb-venus knock-in mice. J. Biochem. 2015, 158, 485–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, S.-C. The non-canonical NF-ΚB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Snell, L.M.; Lin, G.H.Y.; McPherson, A.J.; Moraes, T.J.; Watts, T.H. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol. Rev. 2011, 244, 197–217. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Sharma, R.K.; Yolcu, E.S.; Ulker, V.; MacLeod, K.; Dinc, G.; Shirwan, H. Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner. PLoS ONE 2012, 7, e48463. [Google Scholar] [CrossRef]
- Garza-Morales, R.; Perez-Trujillo, J.J.; Martinez-Jaramillo, E.; Saucedo-Cardenas, O.; Loera-Arias, M.J.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Yolcu, E.; Shirwan, H.; Gomez-Gutierrez, J.G.; et al. A DNA vaccine encoding SA-4-1BBL fused to HPV-16 E7 antigen has prophylactic and therapeutic efficacy in a cervical cancer mouse model. Cancers 2019, 11, 96. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Dinc, G.; Sharma, R.K.; Yolcu, E.S.; Zhao, H.; Shirwan, H. SA-4-1BBL and monophosphoryl lipid a constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res. 2014, 74, 6441–6451. [Google Scholar] [CrossRef]
- Martinez-Perez, A.G.; Perez-Trujillo, J.J.; Garza-Morales, R.; Ramirez-Avila, N.E.; Loera-Arias, M.J.; Gomez-Gutierrez, J.G.; Saucedo-Cardenas, O.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Montes-de-Oca-Luna, R. An oncolytic adenovirus encoding SA-4-1BBL adjuvant fused to HPV-16 E7 antigen produces a specific antitumor effect in a cancer mouse model. Vaccines 2021, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018, 18, 139–147. [Google Scholar] [CrossRef]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Tormoen, G.W.; Crittenden, M.R.; Gough, M.J. Role of the immunosuppressive microenvironment in immunotherapy. Adv. Radiat. Oncol. 2018, 3, 520–526. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.; Choi, M.; Kang, S.; Kim, J.Y.; Kim, S.; Jon, S. Targeted cancer therapy using fusion protein of TNFα and tumor-associated fibronectin-specific aptide. Mol. Pharm. 2017, 14, 3772–3779. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, J.; Cao, J. A novel recombinant fusion protein with soluble PD-1 and TIM-3 domains effectively binds to cancer cells. Indian J. Pharm. Sci. 2020, 82, 537–542. [Google Scholar] [CrossRef]
- Sun, C.; He, D.; Ma, C.; Gao, Z.; Chen, Y.; Wang, S. Bifunctional fusion proteins derived from tumstatin and 4-1BBL for targeted cancer therapy. Mol. Pharm. 2019, 16, 867–876. [Google Scholar] [CrossRef]
- Gozlan, Y.M.; Hilgendorf, S.; Aronin, A.; Sagiv, Y.; Ben-gigi-Tamir, L.; Amsili, S.; Tamir, A.; Pecker, I.; Greenwald, S.; Chajut, A.; et al. Abstract A076: DSP107—A novel SIRPα-4-1BBL dual signaling protein (DSP) for cancer immunotherapy. Cancer Immunol. Res. 2019, 7, A076. [Google Scholar] [CrossRef]
- Sharma, R.K.; Elpek, K.G.; Yolcu, E.S.; Schabowsky, R.-H.; Zhao, H.; Bandura-Morgan, L.; Shirwan, H. Costimulation as a platform for the development of vaccines: A peptide-based vaccine containing a novel form of 4-1BBL eradicates established tumors. Cancer Res. 2009, 69, 4319–4326. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral t cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Kamensek, U.; Cemazar, M.; Lampreht Tratar, U.; Ursic, K.; Sersa, G. Antitumor in situ vaccination effect of TNFα and IL-12 plasmid DNA electrotransfer in a murine melanoma model. Cancer Immunol. Immunother. 2018, 67, 785–795. [Google Scholar] [CrossRef]
- Sznol, M.; Hodi, F.S.; Margolin, K.; McDermott, D.F.; Ernstoff, M.S.; Wojtaszek, J.M.K.; Feltquate, D.; Logan, T. Phase I study of BMS-663513, a fully human Anti-CD137 agonist monoclonal antibody, in patients (Pts) with advanced cancer (CA). J. Clin. Oncol. 2008, 26, 3007. Available online: https://ascopubs.org/doi/10.1200/jco.2008.26.15_suppl.3007 (accessed on 16 April 2021). [CrossRef]
- Bartkowiak, T.; Curran, M.A. 4-1BB Agonists: Multi-potent potentiators of tumor immunity. Front. Oncol. 2015, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; He, A.R.; Doi, T.; Levy, R.; Bhatia, S.; Pishvaian, M.J.; Cesari, R.; Chen, Y.; Davis, C.B.; Huang, B.; et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1816–1823. [Google Scholar] [CrossRef]
