Magnetically Controlled Carbonate Nanocomposite with Ciprofloxacin for Biofilm Eradication
Abstract
1. Introduction
2. Results
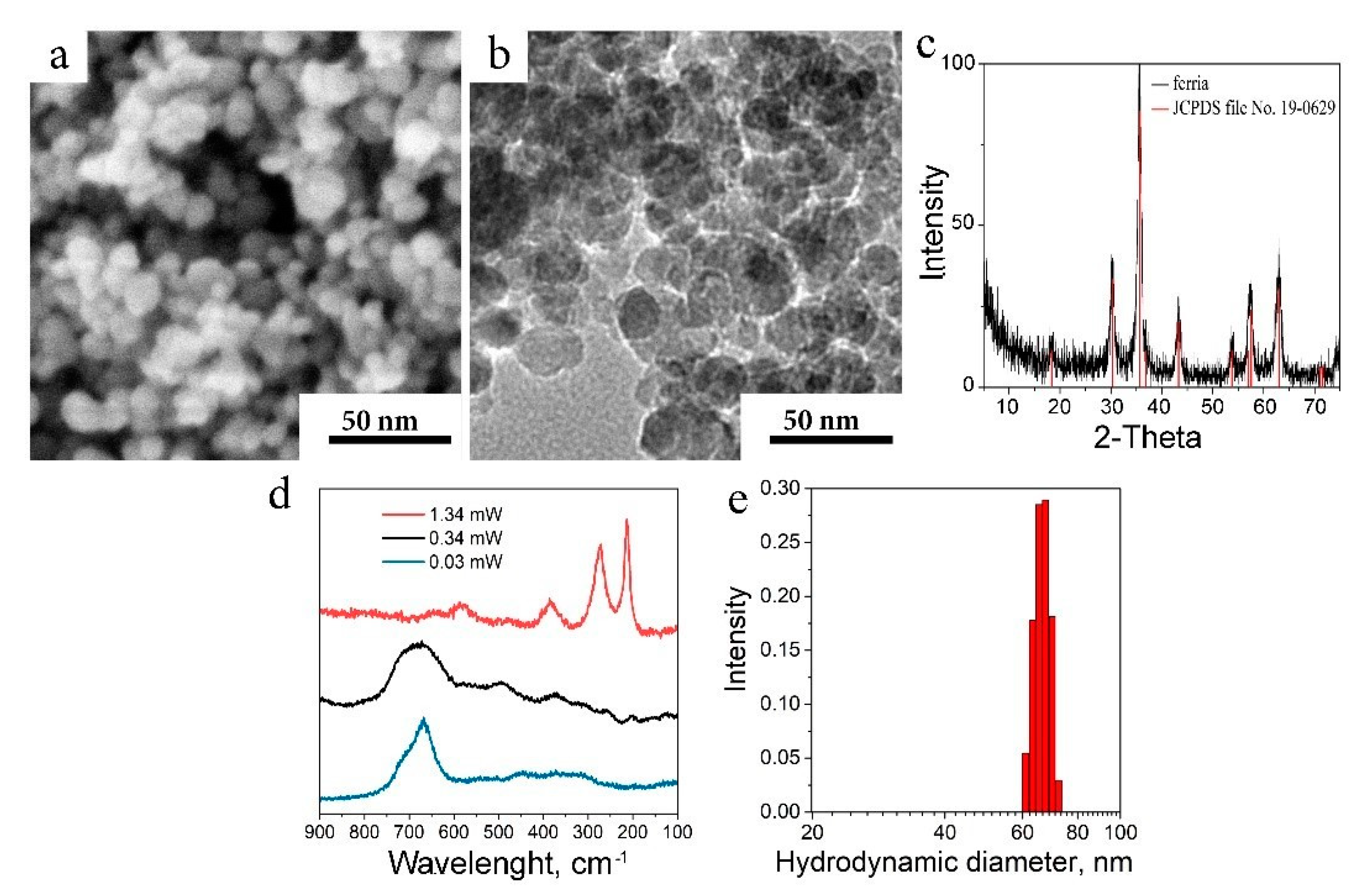
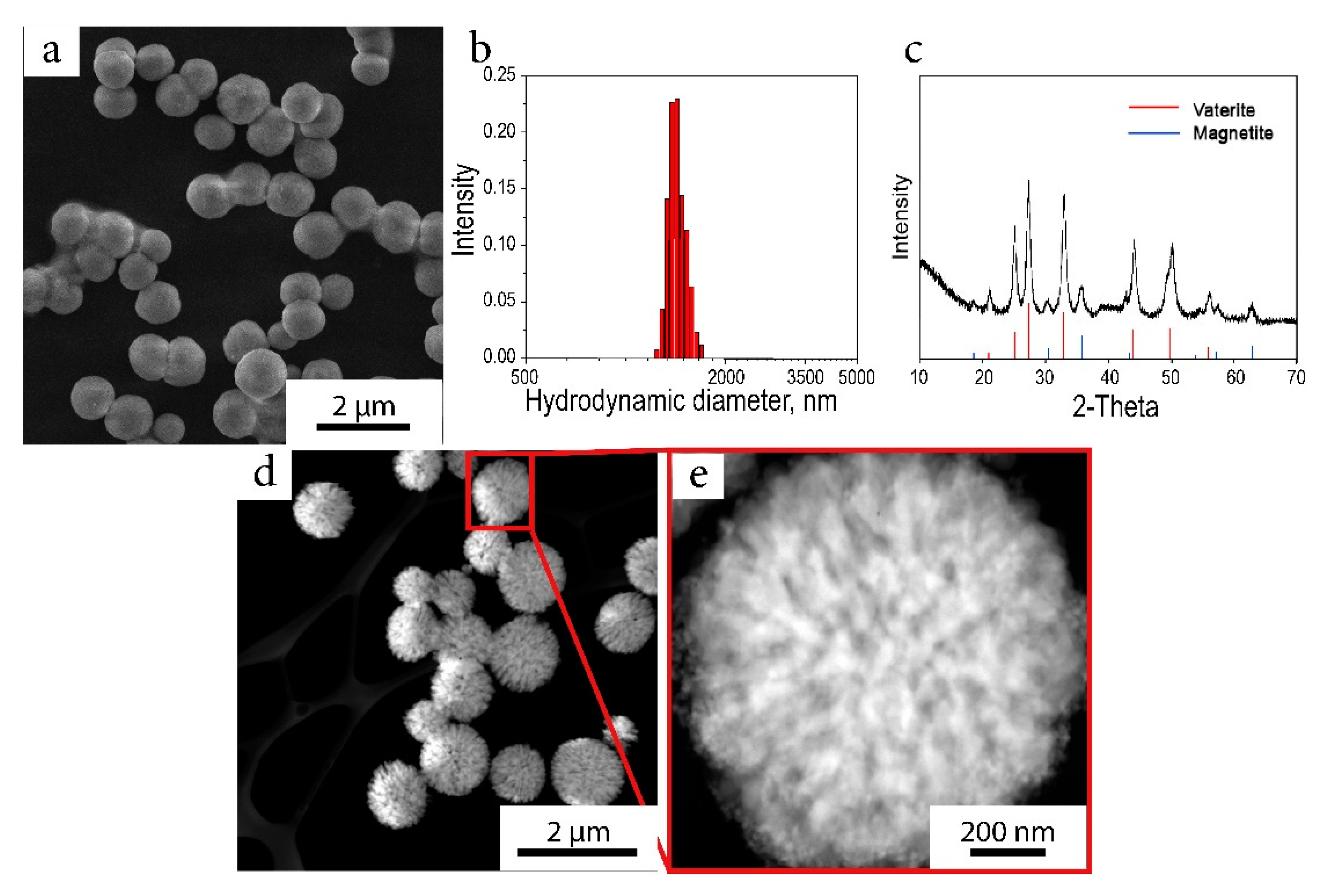
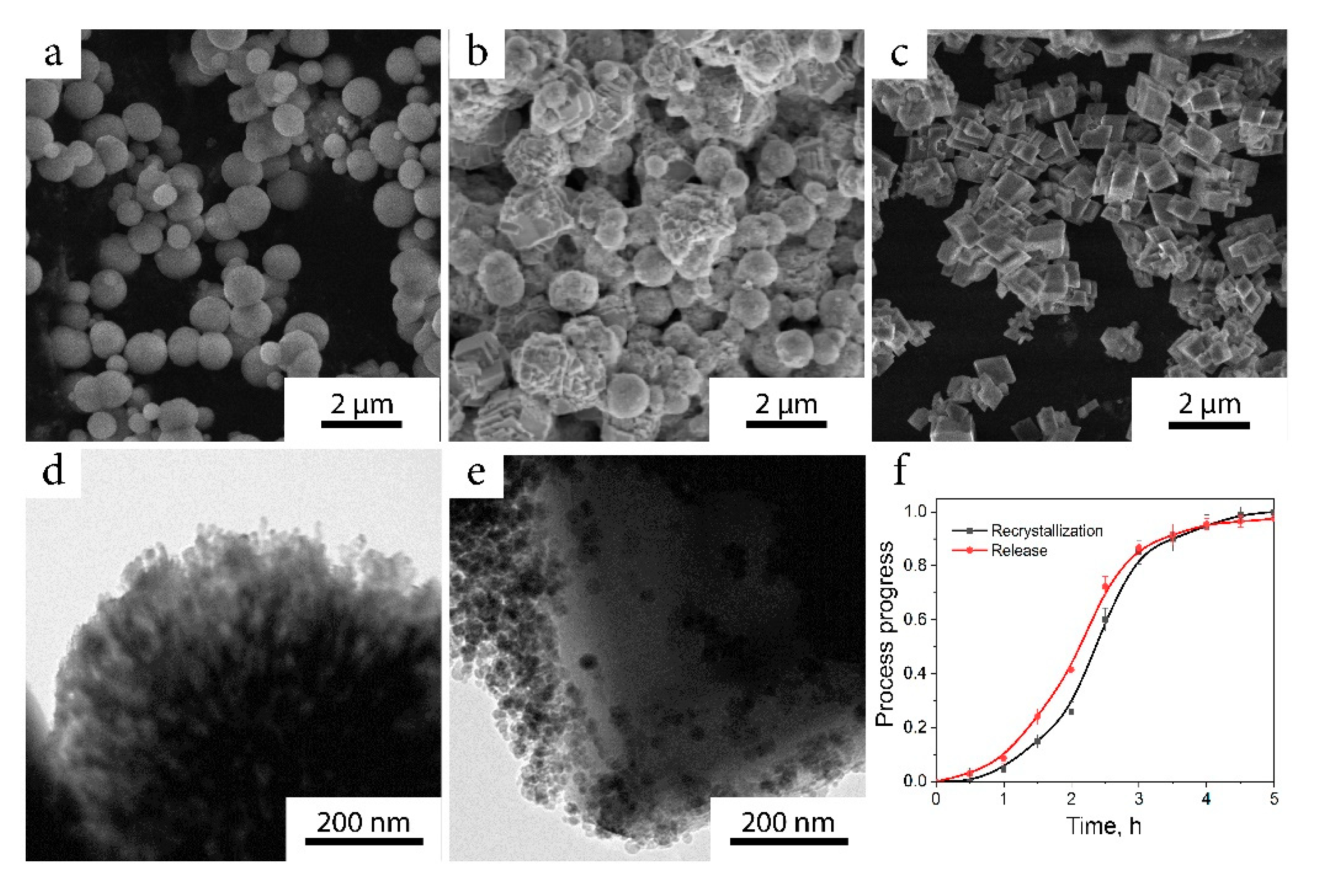
2.1. Characterization of the Nanocomposite
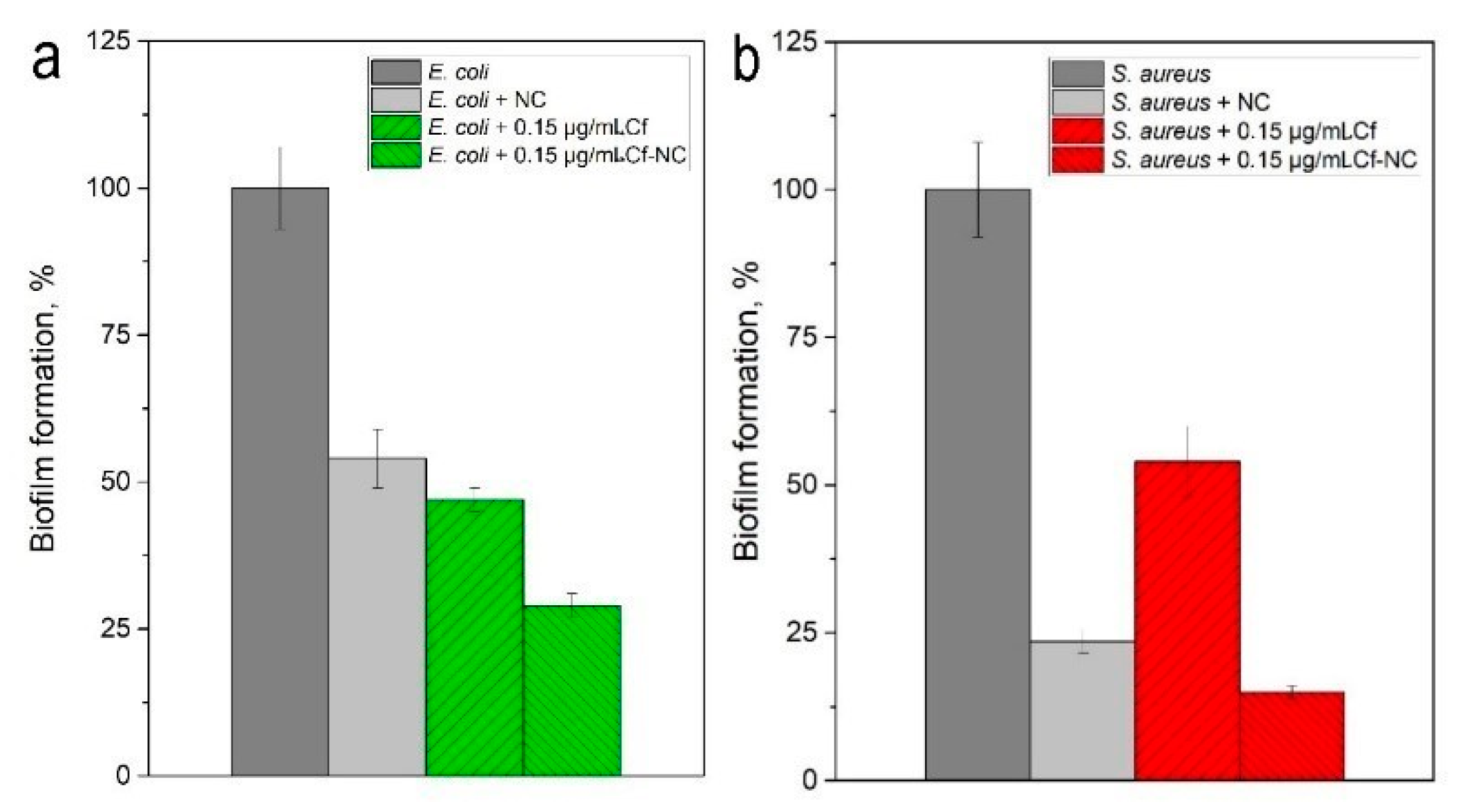
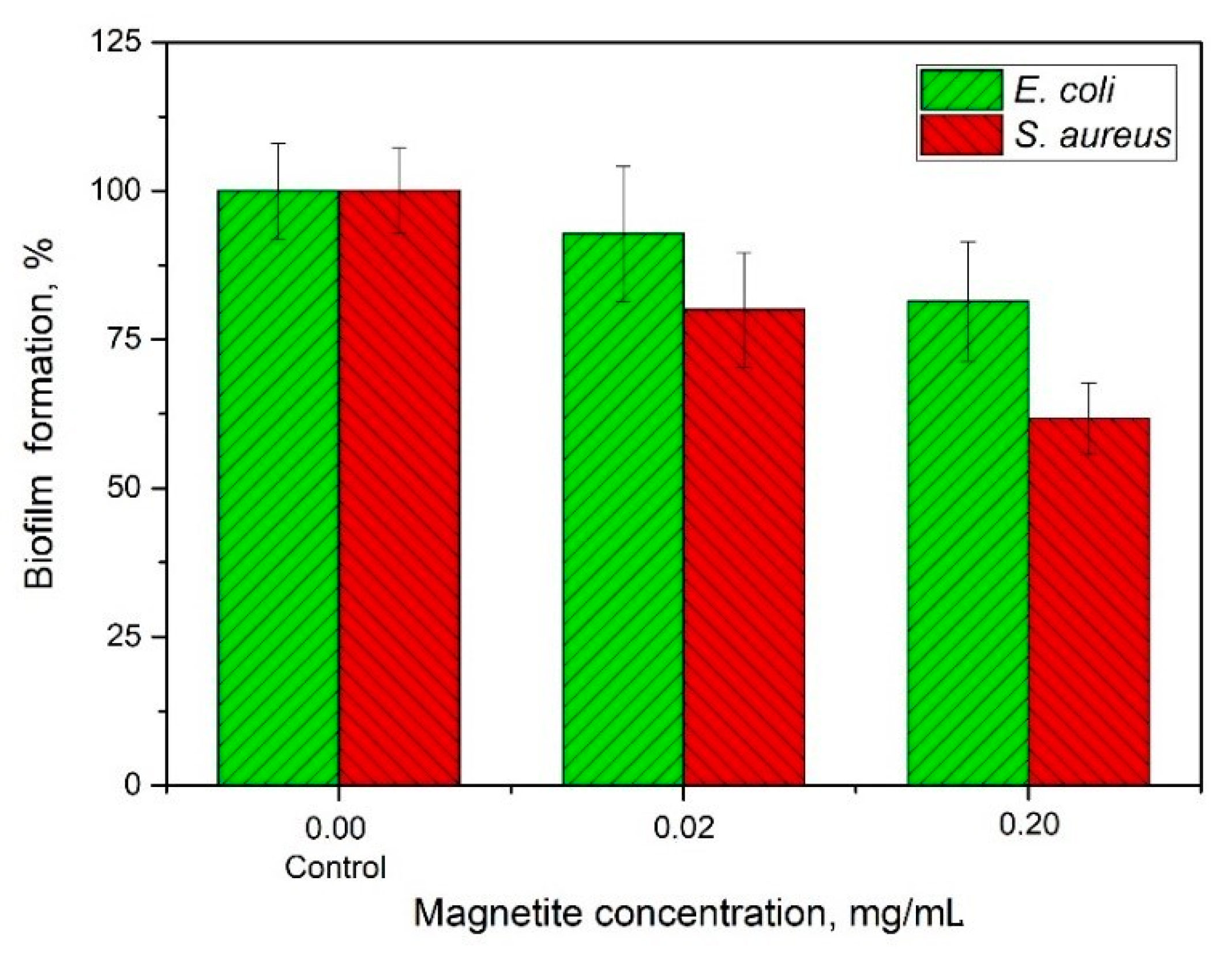
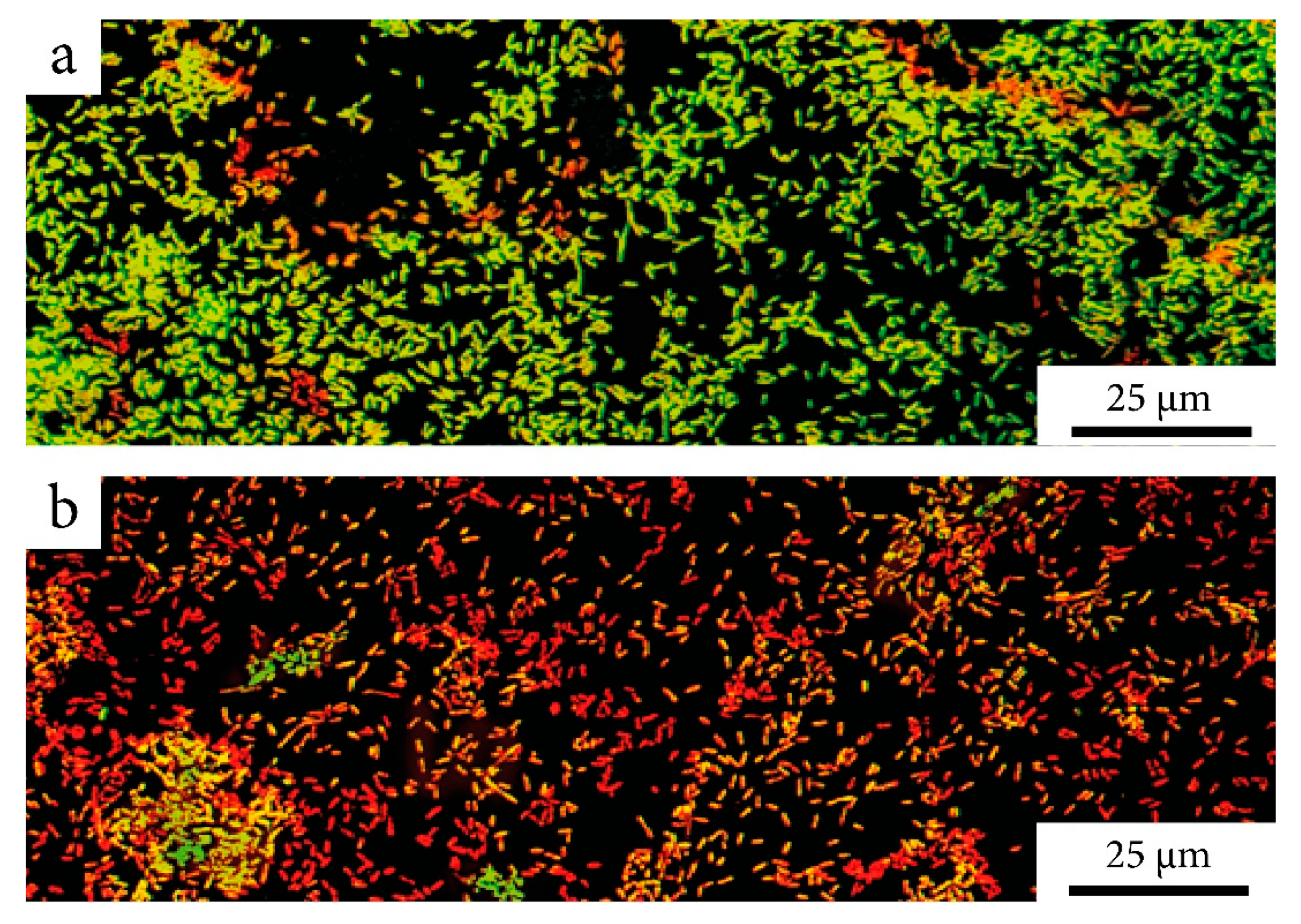
2.2. Nanocomposite Effectiveness Against the Formed Biofilms
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strain, Media, and Culture Conditions
4.3. MNPs Synthesis
4.4. Nanocomposite Synthesis
4.5. Antibiotic Release
4.6. Characterization
4.7. Studies of the Nanocomposite Effectiveness Against Formed Biofilms
4.8. Studies of MNPs Against Formed Biofilms
4.9. Quantitative Evaluation of Biofilm
4.10. Microscopic Analysis of Biofilm
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-based implant infections in orthopaedics. In Biofilm-Based Healthcare-Associated Infections, 1st ed.; Donelli, G., Ed.; Springer: Cham, Switzerland, 2015; Volume 830, pp. 29–46. [Google Scholar]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Montanaro, L.; Speziale, P.; Campoccia, D.; Ravaioli, S.; Cangini, I.; Pietrocola, G.; Giannini, S.; Arciola, C.R. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 2011, 6, 1329–1349. [Google Scholar] [CrossRef]
- Bouza, E.; Garcia-Garrote, F.; Cercenado, E.; Marin, M.; Diaz, M.S. Pseudomonas aeruginosa: A Survey of Resistance in 136 Hospitals in Spain. Antimicrob. Agents Chemother. 1999, 43, 981–982. [Google Scholar] [CrossRef] [PubMed]
