Ubiquitin-Specific Protease 29 Regulates Cdc25A-Mediated Tumorigenesis
Abstract
1. Introduction
2. Results
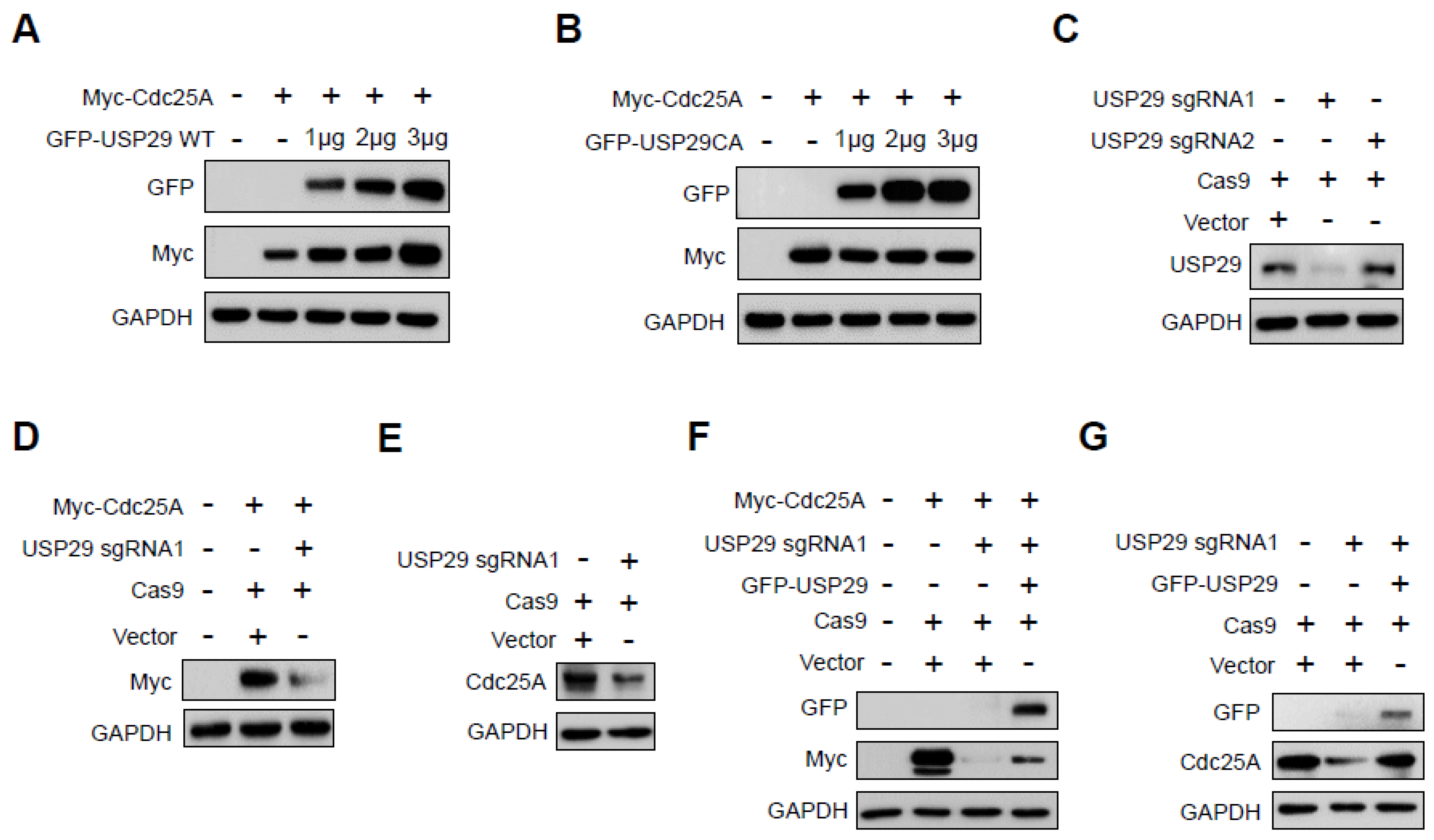
2.1. USP29 Regulates Cdc25A Protein Stability
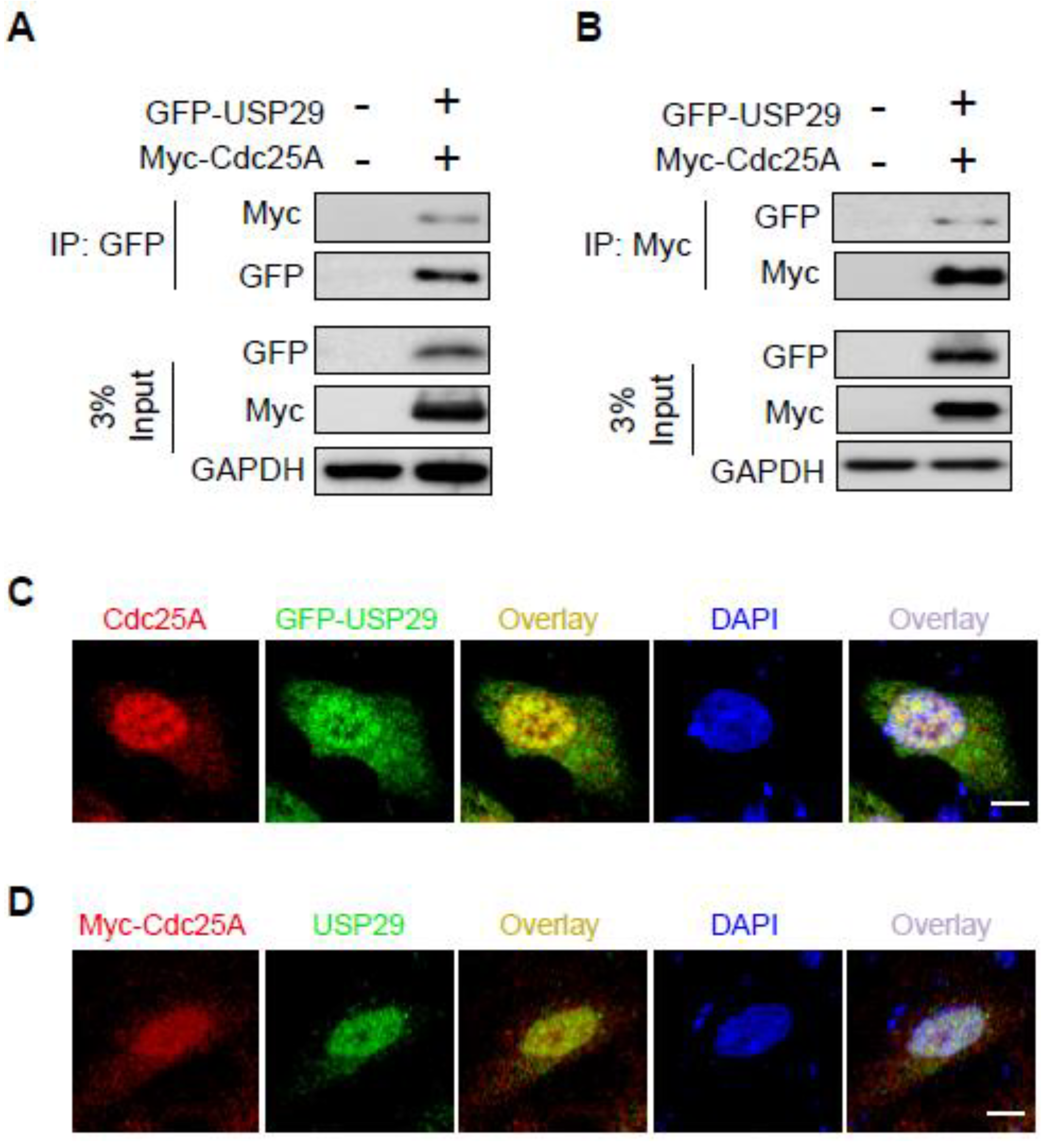
2.2. USP29 Interacts and Co-Localizes with Cdc25A
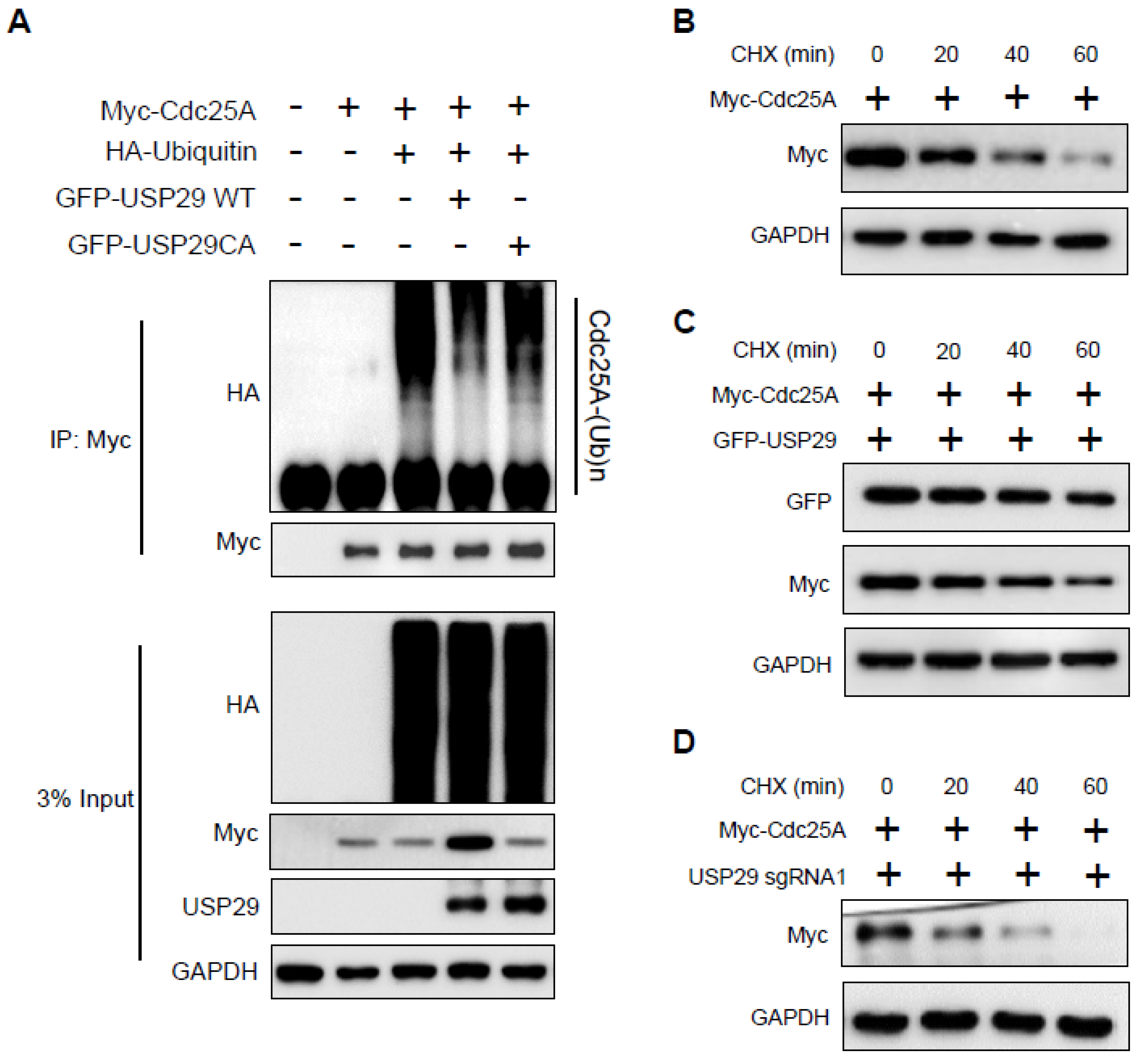
2.3. USP29 Deubiquitinates and Extends the Half-Life of Cdc25A
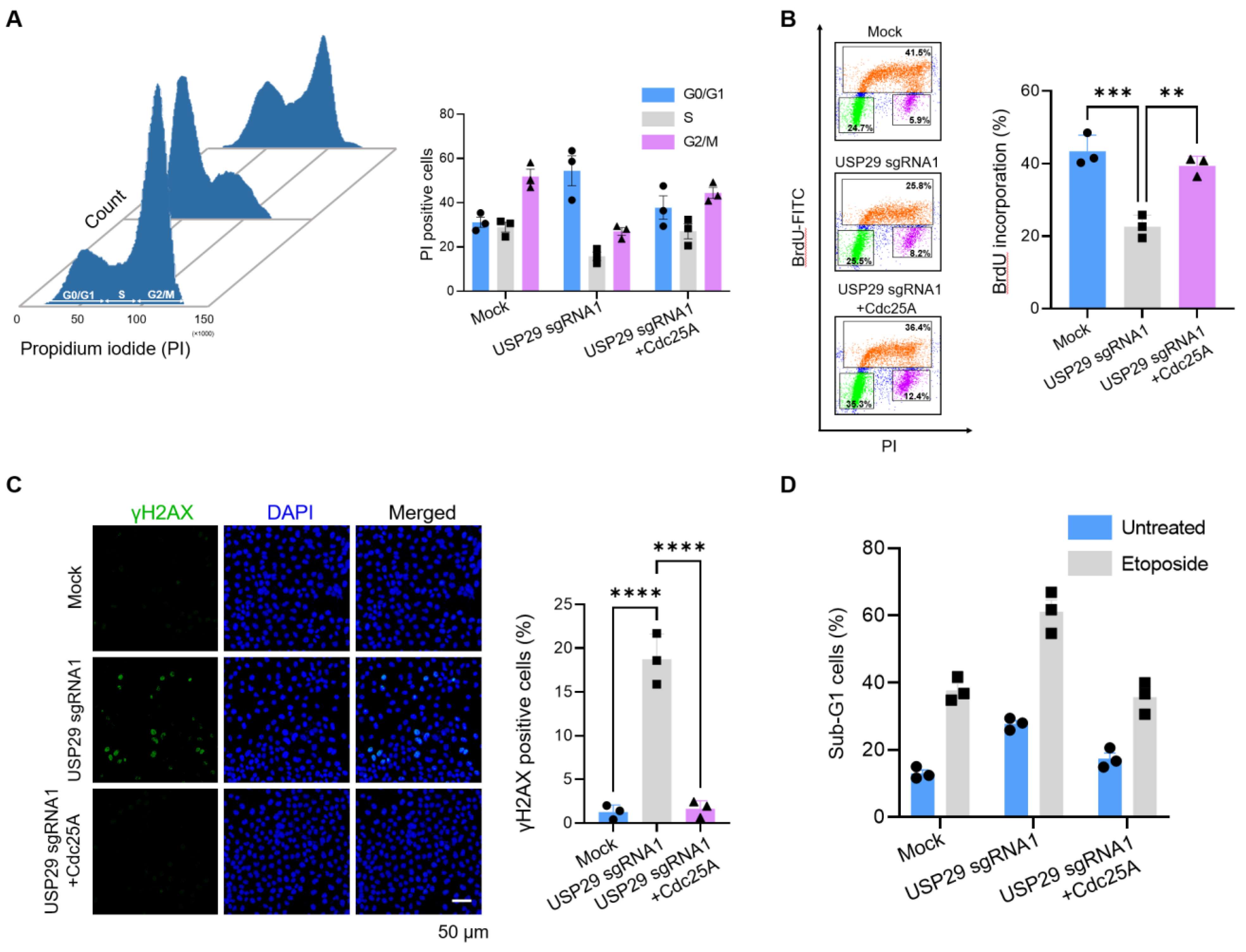
2.4. Loss of USP29 Leads to Cdc25A-Mediated Cell Cycle Arrest and Induces Apoptosis
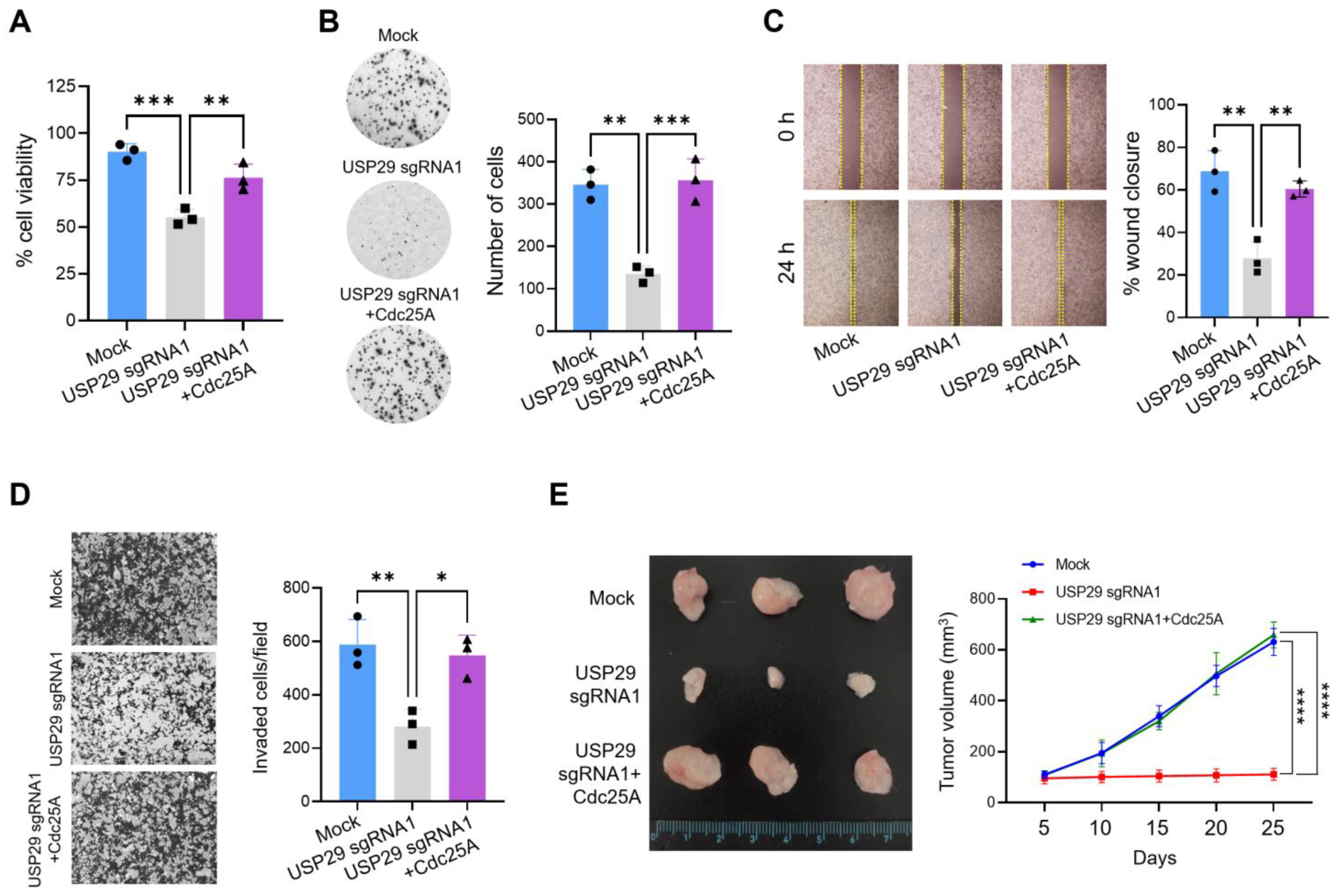
2.5. Knockdown of USP29 Reduces Cdc25A-Dependent Cancer Growth In Vitro and In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Plasmids, Antibodies, and Reagents
4.3. Cas9 and sgRNA Constructs
4.4. Transfection
4.5. Immunoprecipitation
4.6. Immunofluorescence
4.7. Deubiquitination Assay
4.8. Cell Cycle and Apoptosis Assays
4.9. Cell Viability Assay
4.10. Soft Agar Assay
4.11. Wound Healing Assay
4.12. Matrigel Invasion Assay
4.13. Xenograft Tumor Experiment
4.14. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stoeber, K.; Tlsty, T.D.; Happerfield, L.; Thomas, G.A.; Romanov, S.; Bobrow, L.; Williams, E.D.; Williams, G.H. DNA replication licensing and human cell proliferation. J. Cell Sci. 2001, 114, 2027–2041. [Google Scholar] [CrossRef]
