Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina
Abstract
1. Introduction
2. Neural Circuitries and Glial Cell Types in the Retina
3. Cellular Biology of Microglia in Neural and Retinal Tissues
4. Purinergic Modulation of Neural Tissues
4.1. Heterogeneity of Purinoceptors
4.2. Purinoceptors in the Retina
4.3. Purinergic Regulation of Microglia
5. Glycinergic Transmission in the Retina
5.1. Heterogeneity of NMDA Receptors in the Retina
5.2. Glycinergic Regulation of Microglia
6. Neurodegenerative and Neuroinflammatory Disorders in the Retina
6.1. Enhanced Purinergic Signaling in Neurodegenerative/Neuroinflammatory Disorders of the Retina
6.2. Enhanced Glutamatergic–Glycinergic Tone in Neurodegenerative/Neuroinflammatory Disorders of the Retina
6.3. Activated Microglial Cells in Neurodegenerative Disorders of the Retina
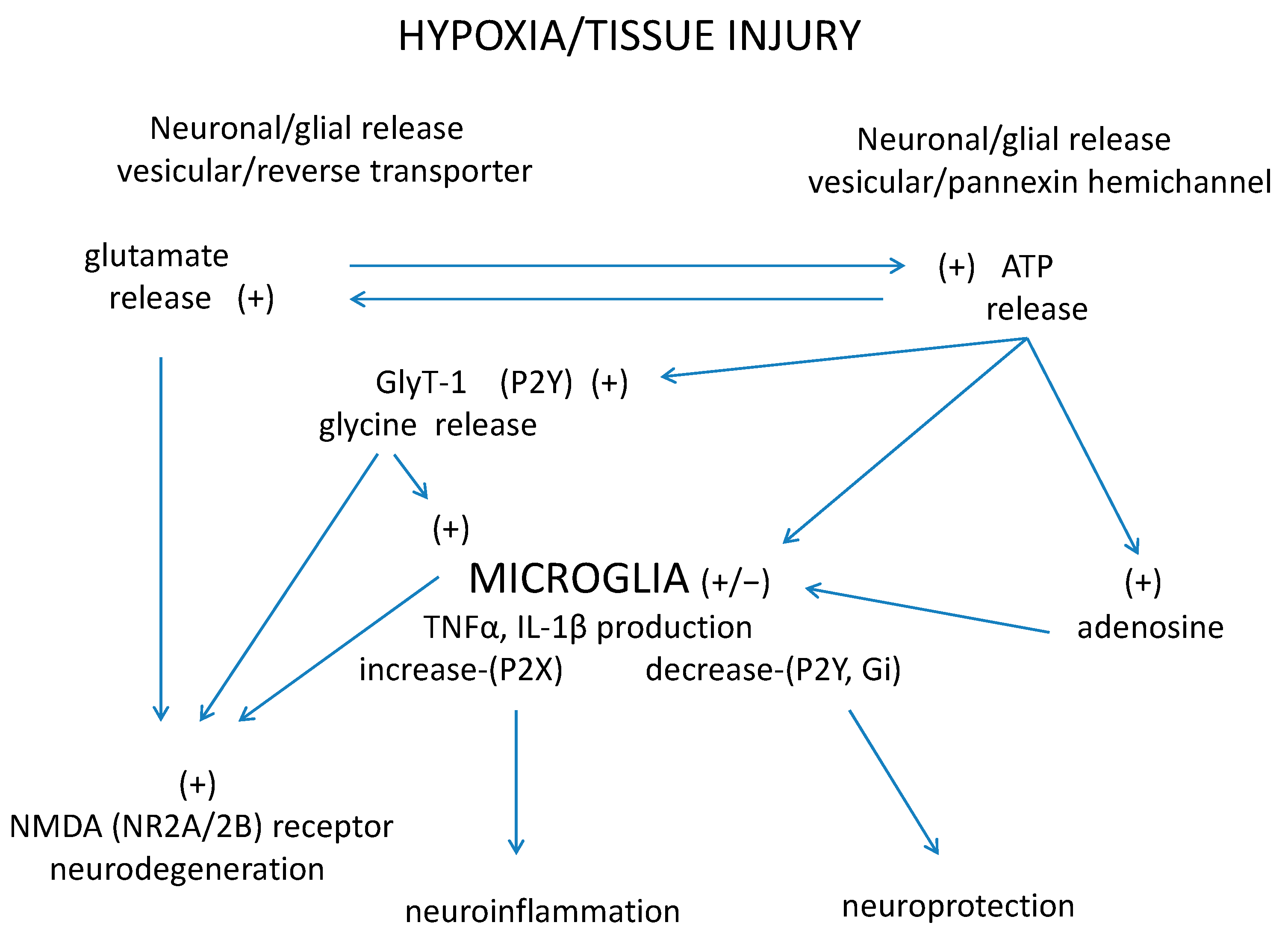
7. A Tripartite Interaction: Purinergic–Glycinergic Cross-Talk and Microglia Activation in Neurodegenerative–Neuroinflammatory Disorders of the Retina
8. Conclusions: Possible Therapeutic Consequences
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| GFAP | glial fibrillary acidic protein |
| GlyR | glycine receptor |
| GlyT-1 | glycine transporter-1 |
| IL | interleukin |
| IP3 | inositol trisphosphate |
| LPS | lipopolysaccharide |
| NMDA | N-methyl-D-aspartate |
| NAAT | neutral amino acid transporter |
| TNFα | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
References
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Gasull, X.; Diebold, Y.; Pintor, J. Purinergic receptors in ocular inflammation. Mediat. Inflamm. 2014, 2014, 320906. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Sperlágh, B. ATP-Mediated Signaling in the Nervous System. In Handbook of Neurochemistry and Molecular Neurobiology: Neurotransmitter Systems; Lajtha, A., Vizi, E.S., Eds.; Springer: Boston, MA, USA, 2008; pp. 227–254. [Google Scholar]
- Illes, P.; Verkhratsky, A.; Burnstock, G.; Sperlagh, B. Purines in neurodegeneration and neuroregeneration. Neuropharmacology 2016, 104, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P.D.; Demb, J.B. Retina. In The Synaptic Organization of the Brain; Shepherd, G.M., Ed.; Oxford University Press: Oxford, UK, 2004; pp. 217–270. [Google Scholar]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrosio, A.F.; Santiago, A.R. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.F. Glycinergic neurons process images. J. Physiol. 2012, 590, 239–240. [Google Scholar] [CrossRef]
- Hama, Y.; Katsuki, H.; Tochikawa, Y.; Suminaka, C.; Kume, T.; Akaike, A. Contribution of endogenous glycine site NMDA agonists to excitotoxic retinal damage in vivo. Neurosci. Res. 2006, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Santiago, A.R.; Baptista, F.I.; Santos, P.F.; Cristovao, G.; Ambrosio, A.F.; Cunha, R.A.; Gomes, C.A. Role of microglia adenosine A(2A) receptors in retinal and brain neurodegenerative diseases. Mediat. Inflamm. 2014, 2014, 465694. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology 2016, 104, 194–211. [Google Scholar] [CrossRef]
- Gadea, A.; Lopez, E.; Lopez-Colome, A.M. Characterization of glycine transport in cultured Muller glial cells from the retina. Glia 1999, 26, 273–279. [Google Scholar] [CrossRef]
- Hanuska, A.; Szenasi, G.; Albert, M.; Koles, L.; Varga, A.; Szabo, A.; Matyus, P.; Harsing, L.G., Jr. Some Operational Characteristics of Glycine Release in Rat Retina: The Role of Reverse Mode Operation of Glycine Transporter Type-1 (GlyT-1) in Ischemic Conditions. Neurochem. Res. 2016, 41, 73–85. [Google Scholar] [CrossRef]