- Prax, M.; Bertram, R. Metabolic aspects of bacterial persisters. Front. Cell. Infect. Microbiol. 2014, 4, 148. [Google Scholar] [CrossRef]
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial biofilm formation: A need to act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Głuszek, K.; Wilczewska, A.Z.; Misztalewska, I.; Mystkowska, J.; Michalak, G.; Sodo, A.; Wątek, M. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomedicine 2016, 12, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Cotar, A.I.; Grumezescu, A.M.; Huang, K.-S.; Chifiriuc, C.M.; Radulescu, R. Magnetite nanoparticles influence the efficacy of antibiotics against biofilm embedded Staphylococcus aureus cells. Biointerface Res. Appl. Chem. 2013, 3, 559–565. [Google Scholar]
- Cotar, A.I.; Grumezescu, A.M.; Andronescu, E.; Voicu, G.; Ficai, A.; Ou, K.-L.; Huang, K.-S.; Chifiriuc, M.C. Nanotechnological solution for improving the antibiotic efficiency against biofilms developed by gram-negative bacterial strains. Lett. Appl. NanoBioSci. 2013, 2, 97–104. [Google Scholar]
- Fakhardo, A.F.; Anastasova, E.I.; Gabdullina, S.R.; Solovyeva, A.S.; Saparova, V.B.; Chrishtop, V.V.; Koshevaya, E.D.; Krivoshapkina, E.F.; Krivoshapkin, P.V.; Kiselev, G.O.; et al. Toxicity Patterns of Clinically Relevant Metal Oxide Nanoparticles. ACS Appl. Bio Mater. 2019, 2, 4427–4435. [Google Scholar] [CrossRef]
- Dukhinova, M.S.; Prilepskii, A.Y.; Shtil, A.A.; Vinogradov, V.V. Metal oxide nanoparticles in therapeutic regulation of macrophage functions. Nanomaterials 2019, 9, 1631. [Google Scholar] [CrossRef]
