Erratum: Yamazaki et al. Editing DNA Methylation in Mammalian Embryos. Int. J. Mol. Sci. 2020, 21, 637
| Target | DNA-Binding Module | Effector | References |
|---|---|---|---|
| KLF4, RHOX, HBB | TALE | TET1 CD | [44] |
| ICAM1 | Zinc Finger | TET2 CD | [46] |
| AscI | TALE-CIB1 | TET1 CD-CRY2 | [26] |
| Snrpn, BDNF, MyoD | dCas9 | TET1 CD | [28] |
| Gfap, H19 DMR | dCas9-SunTag | scFv-TET1 CD | [41] |
| BRCA1 | dCas9 | TET1 CD | [45] |
| FMR1 | dCas9 | TET1 CD | [9] |
| Sox1 | dCas9 | TET1 CD | [47] |
| IAP (Agouti) | dCas9 | TET1 CD | [32] |
| hMLH1 | dCas9 + gRNA withPUFa-binding site | PUFa-TET1 CD with GADD45A or NEIL2 | [42] |
Conflicts of Interest
Reference
- Yamazaki, T.; Hatano, Y.; Taniguchi, R.; Kobayashi, N.; Yamagata, K. Editing DNA Methylation in Mammalian Embryos. Int. J. Mol. Sci. 2020, 21, 637. [Google Scholar] [CrossRef]
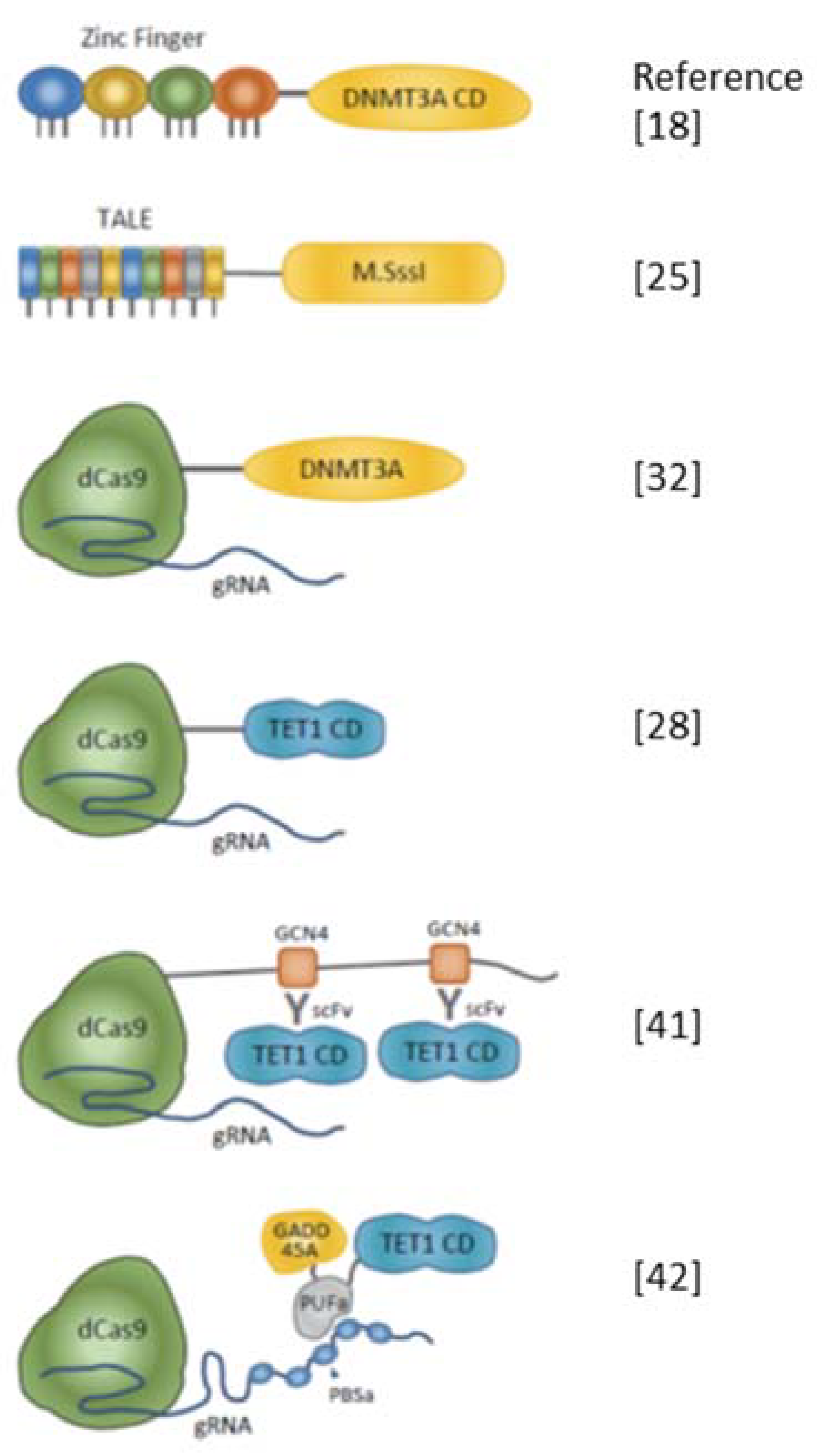
| Target | DNA-Binding Module | Effector | References |
|---|---|---|---|
| Maspin | Zinc Finger | DNMT3A CD | [18] |
| VEGF-A | Zinc Finger | DNMT3A CD-DNMT3L | [19] |
| HBV x promoter | Zinc Finger | DNMT3A C-term | [20] |
| Line1 | Zinc Finger | MIWI2 | [23] |
| P16 (CDKN2A) | TALE | DNMT3A-DNMT3L | [24] |
| Major satellite | TALE, dCas9 | SssI | [25] |
| AsclI | TALE-CIB1 | DNMT3A CD-CRY2 | [26] |
| BACH-2, IL6ST | dCas9 | DNMT3A CD | [27] |
| Snrpn, CTCF | dCas9 | DNMT3A | [28] |