- Chu, D.-T.; Bac, N.D.; Nguyen, K.-H.; Tien, N.L.B.; Thanh, V.V.; Nga, V.T.; Ngoc, V.T.N.; Anh Dao, D.T.; Hoan, L.N.; Hung, N.P.; et al. An update on Anti-CD137 antibodies in immunotherapies for cancer. Int. J. Mol. Sci. 2019, 20, 1822. [Google Scholar] [CrossRef]
- Yeku, O.O.; Brentjens, R.J. Armored CAR T-Cells: Utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem. Soc. Trans. 2016, 44, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.J. Engineered t cell therapy for cancer in the clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef]
- Stephan, M.T.; Ponomarev, V.; Brentjens, R.J.; Chang, A.H.; Dobrenkov, K.V.; Heller, G.; Sadelain, M. T Cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat. Med. 2007, 13, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Condomines, M.; van der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Fukushima, S.; Kimura, T.; Kanemaru, H.; Miyashita, A.; Okada, E.; Kubo, Y.; Nakahara, S.; Tokuzumi, A.; Nishimura, Y.; et al. Immunotherapy with 4-1BBL-expressing IPS cell-derived myeloid lines amplifies antigen-specific t cell infiltration in advanced melanoma. Int. J. Mol. Sci. 2021, 22, 1958. [Google Scholar] [CrossRef] [PubMed]
- Lemos de Matos, A.; Franco, L.S.; McFadden, G. Oncolytic viruses and the immune system: The dynamic duo. Mol. Ther. Methods Clin. Dev. 2020, 17, 349–358. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Qi, F.; Wang, M.; Li, B.; Lu, Z.; Nie, G.; Li, S. Reversal of the immunosuppressive tumor microenvironment by nanoparticle-based activation of immune-associated cells. Acta Pharm. Sin. 2020, 41, 895–901. [Google Scholar] [CrossRef]
- Han, S.; Huang, K.; Gu, Z.; Wu, J. Tumor immune microenvironment modulation-based drug delivery strategies for cancer immunotherapy. Nanoscale 2020, 12, 413–436. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, L.; Chen, W.; Weng, W.; Song, J.; Ji, J. Delivery strategies of cancer immunotherapy: Recent advances and future perspectives. J. Hematol. Oncol. 2019, 12. [Google Scholar] [CrossRef]
- Eriksson, E.; Milenova, I.; Wenthe, J.; Ståhle, M.; Leja-Jarblad, J.; Ullenhag, G.; Dimberg, A.; Moreno, R.; Alemany, R.; Loskog, A. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5846–5857. [Google Scholar] [CrossRef]
- Musher, B.L.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jing, J.; Stanietzky, N.; Lu, J.; Brisco, A.; Wenthe, J.; et al. A phase I/II study combining a TMZ-CD40L/4-1BBL-armed oncolytic adenovirus and nab-paclitaxel/gemcitabine chemotherapy in advanced pancreatic cancer: An interim report. J. Clin. Oncol. 2020, 38, 716. [Google Scholar] [CrossRef]
| Approach | Findings | Immune Signaling Pathway | Year, Ref. |
|---|---|---|---|
| Prophylactic and therapeutic effect of DNA vaccine in a cervical cancer mouse model. | Prophylaxis against TC-1 tumor. Therapeutic effect against established TC-1 tumors. | Increased frequency of E7-specific T cells producing interferon IFN-γ. | 2019 [43] |
| Tumor protection of subunit vaccine in three different tumor types (TC-1, LLC, or 3LL-huMUC1) in a mouse model. | Monotherapy protects mice against tumor challenge. A rapid and lengthy window of protection against the tumor. Protection is tumor-type-independent and does not evolve into a long-lasting immune memory. Prevents post-surgical tumor recurrence. | IFN-γ+ producing CD4+ T and NK cells as predictors of SA-4-1BBL-mediated immune protection against tumors. Protection against the tumor requires IFN-γ as a mediator of crosstalk between NK and CD4+ T cells. | 2019 [31] |
| Vaccine adjuvant system effect in a mouse model of human papilloma virus (HPV)-induced cancer. | SA-4-1BBL/MPL as the adjuvant component of the E7 TAA-based vaccine has robust efficacy in eradicating established TC-1 tumors. SA-4-1BBL/MPL controls 3LL pulmonary metastasis progression. Therapeutic efficacy of the SA-4-1BBL/MPL is achieved in the absence of autoimmunity and detectable clinical toxicity. | The therapeutic efficacy of SA-4-1BBL/MPL is associated with a robust effect of SA-4-1BBL and MPL on the generation of peripheral CD8+ T cell responses. Vaccination with the SA-4-1BBL/MPL results in a favorable intra-tumoral CD8+ T eff/CD4+ Foxp3+ Treg cell ratio. CD8+ T cells and IFN-γ are critical to the therapeutic efficacy of SA-4-1BBL/MPL while Treg cells are detrimental to the efficacy of MPL monotherapy. | 2014 [42] |