- Harsing, L.G., Jr.; Juranyi, Z.; Gacsalyi, I.; Tapolcsanyi, P.; Czompa, A.; Matyus, P. Glycine transporter type-1 and its inhibitors. Curr. Med. Chem. 2006, 13, 1017–1044. [Google Scholar] [CrossRef]
- Van den Eynden, J.; Ali, S.S.; Horwood, N.; Carmans, S.; Brone, B.; Hellings, N.; Steels, P.; Harvey, R.J.; Rigo, J.M. Glycine and glycine receptor signalling in non-neuronal cells. Front. Mol. Neurosci. 2009, 2, 9. [Google Scholar] [CrossRef]
- Cantaut-Belarif, Y.; Antri, M.; Pizzarelli, R.; Colasse, S.; Vaccari, I.; Soares, S.; Renner, M.; Dallel, R.; Triller, A.; Bessis, A. Microglia control the glycinergic but not the GABAergic synapses via prostaglandin E2 in the spinal cord. J. Cell Biol. 2017, 216, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lam, T.T.; Tso, M.O. Heterogeneous populations of microglia/macrophages in the retina and their activation after retinal ischemia and reperfusion injury. Exp. Eye Res. 2005, 81, 700–709. [Google Scholar] [CrossRef]
- Okunuki, Y.; Mukai, R.; Nakao, T.; Tabor, S.J.; Butovsky, O.; Dana, R.; Ksander, B.R.; Connor, K.M. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9989–9998. [Google Scholar] [CrossRef] [PubMed]
- Uckermann, O.; Uhlmann, S.; Wurm, A.; Reichenbach, A.; Wiedemann, P.; Bringmann, A. ADPbetaS evokes microglia activation in the rabbit retina in vivo. Purinergic Signal. 2005, 1, 383–387. [Google Scholar] [CrossRef]
- Tanaka, J.; Toku, K.; Matsuda, S.; Sudo, S.; Fujita, H.; Sakanaka, M.; Maeda, N. Induction of resting microglia in culture medium devoid of glycine and serine. Glia 1998, 24, 198–215. [Google Scholar] [CrossRef]
- Beamer, E.; Goloncser, F.; Horvath, G.; Beko, K.; Otrokocsi, L.; Kovanyi, B.; Sperlagh, B. Purinergic mechanisms in neuroinflammation: An update from molecules to behavior. Neuropharmacology 2016, 104, 94–104. [Google Scholar] [CrossRef]
- Moller, T.; Kann, O.; Verkhratsky, A.; Kettenmann, H. Activation of mouse microglial cells affects P2 receptor signaling. Brain Res. 2000, 853, 49–59. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 77. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Imperatore, R.; Napolitano, F.; Gatta, L.; Cristino, L.; Giordano, C.; Siniscalco, D.; Di Marzo, V.; Bellini, G.; et al. The A1 adenosine receptor as a new player in microglia physiology. Glia 2014, 62, 122–132. [Google Scholar] [CrossRef]
- Viviani, B.; Corsini, E.; Galli, C.L.; Marinovich, M. Glia increase degeneration of hippocampal neurons through release of tumor necrosis factor-alpha. Toxicol. Appl. Pharmacol. 1998, 150, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.J. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J. Neurosci. 2014, 34, 10528–10540. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Kaur, C.; Foulds, W.S.; Ling, E.A. Hypoxia-ischemia and retinal ganglion cell damage. Clin. Ophthalmol. 2008, 2, 879–889. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Nussenblatt, R.B.; Lightman, S.L.; Hollander, D.A. Inflammation in retinal disease. Int. J. Inflamm. 2013, 2013, 724648. [Google Scholar] [CrossRef]
- Frade, J.M.; Barde, Y.A. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron 1998, 20, 35–41. [Google Scholar] [CrossRef]
