Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits
Abstract
1. Introduction
2. Results
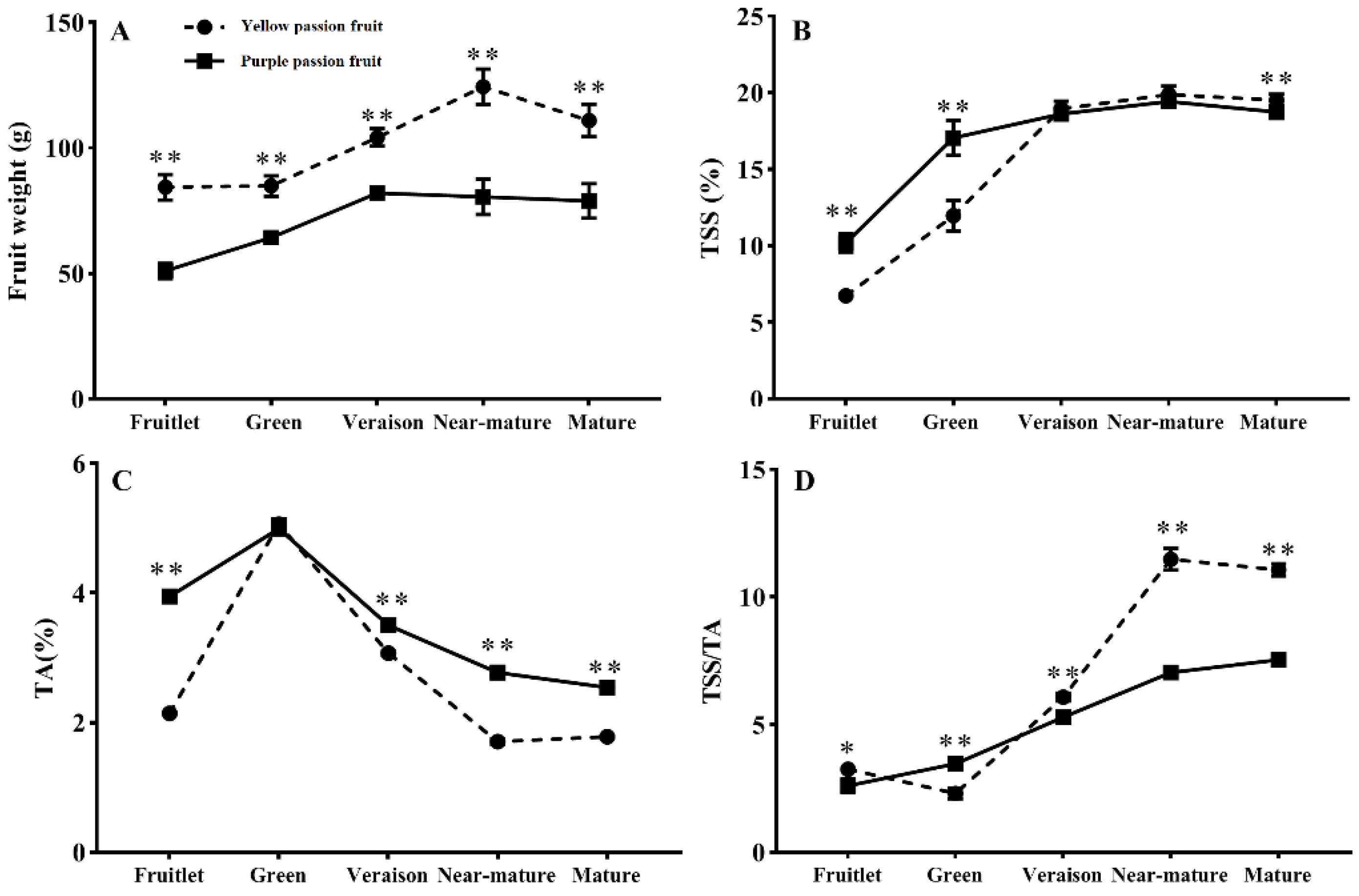
2.1. Fruit Weight, Total Soluble Solids, Titratable Acidity and Sugar-Acid Ratio
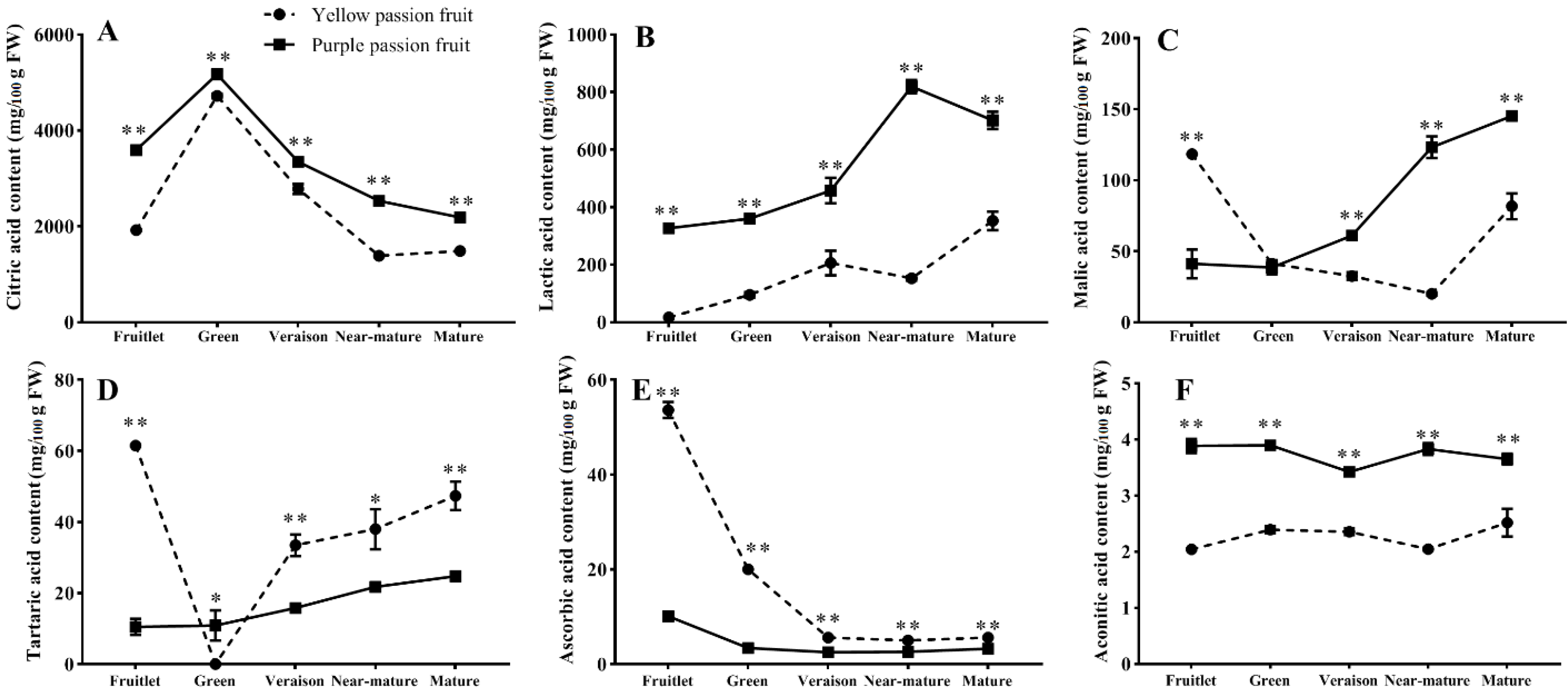
2.2. Organic Acids
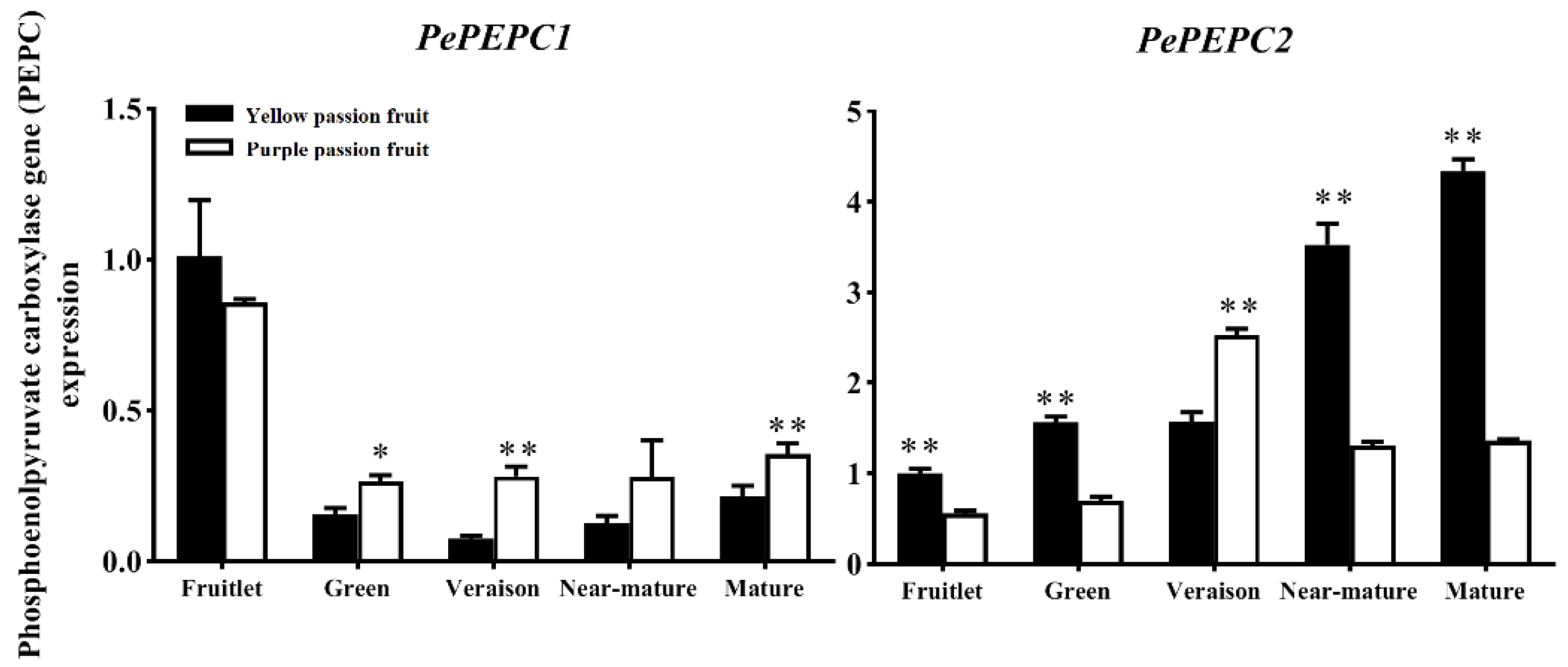
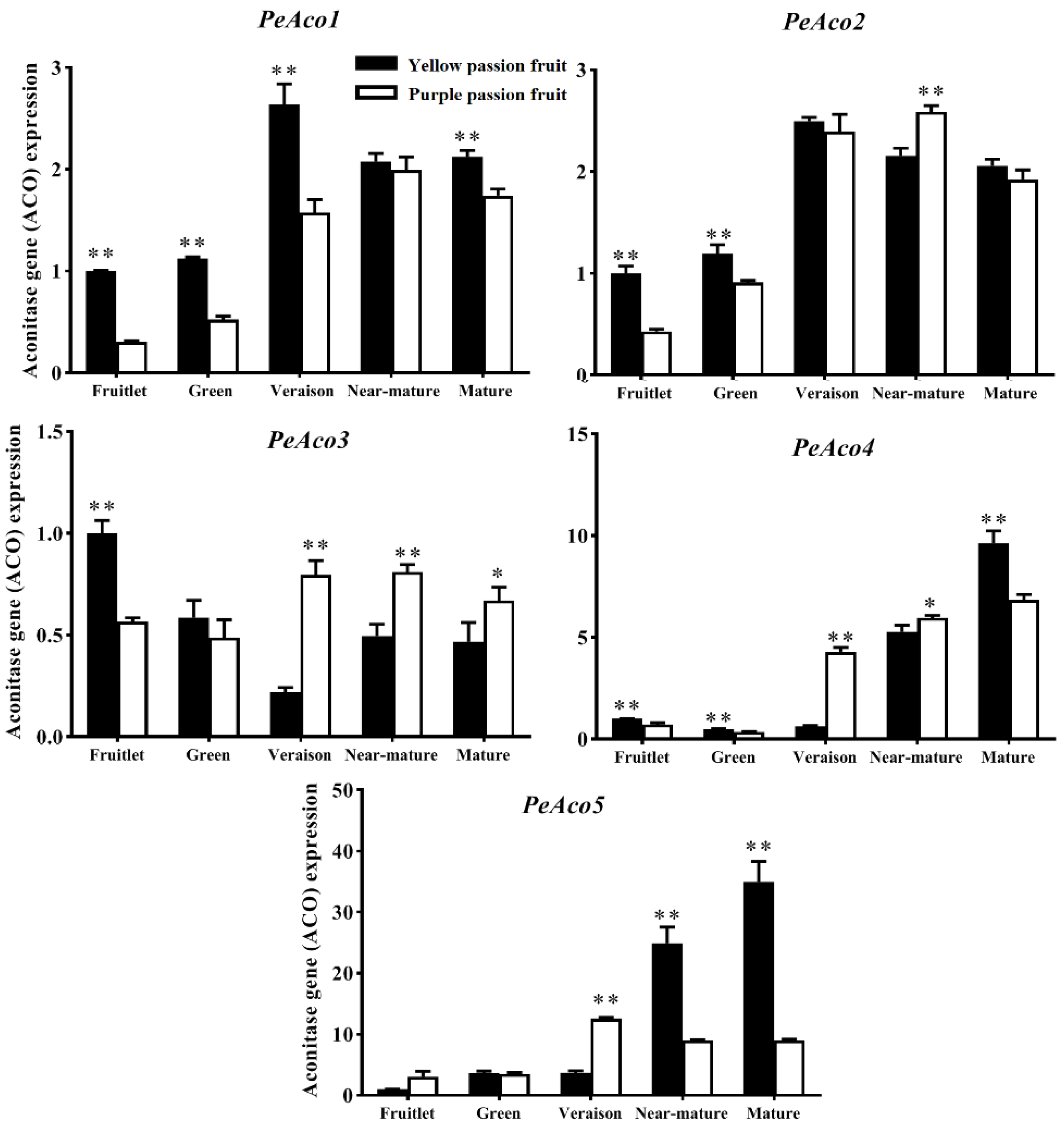
2.3. Key Enzymes Involved in Citric Acid Metabolism
2.4. Expression Analysis of Genes Encoding Key Enzymes for Citric Acid Metabolism
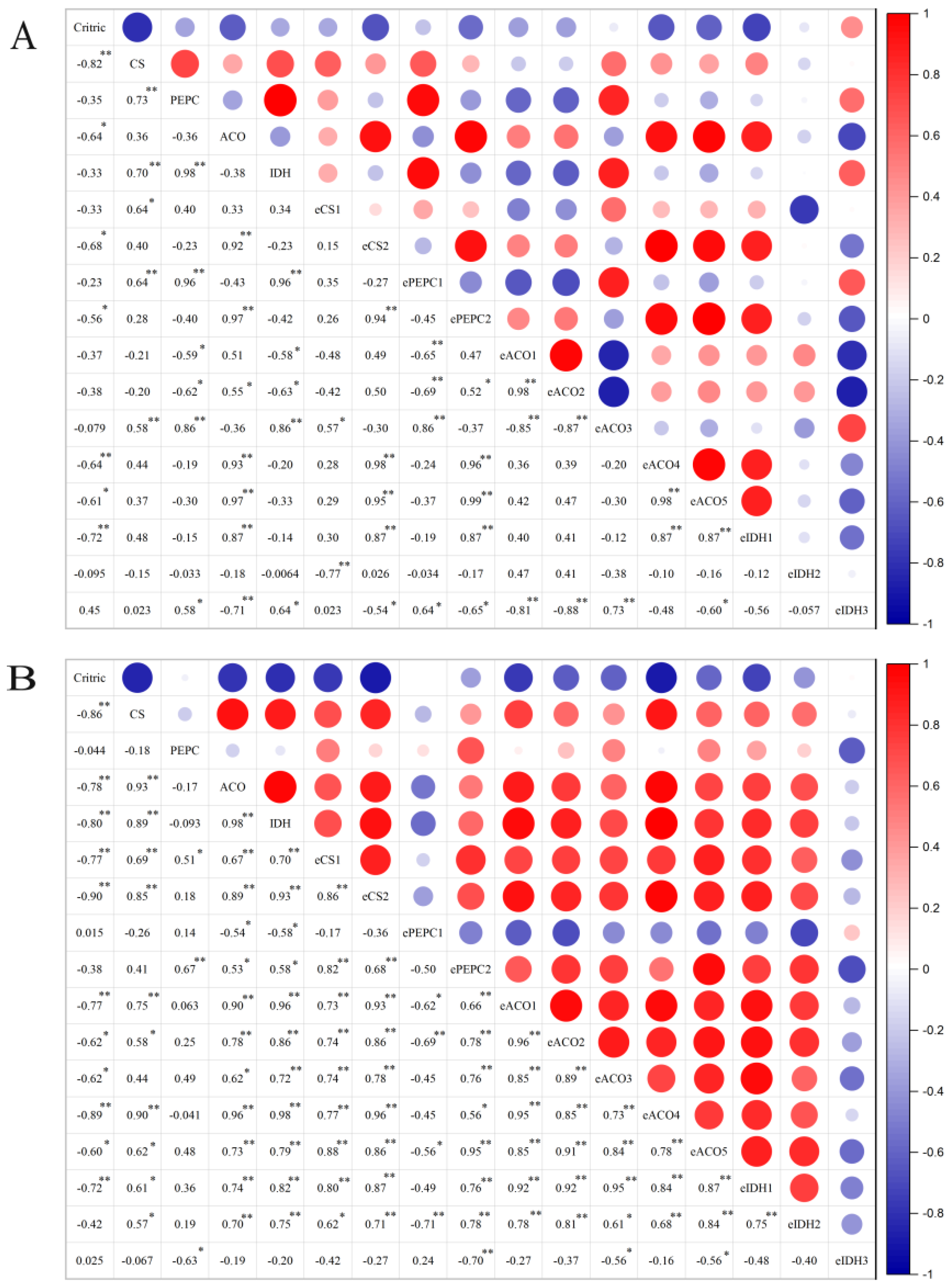