| Therapeutic efficacy of subunit vaccine in 3LL lung carcinoma in mice. | Eradicating 3LL tumors in mice. | The therapeutic efficacy of the vaccine is associated with robust CD8+ T cells and NK cells’ effector responses. CD8+ T cells play an obligatory role, while NK cells play a moderate, but significant, role in the therapeutic efficacy of the vaccine. | 2012 [44] |
| Therapeutic efficacy of a protein-based vaccine in a mouse cervical cancer model. | Eradication of established TC-1 tumors and generates long-term tumor-specific memory response. | Vaccination with E7 protein and SA-4-1BBL generates primary T cell responses. Generation of robust T cell proliferative and effector responses. Long-term T cell memory pool and enhanced intra-tumoral CD4+ and CD8+ T cells. NK cells play a critical role in vaccine efficacy. | 2010 [13] |
| Intervention Model | Year; Phase |
|---|---|
| An open-label, multicenter, multidose, first-in-human study of RTX-240 for the treatment of patients with relapsed/refractory R/R or locally advanced solid tumors. RTX-240 is a cellular therapy that co-expressed 4-1BBL and IL-15TP, a fusion of IL-15 and IL-15 receptor alpha as monotherapy. | 2020; I, II |
| Study of DSP107 in subjects with advanced solid tumors including a dose-escalation safety study (part 1) and preliminary efficacy assessment of DSP107 as monotherapy and in combination with atezolizumab (part 2). DSP107 (SIRPα–4-1BBL) is a bi-functional, trimeric, fusion protein. | 2020; I, II |
| LOAd703 in combination with atezolizumab in malignant melanoma. LOAd703 is an oncolytic adenovirus encoding trimerized membrane-bound (TMZ)-CD40L and 4-1BBL. | 2019; I, II |
| An open-label study to evaluate the safety, pharmacokinetics, and preliminary antitumor activity of RO7227166 (a CD19-targeted 4-1BB ligand) in combination with obinutuzumab and in combination with glofitamab following a pre-treatment dose of obinutuzumab administered in participants with relapsed/refractory B-cell non-Hodgkin’s lymphoma. | 2019; I |
| Evaluating the effect of LOAd703 in patients with pancreatic cancer, biliary cancer, ovarian cancer, and colorectal cancer. LOAd703 is an oncolytic adenovirus serotype 5/35 encoding immunostimulatory transgenes: TMZ-CD40L and 41BBL. | 2017; I, II |
| A CD19-targeted EGFRt/19-28z/4-1BBL “armored” Chimeric Antigen Receptor (CAR) modified T cells in patients with relapsed or refractory CD19+ hematologic malignancies. | 2017; I |
| Evaluating safety of LOAd703, an armed oncolytic adenovirus for pancreatic cancer. Delolimogene mupadenorepvec oncolytic virus encoding TMZ-CD40L and 4-1BBL. | 2016; I, II |
| Allogeneic vaccine modified to express HLA A2/4-1BB ligand for high-risk or low residual disease melanoma patients. | 2013; I, II |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Perez, A.G.; Perez-Trujillo, J.J.; Garza-Morales, R.; Loera-Arias, M.J.; Saucedo-Cardenas, O.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Montes-de-Oca-Luna, R. 4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer. Int. J. Mol. Sci. 2021, 22, 6210. https://doi.org/10.3390/ijms22126210
Martinez-Perez AG, Perez-Trujillo JJ, Garza-Morales R, Loera-Arias MJ, Saucedo-Cardenas O, Garcia-Garcia A, Rodriguez-Rocha H, Montes-de-Oca-Luna R. 4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer. International Journal of Molecular Sciences. 2021; 22(12):6210. https://doi.org/10.3390/ijms22126210
Chicago/Turabian StyleMartinez-Perez, Alejandra G., Jose J. Perez-Trujillo, Rodolfo Garza-Morales, Maria J. Loera-Arias, Odila Saucedo-Cardenas, Aracely Garcia-Garcia, Humberto Rodriguez-Rocha, and Roberto Montes-de-Oca-Luna. 2021. "4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer" International Journal of Molecular Sciences 22, no. 12: 6210. https://doi.org/10.3390/ijms22126210
APA StyleMartinez-Perez, A. G., Perez-Trujillo, J. J., Garza-Morales, R., Loera-Arias, M. J., Saucedo-Cardenas, O., Garcia-Garcia, A., Rodriguez-Rocha, H., & Montes-de-Oca-Luna, R. (2021). 4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer. International Journal of Molecular Sciences, 22(12), 6210. https://doi.org/10.3390/ijms22126210