- Farber, K.; Kettenmann, H. Purinergic signaling and microglia. Pflüg. Arch. 2006, 452, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Reigada, D.; Lu, W.; Zhang, M.; Mitchell, C.H. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 2008, 157, 396–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Sperlagh, B.; Szabo, G.; Erdelyi, F.; Baranyi, M.; Vizi, E.S. Homo- and heteroexchange of adenine nucleotides and nucleosides in rat hippocampal slices by the nucleoside transport system. Br. J. Pharmacol. 2003, 139, 623–633. [Google Scholar] [CrossRef]
- Battista, A.G.; Ricatti, M.J.; Pafundo, D.E.; Gautier, M.A.; Faillace, M.P. Extracellular ADP regulates lesion-induced in vivo cell proliferation and death in the zebrafish retina. J. Neurochem. 2009, 111, 600–613. [Google Scholar] [CrossRef]
- Latini, S.; Pedata, F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Molina-Arcas, M.; Casado, F.J.; Pastor-Anglada, M. Nucleoside transporter proteins. Curr. Vasc. Pharmacol. 2009, 7, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Rosenbaum, P.S.; Osinski, J.; Park, S.S.; Toledano, A.Y.; Li, B.; Moshfeghi, A.A. Ischemia induces significant changes in purine nucleoside concentration in the retina-choroid in rats. Exp. Eye Res. 1997, 65, 771–779. [Google Scholar] [CrossRef]
- Ghiardi, G.J.; Gidday, J.M.; Roth, S. The purine nucleoside adenosine in retinal ischemia-reperfusion injury. Vis. Res. 1999, 39, 2519–2535. [Google Scholar] [CrossRef][Green Version]
- Jacobson, K.A.; Civan, M.M. Ocular Purine Receptors as Drug Targets in the Eye. J. Ocul. Pharmacol. Ther. 2016, 32, 534–547. [Google Scholar] [CrossRef]
- Housley, G.D.; Bringmann, A.; Reichenbach, A. Purinergic signaling in special senses. Trends Neurosci. 2009, 32, 128–141. [Google Scholar] [CrossRef]
- Wurm, A.; Erdmann, I.; Bringmann, A.; Reichenbach, A.; Pannicke, T. Expression and function of P2Y receptors on Muller cells of the postnatal rat retina. Glia 2009, 57, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. Adenosine Neuromodulation and Neuroprotection. In Handbook of Neurochemistry and Molecular Neurobiology: Neurotransmitter Systems; Lajtha, A., Vizi, E.S., Eds.; Springer: Boston, MA, USA, 2008; pp. 255–273. [Google Scholar]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Norenberg, W.; Gebicke-Haerter, P.J. Molecular mechanisms of microglial activation. B. Voltage- and purinoceptor-operated channels in microglia. Neurochem. Int. 1996, 29, 13–24. [Google Scholar] [CrossRef]
- Sanderson, J.; Dartt, D.A.; Trinkaus-Randall, V.; Pintor, J.; Civan, M.M.; Delamere, N.A.; Fletcher, E.L.; Salt, T.E.; Grosche, A.; Mitchell, C.H. Purines in the eye: Recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Muller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp. Eye Res. 2014, 127, 270–279. [Google Scholar] [CrossRef]
- Jimenez, E.; Zafra, F.; Perez-Sen, R.; Delicado, E.G.; Miras-Portugal, M.T.; Aragon, C.; Lopez-Corcuera, B. P2Y purinergic regulation of the glycine neurotransmitter transporters. J. Biol. Chem. 2011, 286, 10712–10724. [Google Scholar] [CrossRef]
- Pannicke, T.; Frommherz, I.; Biedermann, B.; Wagner, L.; Sauer, K.; Ulbricht, E.; Hartig, W.; Krugel, U.; Ueberham, U.; Arendt, T.; et al. Differential effects of P2Y1 deletion on glial activation and survival of photoreceptors and amacrine cells in the ischemic mouse retina. Cell Death Dis. 2014, 5, e1353. [Google Scholar] [CrossRef] [PubMed]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Pannicke, T.; Iandiev, I.; Francke, M.; Hollborn, M.; Wiedemann, P.; Reichenbach, A.; Osborne, N.N.; Bringmann, A. Purinergic signaling involved in Muller cell function in the mammalian retina. Prog. Retin. Eye Res. 2011, 30, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Solino, M.; Lopez, E.M.; Rey-Funes, M.; Loidl, C.F.; Larrayoz, I.M.; Martinez, A.; Girardi, E.; Lopez-Costa, J.J. Adenosine A1 receptor: A neuroprotective target in light induced retinal degeneration. PLoS ONE 2018, 13, e0198838. [Google Scholar] [CrossRef]