- Vassilev, A.; DePamphilis, M. Links between DNA Replication, Stem Cells and Cancer. Genes 2017, 8, 45. [Google Scholar] [CrossRef]
- Ubhi, T.; Brown, G.W. Exploiting DNA Replication Stress for Cancer Treatment. Cancer Res. 2019, 79, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, T.; Said, M.; Naim, V. DNA Replication Stress and Chromosomal Instability: Dangerous Liaisons. Genes 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Strausfeld, U.; Labbé, J.C.; Fesquet, D.; Cavadore, J.C.; Picard, A.; Sadhu, K.; Russell, P.; Dorée, M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature 1991, 351, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Cangi, M.G.; Cukor, B.; Soung, P.; Signoretti, S.; Moreira, G.; Ranashinge, M.; Cady, B.; Pagano, M.; Loda, M. Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Investig. 2000, 106, 753–761. [Google Scholar] [CrossRef]
- Wu, W.; Fan, Y.H.; Kemp, B.L.; Walsh, G.; Mao, L. Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res. 1998, 58, 4082–4085. [Google Scholar] [PubMed]
- Galaktionov, K.; Lee, A.; Eckstein, J.; Draetta, G.; Meckler, J.; Loda, M.; Beach, D. CDC25 phosphatases as potential human oncogenes. Science 1995, 269, 1575–1577. [Google Scholar] [CrossRef]
- Blomberg, I.; Hoffmann, I. Ectopic Expression of Cdc25A Accelerates the G1/S Transition and Leads to Premature Activation of Cyclin E- and Cyclin A-Dependent Kinases. Mol. Cell. Biol. 1999, 19, 6183–6194. [Google Scholar] [CrossRef]
- Kabakci, Z.; Käppeli, S.; Cantù, C.; Jensen, L.D.; König, C.; Toggweiler, J.; Gentili, C.; Ribaudo, G.; Zagotto, G.; Basler, K.; et al. Pharmacophore-guided discovery of CDC25 inhibitors causing cell cycle arrest and tumor regression. Sci. Rep. 2019, 9, 1335. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Hirai, G.; Koyama, Y.; Oonuma, K.; Otani, Y.; Osada, H.; Sodeoka, M. Dual-Specificity Phosphatase CDC25A/B Inhibitor Identified from a Focused Library with Nonelectrophilic Core Structure. ACS Med. Chem. Lett. 2012, 3, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, M. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 2002, 21, 4875–4884. [Google Scholar] [CrossRef]