- Shkodenko, L.A.; Kassirov, I.S.; Koshel, E.I. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Paramonova, A.P.; Kiselev, G.O.; Fakhardo, A.F.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Synthesis of upconversion zirconia nanoparticles for bioimaging. IOP Conf. Ser. Mater. Sci. Eng. 2019, 525, 012028. [Google Scholar] [CrossRef]
- Andreeva, Y.I.; Drozdov, A.S.; Solovyeva, A.S.; Fakhardo, A.F.; Vinogradov, V.V. Polyelectrolyte-based magnetic photonic crystals with anticoagulant activity. Mater. Today Chem. 2020, 17, 100292. [Google Scholar] [CrossRef]
- Yan, X.; Li, J.; Möhwald, H. Templating assembly of multifunctional hybrid colloidal spheres. Adv. Mater. 2012, 24, 2663–2667. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, L.F.; Dobrinskaya, M.N.; Kamantsev, I.S. Doped nanocrystalline calcium carbonate-phosphate—A biomaterial for bone repair and strengtheining by drug delivery. Diagn. Resour. Mech. Mater. Struct. 2015, 5, 147–157. [Google Scholar] [CrossRef]
- Ueno, Y.; Futagawa, H.; Takagi, Y.; Ueno, A.; Mizushima, Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J. Control. Release 2005, 103, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium carbonate precipitation for CO2 storage and utilization: A review of the carbonate crystallization and polymorphism. Front. Energy Res. 2017, 5, 17. [Google Scholar] [CrossRef]
- Ataee, R.A.; Derakhshanpour, J.; Mehrabi Tavana, A.; Eydi, A. Antibacterial effect of calcium carbonate nanoparticles on Agrobacterium tumefaciens. J. Mil. Med. 2011, 13, 65–70. [Google Scholar]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.-S.; Adibkia, K. Physicochemical characterization and antimicrobial evaluation of gentamicin-loaded CaCO3 nanoparticles prepared via microemulsion method. J. Drug Deliv. Sci. Technol. 2016, 35, 16–23. [Google Scholar] [CrossRef]