- Wang, X.; Franciosi, S.; Bae, J.H.; Kim, S.U.; McLarnon, J.G. Expression of P2y and P2x receptors in cultured human microglia. Proc. West. Pharmacol. Soc. 1999, 42, 79–81. [Google Scholar]
- Ogata, T.; Chuai, M.; Morino, T.; Yamamoto, H.; Nakamura, Y.; Schubert, P. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Res. 2003, 981, 174–183. [Google Scholar] [CrossRef]
- Wang, M.; Wong, W.T. Microglia-Müller cell interactions in the retina. Adv. Exp. Med. Biol. 2014, 801, 333–338. [Google Scholar] [CrossRef]
- Inoue, K. UDP facilitates microglial phagocytosis through P2Y6 receptors. Cell Adhes. Migr. 2007, 1, 131–132. [Google Scholar] [CrossRef]
- Orr, A.G.; Orr, A.L.; Li, X.J.; Gross, R.E.; Traynelis, S.F. Adenosine A(2A) receptor mediates microglial process retraction. Nat. Neurosci. 2009, 12, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Relvas, L.J.; Bouffioux, C.; Marcet, B.; Communi, D.; Makhoul, M.; Horckmans, M.; Blero, D.; Bruyns, C.; Caspers, L.; Boeynaems, J.M.; et al. Extracellular nucleotides and interleukin-8 production by ARPE cells: Potential role of danger signals in blood-retinal barrier activation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Villalba, M.; Chiozzi, P.; Falzoni, S.; Ricciardi-Castagnoli, P.; Di Virgilio, F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol. 1996, 156, 1531–1539. [Google Scholar] [PubMed]
- Hide, I.; Tanaka, M.; Inoue, A.; Nakajima, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J. Neurochem. 2000, 75, 965–972. [Google Scholar] [CrossRef]
- Shigemoto-Mogami, Y.; Koizumi, S.; Tsuda, M.; Ohsawa, K.; Kohsaka, S.; Inoue, K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J. Neurochem. 2001, 78, 1339–1349. [Google Scholar] [CrossRef]
- Madeira, M.H.; Rashid, K.; Ambrosio, A.F.; Santiago, A.R.; Langmann, T. Blockade of microglial adenosine A2A receptor impacts inflammatory mechanisms, reduces ARPE-19 cell dysfunction and prevents photoreceptor loss in vitro. Sci. Rep. 2018, 8, 2272. [Google Scholar] [CrossRef]
- Ahmad, S.; Fatteh, N.; El-Sherbiny, N.M.; Naime, M.; Ibrahim, A.S.; El-Sherbini, A.M.; El-Shafey, S.A.; Khan, S.; Fulzele, S.; Gonzales, J.; et al. Potential role of A2A adenosine receptor in traumatic optic neuropathy. J. Neuroimmunol. 2013, 264, 54–64. [Google Scholar] [CrossRef]
- Santiago, A.R.; Madeira, M.H.; Boia, R.; Aires, I.D.; Rodrigues-Neves, A.C.; Santos, P.F.; Ambrosio, A.F. Keep an eye on adenosine: Its role in retinal inflammation. Pharmacol. Ther. 2020, 210, 107513. [Google Scholar] [CrossRef]
- Bucheimer, R.E.; Linden, J. Purinergic regulation of epithelial transport. J. Physiol. 2004, 555, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Krugel, U.; Illes, P. P2 receptors and neuronal injury. Pflüg. Arch. 2006, 452, 622–644. [Google Scholar] [CrossRef]