- Bernardi, R.; Liebermann, D.A.; Hoffman, B. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 2000, 19, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Wei, Y.; Honaker, Y.; Yamaguchi, H.; Appella, E.; Hung, M.-C.; Piwnica-Worms, H. GSK-3β Targets Cdc25A for Ubiquitin-Mediated Proteolysis, and GSK-3β Inactivation Correlates with Cdc25A Overproduction in Human Cancers. Cancer Cell 2008, 13, 36–47. [Google Scholar] [CrossRef]
- Antao, A.M.; Tyagi, A.; Kim, K.-S.; Ramakrishna, S. Advances in Deubiquitinating Enzyme Inhibition and Applications in Cancer Therapeutics. Cancers 2020, 12, 1579. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Das, S.; Kim, D.-H.; Chandrasekaran, A.P.; Hong, S.-H.; Kim, K.-S.; Ramakrishna, S. The stability and oncogenic function of LIN28A are regulated by USP28. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Poondla, N.; Chandrasekaran, A.P.; Kim, K.-S.; Ramakrishna, S. Deubiquitinating enzymes as cancer biomarkers: New therapeutic opportunities? BMB Rep. 2019, 52, 181–189. [Google Scholar] [CrossRef]
- Dong, L.; Yu, L.; Bai, C.; Liu, L.; Long, H.; Shi, L.; Lin, Z. USP27-mediated Cyclin E stabilization drives cell cycle progression and hepatocellular tumorigenesis. Oncogene 2018, 37, 2702–2713. [Google Scholar] [CrossRef]