- Rumyantceva, V.I.; Rumyantceva, V.I.; Koshel, E.I.; Vinogradov, V.V. Biocide-conjugated magnetite nanoparticles as an advanced platform for biofilm treatment. Ther Deliv. 2019, 10, 241–250. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cristescu, R.; Chifiriuc, M.C.; Dorcioman, G.; Socol, G.; Mihailescu, I.N.; Mihaiescu, D.E.; Ficai, A.; Vasile, O.R.; Enculescu, M.; et al. Fabrication of magnetite-based core–shell coated nanoparticles with antibacterial properties. Biofabrication 2015, 7, 015014. [Google Scholar] [CrossRef]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Scialabba, C.; Licciardi, M.; Mauro, N.; Rocco, F.; Ceruti, M.; Giammona, G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. Eur. J. Pharm. Biopharm. 2014, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y.; et al. Urokinase-conjugated magnetite nanoparticles as a promising drug delivery system for targeted thrombolysis: Synthesis and preclinical evaluation. ACS Appl. Mater. Interfaces 2018, 10, 36764–36775. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Schekina, A.N.; Vinogradov, V.V. Magnetically controlled protein nanocontainers as a drug depot for the hemostatic agent. Nanotechnol. Sci. Appl. 2019, 12, 11. [Google Scholar] [CrossRef]
- Anastasova, E.I.; Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V. Magnetite nanocontainers: Toward injectable highly magnetic materials for targeted drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 30040–30044. [Google Scholar] [CrossRef]