- Wassle, H.; Heinze, L.; Ivanova, E.; Majumdar, S.; Weiss, J.; Harvey, R.J.; Haverkamp, S. Glycinergic transmission in the Mammalian retina. Front. Mol. Neurosci. 2009, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.W.; Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Harsing, L.G., Jr.; Albert, M.; Matyus, P.; Szenasi, G. Inhibition of hypoxia-induced [(3)H]glycine release from chicken retina by the glycine transporter type-1 (GlyT-1) inhibitors NFPS and Org-24461. Exp. Eye Res. 2012, 94, 6–12. [Google Scholar] [CrossRef]
- Eulenburg, V.; Knop, G.; Sedmak, T.; Schuster, S.; Hauf, K.; Schneider, J.; Feigenspan, A.; Joachimsthaler, A.; Brandstatter, J.H. GlyT1 determines the glycinergic phenotype of amacrine cells in the mouse retina. Brain Struct. Funct. 2018, 223, 3251–3266. [Google Scholar] [CrossRef]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Dumitrescu, O.N.; Protti, D.A.; Majumdar, S.; Zeilhofer, H.U.; Wassle, H. Ionotropic glutamate receptors of amacrine cells of the mouse retina. Vis. Neurosci. 2006, 23, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Muller, U.; Wassle, H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur. J. Neurosci. 2006, 23, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Mann, L.B.; Rickman, D.W.; Lim, E.-J.; Chun, M.-H.; Grzywacz, N.M. AII amacrine cells in the distal inner nuclear layer of the mouse retina. J. Comp. Neurol. 2006, 494, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Eder, C. A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J. Physiol. 2004, 559, 35–40. [Google Scholar] [CrossRef]
- Harsing, L.G., Jr.; Matyus, P. Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: The role of synaptic and non-synaptic glycine transporters. Brain Res. Bull. 2013, 93, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zafra, F.; Aragon, C.; Olivares, L.; Danbolt, N.C.; Gimenez, C.; Storm-Mathisen, J. Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 1995, 15, 3952–3969. [Google Scholar] [CrossRef]
- Oyama, M.; Kuraoka, S.; Watanabe, S.; Iwai, T.; Tanabe, M. Electrophysiological evidence of increased glycine receptor-mediated phasic and tonic inhibition by blockade of glycine transporters in spinal superficial dorsal horn neurons of adult mice. J. Pharmacol. Sci. 2017, 133, 162–167. [Google Scholar] [CrossRef]
- Pena-Rangel, M.T.; Riesgo-Escovar, J.R.; Sanchez-Chavez, G.; Salceda, R. Glycine transporters (glycine transporter 1 and glycine transporter 2) are expressed in retina. NeuroReport 2008, 19, 1295–1299. [Google Scholar] [CrossRef]
- Thomsen, C. Glycine transporter inhibitors as novel antipsychotics. Drug Discov. Today Ther. Strateg. 2006, 3, 539–545. [Google Scholar] [CrossRef]
- Fletcher, E.L.; Kalloniatis, M. Neurochemical architecture of the normal and degenerating rat retina. J. Comp. Neurol. 1996, 376, 343–360. [Google Scholar] [CrossRef]
- Kalbaugh, T.L.; Zhang, J.; Diamond, J.S. Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J. Neurosci. 2009, 29, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Ladepeche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; Groc, L.; Pollegioni, L.; Mothet, J.P.; Oliet, S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012, 150, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.R.; Esguerra, M.; Kim, P.M.; Newman, E.A.; Snyder, S.H.; Zahs, K.R.; Miller, R.F. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 6789–6794. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.T.; Sullivan, S.J.; Tsai, G.; Coyle, J.T.; Esguerra, M.; Miller, R.F. The glycine transporter GlyT1 controls N-methyl-D-aspartic acid receptor coagonist occupancy in the mouse retina. Eur. J. Neurosci. 2009, 30, 2308–2317. [Google Scholar] [CrossRef]