- Leznicki, P.; Natarajan, J.; Bader, G.; Spevak, W.; Schlattl, A.; Abdul Rehman, S.A.; Pathak, D.; Weidlich, S.; Zoephel, A.; Bordone, M.C.; et al. Expansion of DUB functionality generated by alternative isoforms–USP35, a case study. J. Cell Sci. 2018, 131, jcs212753. [Google Scholar] [CrossRef]
- Pereg, Y.; Liu, B.Y.; O’Rourke, K.M.; Sagolla, M.; Dey, A.; Komuves, L.; French, D.M.; Dixit, V.M. Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat. Cell Biol. 2010, 12, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chandrasekaran, A.P.; Jo, K.-S.; Ko, N.R.; Oh, S.J.; Kim, K.-S.; Ramakrishna, S. HAUSP stabilizes Cdc25A and protects cervical cancer cells from DNA damage response. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118835. [Google Scholar] [CrossRef]
- Das, S.; Chandrasekaran, A.P.; Suresh, B.; Haq, S.; Kang, J.-H.; Lee, S.-J.; Kim, J.; Kim, J.; Lee, S.; Kim, H.H.; et al. Genome-scale screening of deubiquitinase subfamily identifies USP3 as a stabilizer of Cdc25A regulating cell cycle in cancer. Cell Death Differ. 2020, 27, 3004–3020. [Google Scholar] [CrossRef]