- Inozemtseva, O.A.; German, S.V.; Navolokin, N.A.; Bucharskaya, A.B.; Maslyakova, G.N.; Gorin, D.A. Encapsulated Magnetite Nanoparticles: Preparation and Application as Multifunctional Tool for Drug Delivery Systems. In Nanotechnology and Biosensors, 1st ed.; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–192. [Google Scholar]
- Taylor, E.N.; Kummer, K.M.; Durmus, N.G.; Leuba, K.; Tarquinio, K.M.; Webster, T.J. Superparamagnetic iron oxide nanoparticles (SPION) for the treatment of antibiotic-resistant biofilms. Small 2012, 8, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Ranmadugala, D.; Ebrahiminezhad, A.; Manley-Harris, M.; Ghasemi, Y.; Berenjian, A. The effect of iron oxide nanoparticles on Bacillus subtilis biofilm, growth and viability. Process Biochem. 2017, 62, 231–240. [Google Scholar] [CrossRef]
- Davis, R.; Markham, A.; Balfour, J.A. Ciprofloxacin. Drugs 1996, 51, 1019–1074. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Ivanovski, V.; Avnir, D.; Vinogradov, V.V. A universal magnetic ferrofluid: Nanomagnetite stable hydrosol with no added dispersants and at neutral pH. J. Colloid Interface Sci. 2016, 468, 307–312. [Google Scholar] [CrossRef]
- Andreeva, Y.I.; Drozdov, A.S.; Avnir, D.; Vinogradov, V.V. Enzymatic nanocomposites with radio frequency field-modulated activity. ACS Biomater. Sci. Eng. 2018, 4, 3962–3967. [Google Scholar] [CrossRef]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37, S59–S66. [Google Scholar] [CrossRef]