- Eggers, E.D.; Lukasiewicz, P.D. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis. Neurosci. 2011, 28, 95–108. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef]
- Carmans, S.; Hendriks, J.J.; Thewissen, K.; Van den Eynden, J.; Stinissen, P.; Rigo, J.M.; Hellings, N. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J. Neurosci. Res. 2010, 88, 2420–2430. [Google Scholar] [CrossRef]
- Van den Eynden, J.; Notelaers, K.; Brone, B.; Janssen, D.; Nelissen, K.; Sahebali, S.; Smolders, I.; Hellings, N.; Steels, P.; Rigo, J.M. Glycine enhances microglial intracellular calcium signaling. A role for sodium-coupled neutral amino acid transporters. Pflüg. Arch. 2011, 461, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Raouf, R.; Chabot-Dore, A.J.; Ase, A.R.; Blais, D.; Seguela, P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology 2007, 53, 496–504. [Google Scholar] [CrossRef]
- Spittler, A.; Reissner, C.M.; Oehler, R.; Gornikiewicz, A.; Gruenberger, T.; Manhart, N.; Brodowicz, T.; Mittlboeck, M.; Boltz-Nitulescu, G.; Roth, E. Immunomodulatory effects of glycine on LPS-treated monocytes: Reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999, 13, 563–571. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Thurman, R.G. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J. Physiol. 1999, 277, L952–L959. [Google Scholar] [CrossRef]
- Hayashi, Y.; Ishibashi, H.; Hashimoto, K.; Nakanishi, H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia 2006, 53, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Juranyi, Z.; Sperlagh, B.; Vizi, E.S. Involvement of P2 purinoceptors and the nitric oxide pathway in [3H]purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res. 1999, 823, 183–190. [Google Scholar] [CrossRef]
- Sperlagh, B.; Zsilla, G.; Baranyi, M.; Illes, P.; Vizi, E.S. Purinergic modulation of glutamate release under ischemic-like conditions in the hippocampus. Neuroscience 2007, 149, 99–111. [Google Scholar] [CrossRef]
- Uckermann, O.; Wolf, A.; Kutzera, F.; Kalisch, F.; Beck-Sickinger, A.G.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: Activation by neuropeptide Y. J. Neurosci. Res. 2006, 83, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Rosenbaum, P.S.; Jennings, N.M.; Maxwell, K.M.; Roth, S. Differing roles of adenosine receptor subtypes in retinal ischemia-reperfusion injury in the rat. Exp Eye Res 1999, 68, 9–17. [Google Scholar] [CrossRef]
- Larsen, A.K.; Osborne, N.N. Involvement of adenosine in retinal ischemia. Studies on the rat. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2603–2611. [Google Scholar]
- Schmidt, K.G.; Bergert, H.; Funk, R.H. Neurodegenerative diseases of the retina and potential for protection and recovery. Curr. Neuropharmacol. 2008, 6, 164–178. [Google Scholar] [CrossRef]
- Chidlow, G.; Wood, J.P.; Casson, R.J. Pharmacological neuroprotection for glaucoma. Drugs 2007, 67, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Yagihashi, T.; Kezuka, J.; Muramatsu, D.; Usui, M.; Iwasaki, T. Glutamate levels in aqueous humor of patients with retinal artery occlusion. Retina 2006, 26, 432–436. [Google Scholar] [CrossRef]
- Osborne, N.N.; Melena, J.; Chidlow, G.; Wood, J.P. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: Possible implication for the treatment of glaucoma. Br. J. Ophthalmol 2001, 85, 1252–1259. [Google Scholar] [CrossRef][Green Version]