- Csizmadia, T.; Lőw, P. The Role of Deubiquitinating Enzymes in the Various Forms of Autophagy. Int. J. Mol. Sci. 2020, 21, 4196. [Google Scholar] [CrossRef]
- Li, T.; Zou, C. The Role of Deubiquitinating Enzymes in Acute Lung Injury and Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 4842. [Google Scholar] [CrossRef]
- Shin, J.Y.; Muniyappan, S.; Tran, N.-N.; Park, H.; Lee, S.B.; Lee, B.-H. Deubiquitination Reactions on the Proteasome for Proteasome Versatility. Int. J. Mol. Sci. 2020, 21, 5312. [Google Scholar] [CrossRef]
- Mapa, C.E.; Arsenault, H.E.; Conti, M.M.; Poti, K.E.; Benanti, J.A. A balance of deubiquitinating enzymes controls cell cycle entry. Mol. Biol. Cell 2018, 29, 2821–2834. [Google Scholar] [CrossRef] [PubMed]
- Martín, Y.; Cabrera, E.; Amoedo, H.; Hernández-Pérez, S.; Domínguez-Kelly, R.; Freire, R. USP29 controls the stability of checkpoint adaptor Claspin by deubiquitination. Oncogene 2015, 34, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Kwaku Dad, A.-B.; Beloor, J.; Gopalappa, R.; Lee, S.-K.; Kim, H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014, 24, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, A.P.; Woo, S.H.; Sarodaya, N.; Rhie, B.H.; Tyagi, A.; Das, S.; Suresh, B.; Ko, N.R.; Oh, S.J.; Kim, K.