- Serov, N.S.; Darmoroz, D.D.; Lokteva, A.V.; Chernyshov, I.Y.; Koshel, E.I.; Vinogradov, V.V. One-pot synthesis of template-free hollow anisotropic CaCO3 structures: Towards inorganic shape-mimicking drug delivery systems. Chem. Commun. 2020, 56, 11969–11972. [Google Scholar] [CrossRef]
- Geilich, B.M.; Gelfat, I.; Sridhar, S.; van de Ven, A.L.; Webster, T.J. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials. 2017, 119, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Nguyen, D.; Mogarala, S.; Osinski, M.; Smyth, H.D.C. Magnetic fields suppress Pseudomonas aeruginosa biofilms and enhance ciprofloxacin activity. Biofouling. 2015, 31, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, A.; Huang, L.-Y.; Yang, M.-C.; Liu, T.-Y.; Tsai, S.-C.; Yang, C.-Y.; Kuo, C.-Y.; Chan, T.-Y.; Zou, H.-M.; Lian, W.-N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef]
- Bigham, A.; Aghajanian, A.H.; Behzadzadeh, S.; Sokhani, Z.; Shojaei, S.; Kaviani, Y.; Hassanzadeh-Tabrizi, S.A. Nanostructured magnetic Mg2SiO4-CoFe2O4 composite scaffold with multiple capabilities for bone tissue regeneration. Mater. Sci. Eng. C 2019, 99, 83–95. [Google Scholar] [CrossRef]
- Huang, W.C.; Hu, S.-H.; Liu, K.-H.; Chen, S.-Y.; Liu, D.-M. A flexible drug delivery chip for the magnetically-controlled release of anti-epileptic drugs. J. Control. Release. 2009, 139, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.; Noble, M.; Francolini, I.; Vinogradov, A.M.; Stewart, P.S.; Ratner, B.D.; Costerton, J.W.; Stoodley, P. Ultrasonically controlled release of ciprofloxacin from self-assembled coatings on poly (2-hydroxyethyl methacrylate) hydrogels for Pseudomonas aeruginosa biofilm prevention. Antimicrob. Agents Chemother. 2005, 49, 4272–4279. [Google Scholar] [CrossRef]