| Hox genes, Runx1, H19 | dCas9 | SssI (Q147L) | [29] |
| SALL2, HBG | dCas9 | Split SssI | [30] |
| HoxA5, KLF4 | dCas9-SunTag | scFv-DNMT3A | [31] |
| IAP (Agouti), H19,IG-DMR, Snrpn DMR | dCas9 | DNMT3A | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, T.; Hatano, Y.; Taniguchi, R.; Kobayashi, N.; Yamagata, K. Erratum: Yamazaki et al. Editing DNA Methylation in Mammalian Embryos. Int. J. Mol. Sci. 2020, 21, 637. Int. J. Mol. Sci. 2021, 22, 6175. https://doi.org/10.3390/ijms22126175
Yamazaki T, Hatano Y, Taniguchi R, Kobayashi N, Yamagata K. Erratum: Yamazaki et al. Editing DNA Methylation in Mammalian Embryos. Int. J. Mol. Sci. 2020, 21, 637. International Journal of Molecular Sciences. 2021; 22(12):6175. https://doi.org/10.3390/ijms22126175
Chicago/Turabian StyleYamazaki, Taiga, Yu Hatano, Ryoya Taniguchi, Noritada Kobayashi, and Kazuo Yamagata. 2021. "Erratum: Yamazaki et al. Editing DNA Methylation in Mammalian Embryos. Int. J. Mol. Sci. 2020, 21, 637" International Journal of Molecular Sciences 22, no. 12: 6175. https://doi.org/10.3390/ijms22126175
APA StyleYamazaki, T., Hatano, Y., Taniguchi, R., Kobayashi, N., & Yamagata, K. (2021). Erratum: Yamazaki et al. Editing DNA Methylation in Mammalian Embryos. Int. J. Mol. Sci. 2020, 21, 637. International Journal of Molecular Sciences, 22(12), 6175. https://doi.org/10.3390/ijms22126175