-S.; et al. Ubiquitin-Specific Protease 29 Regulates Cdc25A-Mediated Tumorigenesis. Int. J. Mol. Sci. 2021, 22, 5766. https://doi.org/10.3390/ijms22115766
Chandrasekaran AP, Woo SH, Sarodaya N, Rhie BH, Tyagi A, Das S, Suresh B, Ko NR, Oh SJ, Kim K-S, et al. Ubiquitin-Specific Protease 29 Regulates Cdc25A-Mediated Tumorigenesis. International Journal of Molecular Sciences. 2021; 22(11):5766. https://doi.org/10.3390/ijms22115766
Chicago/Turabian StyleChandrasekaran, Arun Pandian, Sang Hyeon Woo, Neha Sarodaya, Byung Ho Rhie, Apoorvi Tyagi, Soumyadip Das, Bharathi Suresh, Na Re Ko, Seung Jun Oh, Kye-Seong Kim, and et al. 2021. "Ubiquitin-Specific Protease 29 Regulates Cdc25A-Mediated Tumorigenesis" International Journal of Molecular Sciences 22, no. 11: 5766. https://doi.org/10.3390/ijms22115766
APA StyleChandrasekaran, A. P., Woo, S. H., Sarodaya, N., Rhie, B. H., Tyagi, A., Das, S., Suresh, B., Ko, N. R., Oh, S. J., Kim, K.-S., & Ramakrishna, S. (2021). Ubiquitin-Specific Protease 29 Regulates Cdc25A-Mediated Tumorigenesis. International Journal of Molecular Sciences, 22(11), 5766. https://doi.org/10.3390/ijms22115766