- Ghanwate, N.A. Biofilm eradication studies on uropathogenic E. coli using ciprofloxacin and nitrofurantoin. Int. J. Pharm. Biomed. Res. 2012, 3, 127–131. [Google Scholar]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta Biomembr. 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Giulio, M.D.; D′Arrigo, M.; Marino, A.; Blanco, A.R.; Favaloro, A.; Cutroneo, G.; Bisignano, G. Effect of alkaline pH on staphylococcal biofilm formation. Apmis 2012, 120, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Tolba, E.; Müller, W.E.G.; Abd El-Hady, B.M.; Neufurth, M.; Wurm, F.; Wang, S.; Schröder, H.C.; Wang, X. High biocompatibility and improved osteogenic potential of amorphous calcium carbonate/vaterite. J. Mater. Chem. B 2016, 4, 376–386. [Google Scholar] [CrossRef]
- Serov, N.S.; Prilepskii, A.Y.; Sokolov, A.; Vinogradov, V.V. Synthesis of Plasmin-Loaded Fe3O4@CaCO3 Nanoparticles: Towards Next-Generation Thrombolytic Drugs. ChemNanoMat 2019, 5, 1267–1271. [Google Scholar] [CrossRef]
- Shapovalova, O.E.; Drozdov, A.S.; Brushkova, E.A.; Morozov, M.I.; Vinogradov, V.V. Room-temperature fabrication of magnetite-boehmite sol-gel composites for heavy metal ions removal. Arab. J. Chem. 2020, 13, 1933–1944. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2005, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]

| Compound | Biofilm Mass after Treatment (М ± SD) | The Efficiency of CF in NC Compared To the Original Form of CF, % | |
|---|---|---|---|
| CF 1 in Its Original Form (Free) | CF in NC 2 | ||
| E. coli | |||
| Without compound | 0.951 ± 0.07 | - | |