- Ozawa, Y.; Kamoshita, M.; Narimatsu, T.; Ban, N.; Toda, N.; Okamoto, D.; Yuki, K.; Miyake, S.; Tsubota, K. Neuroinflammation and Neurodegenerative Disorders of the Retina. Endocrinol. Metab. Synd. 2013, 2. [Google Scholar] [CrossRef]
- Hayreh, S.S. Retinal and optic nerve head ischemic disorders and atherosclerosis: Role of serotonin. Prog. Retin. Eye Res. 1999, 18, 191–221. [Google Scholar] [CrossRef]
- Harsing, L.G.; Zsilla, G.; Matyus, P.; Nagy, K.M.; Marko, B.; Gyarmati, Z.; Timar, J. Interactions between glycine transporter type 1 (GlyT-1) and some inhibitor molecules—Glycine transporter type 1 and its inhibitors (review). Acta Physiol. Hung. 2012, 99, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W.; Wolkenberg, S.E.; Kinney, G.G. Progress in the preparation and testing of glycine transporter type-1 (GlyT1) inhibitors. Curr. Top. Med. Chem. 2006, 6, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Harsing, L.G., Jr.; Albert, M.; Mátyus, P.; Szénási, G. Glycine transporter 1 inhibitors may exert neuroprotective effects in hypoxic retina. Intrinsic Act. 2016, 4 (Suppl. 3), A4.19. [Google Scholar] [CrossRef]
- NHangai, M.; Yoshimura, N.; Yoshida, M.; Yabuuchi, K.; Honda, Y. Interleukin-1 gene expression in transient retinal ischemia in the rat. Investig. Ophthalmol. Vis. Sci. 1995, 36, 571–578. [Google Scholar]
- Porter, R.A.; Dawson, L.A. GlyT-1 Inhibitors: From Hits to Clinical Candidates. In Small Molecule Therapeutics for Schizophrenia; Celanine, S., Poli, S., Eds.; Topics in Medicinal Chemistry; Springer: Cham, Swizterland, 2015; Volume 13, pp. 51–99. [Google Scholar]
- Cukras, C.A.; Petrou, P.; Chew, E.Y.; Meyerle, C.B.; Wong, W.T. Oral minocycline for the treatment of diabetic macular edema (DME): Results of a phase I/II clinical study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3865–3874. [Google Scholar] [CrossRef]

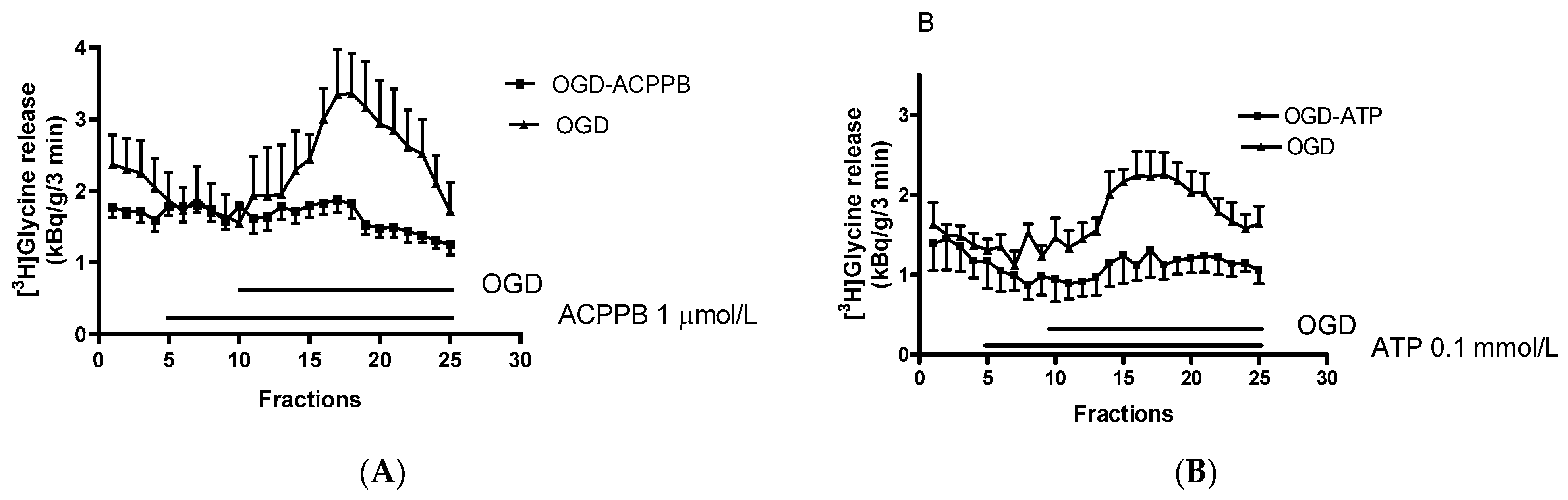
| Drugs | Mode of Action |
|---|---|
| P2 nucleotide purinoceptors | |
| α,β-Methylene-ATP | P2 agonist |
| PPADS | P2 antagonist |
| Suramin | non-specific P2 antagonist |
| P2Y nucleotide purinoceptors | |
| 2-MeS-ATP | P2 agonist |
| 2-MeS-ADP | P2Y1,12,13 agonist |
| MRS 2365 | P2Y1 agonist |
| MRS 2179 | P2Y1 antagonist |
| MRS 2211 | P2Y13 antagonist |
| P2X nucleotide purinoceptors | |
| β,γ-Methylene-ATP | P2X agonist |
| BzATP | P2X7 agonist |
| TNP-ATP | P2X antagonist |
| NF449 | P2X1 antagonist |
| Brillant Blue G (BBG) | P2X7 antagonist |
| P1 nucleoside (adenosine) purinoceptors | |
| R-PIA | A1 agonist |
| CCPA | A1 agonist |
| CPA | A1 agonist |
| DPCPX | A1 antagonist |
| CGS21680 | A2A agonist |
| SCH58261 | A2A antagonist |
| ZM-241,385 | A2A antagonist |
| BAY 60-6583 | A2B agonist |
| LUF-5835 | A2B agonist |
| MRS-1706 | A2B antagonist |
| CF101 | A3 agonist |
| CP-532,903 | A3 agonist |
| MRE 3008F20 | A3 antagonist |
| Other drugs influencing purinergic signaling | |
| ARL67156 | ecto-ATPase inhibitor |
| NBMPR | adenosine reuptake inhibitor |
| Abbreviations | |
| ARL 67156 | 6-N,N-diethyl-D-β,γ-dibromomethylene ATP |
| BAY 60-6583 | 2-[[6-Amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]-2-pyridinyl]thio] acetamide |
| BzATP | 2′,5′-O-4-benzo-yl)-ATP |
| CCPA | 2-chloro-N6-cyclopentyladenosine |
| CF101 | (N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine |
| CGS 21680 | 4-[2][6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl] benzeneproprionic acid |
| CP-532,903 | (2S,3S,4R,5R)-3-amino-5-[6-(2,5-dichlorobenzylamino)purin-9-yl]-4 hydroxytetrahydrofuran-2-carboxylic acid methylamide |