| NC | 0.516 ± 0.05 | - | |
| NC with 0.15 µg/mL CF | 0.443 ± 0.02 | 0.275 ± 0.02 | 38 |
| S. aureus | |||
| Without compound | 1.575 ± 0.08 | - | |
| NC | 0.370 ± 0.02 | - | |
| NC with 2.5 µg/mL CF | 0.853 ± 0.06 | 0.239 ± 0.01 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumyantceva, V.; Rumyantceva, V.; Andreeva, Y.; Tsvetikova, S.; Radaev, A.; Vishnevskaya, M.; Vinogradov, V.; Drozdov, A.S.; Koshel, E. Magnetically Controlled Carbonate Nanocomposite with Ciprofloxacin for Biofilm Eradication. Int. J. Mol. Sci. 2021, 22, 6187. https://doi.org/10.3390/ijms22126187
Rumyantceva V, Rumyantceva V, Andreeva Y, Tsvetikova S, Radaev A, Vishnevskaya M, Vinogradov V, Drozdov AS, Koshel E. Magnetically Controlled Carbonate Nanocomposite with Ciprofloxacin for Biofilm Eradication. International Journal of Molecular Sciences. 2021; 22(12):6187. https://doi.org/10.3390/ijms22126187
Chicago/Turabian StyleRumyantceva, Viktoriya, Valeriya Rumyantceva, Yulia Andreeva, Sofia Tsvetikova, Anton Radaev, Maria Vishnevskaya, Vladimir Vinogradov, Andrey S. Drozdov, and Elena Koshel. 2021. "Magnetically Controlled Carbonate Nanocomposite with Ciprofloxacin for Biofilm Eradication" International Journal of Molecular Sciences 22, no. 12: 6187. https://doi.org/10.3390/ijms22126187
APA StyleRumyantceva, V., Rumyantceva, V., Andreeva, Y., Tsvetikova, S., Radaev, A., Vishnevskaya, M., Vinogradov, V., Drozdov, A. S., & Koshel, E. (2021). Magnetically Controlled Carbonate Nanocomposite with Ciprofloxacin for Biofilm Eradication. International Journal of Molecular Sciences, 22(12), 6187. https://doi.org/10.3390/ijms22126187