| CPA | N6-cyclopentyladenosine |
| DPCPX | Dipropylcyclopenthylxanthine |
| LUF-5835 | 1-[6-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purin2-yl]-N-methylpyrazole-4-carboxamide |
| 2-MeS-ATP | 2-methylthio-ATP |
| 2-MeSADP | 2-methylthio-ADP |
| MRE 3008F20 | N-[2-(2-Furanyl)-8-propyl-8H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin 5-yl]-N′-(4-methoxyphenyl)urea |
| MRS 1706 | N-(4-acetylphenyl)-2-([4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]acetamide |
| MRS 2179 | N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate |
| MRS 2211 | pyridoxal-5′-phosphate-6-azo(2-chloro-5-nitrophenyl)-2,4-disulfonate |
| MRS 2365 | [[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxy bicyclo-[3.1.0]hex-1-yl]methyl] diphosphoric acid monoester |
| NBMPR | S6-(4-nitrobenzyl)mercaptopurine riboside |
| NF449 | 4,4′,4′′,4′′′-[Carbonylbis(imino-5,1,3,-benzenetriyl-bis(carbonylimino))]tetrakis-1,3 benzenedisulfonic acid |
| PPADS | pyridoxalphosphate-6-azaphenyl-2,4-disulfonic acid |
| R-PIA | R-N6-(2-phenylisopropyl)adenosine |
| SCH5826 | 1,7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine |
| TNP-ATP | 2′,3′-(2,4,6,-trinitrophenyl)adenosine-5′-triphosphate |
| ZM241,385 | 4-(2-[7-amino-2-)2-furyl(triazolo-[1,3,5]triazin-5-ylamino]ethyl)phenol |
| Receptor | Signal Transduction | Ligands |
|---|---|---|
| P2Y nucleotide purinoceptors | ||
| P2Y purinoceptors subtypes | ||
| P2Y1,2,4,6,11* | Gq protein-coupled | ATP, ADP and/or UTP, UDP |
| P2Y purinoceptors subtypes | ||
| P2Y12,13,14 | Gi protein-coupled | ADP, UDP |
| P2X nucleotide purinoceptors | ||
| P2X purinoceptors subtypes | ||
| P2X1,2,3,4,5,6,7 | cationic ion channel-coupled | ATP |
| P1 nucleoside purinoceptors | ||
| A1 adenosine receptor | Gi protein-coupled | adenosine |
| A2A, A2B adenosine receptor | Gs protein-coupled | adenosine |
| A3 adenosine receptor | Gi protein-coupled | adenosine |
| Glia Cell Type | Locations in the Retina | Receptor Subtype Expression | GlyT Expression |
|---|---|---|---|
| Macroglia | |||
| Müller cells | Span all retina layers | P2Y1 | GlyT-1 |
| P2X7 | |||
| A2A/2B | |||
| Astroglia | Ganglion cell layer | P2Y1,12,13 | GlyT-1 |
| P2X7 | |||
| Microglia | |||
| In healthy retina | Inner and outer plexiform layers | P2X4,7 A2A/2B | ? |
| In retina pathologies | Ganglion cell layer | P2Y1,2,4,12 | ? |
| Perivascular accumulation | P2X7 | ||
| Outer nuclear layer | A1,2A/2B,3 | ||
| Subretinal space | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harsing, L.G., Jr.; Szénási, G.; Zelles, T.; Köles, L. Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina. Int. J. Mol. Sci. 2021, 22, 6209. https://doi.org/10.3390/ijms22126209
Harsing LG Jr., Szénási G, Zelles T, Köles L. Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina. International Journal of Molecular Sciences. 2021; 22(12):6209. https://doi.org/10.3390/ijms22126209
Chicago/Turabian StyleHarsing, Laszlo G., Jr., Gábor Szénási, Tibor Zelles, and László Köles. 2021. "Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina" International Journal of Molecular Sciences 22, no. 12: 6209. https://doi.org/10.3390/ijms22126209
APA StyleHarsing, L. G., Jr., Szénási, G., Zelles, T., & Köles, L. (2021). Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina. International Journal of Molecular Sciences, 22(12), 6209. https://doi.org/10.3390/ijms22126209