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Fruit Quality Evaluation
4.2. Extraction and Determination of Organic Acids
4.3. Enzymes Extraction and Activity Assay
4.4. RNA Extraction and Real-Time Quantitative PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rodriguez-Amaya, D.B. PASSION FRUITS. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Cambridge, MA, USA, 2003; pp. 4368–4373. [Google Scholar]
- Rinaldi, M.M.; Costa, A.M.; Faleiro, F.G.; Junqueira, N.T.V. Conservação pós-colheita de frutos de Passiflora setacea DC. submetidos a diferentes sanitizantes e temperaturas de armazenamento. Braz. J. Food Technol. 2017, 20, e2016046. [Google Scholar] [CrossRef]
- Maniwara, P.; Nakano, K.; Boonyakiat, D.; Ohashi, S.; Hiroi, M.; Tohyama, T. The use of visible and near infrared spectroscopy for evaluating passion fruit postharvest quality. J. Food Eng. 2014, 143, 33–43. [Google Scholar] [CrossRef]
- Wu, B.H.; Zhao, J.B.; Chen, J.; Xi, H.F.; Jiang, Q.; Li, S.H. Maternal inheritance of sugars and acids in peach (P. persica (L.) Batsch) fruit. Euphytica 2012, 188, 333–345. [Google Scholar] [CrossRef]
- Ashoor, S.H.; Welty, J. Determination of organic acids in food by high-performance liquid chromatography: Lactic acid. J. Chromatogr. A 1984, 287, 452–456. [Google Scholar] [CrossRef]
- Hudina, M.; Śtampar, F. Sugars and organic acids contents of European Pyrus comminus L. and Asian Pyrus serotina r Rehd. pear cultivars. Acta Aliment. 2000, 29, 217–230. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of Sugar and Organic Acid of Fruit Juices: A Comparative Study and Implication for Authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Gaifang, Y.; Shaoling, Z.; Jun, W. Analysis of components and contents of soluble sugars and organic acids in ten cultivars of pear by high performance liquid chromatography (In Chinese). J. Nanjing Agric. Univ. 2011, 34, 25–31. [Google Scholar]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of Bagging on the Development and Quality of Fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef]
- Werkhoff, P.; Güntert, M.; Krammer, G.; Sommer, H.; Kaulen, J. Vacuum Headspace Method in Aroma Research: Flavor Chemistry of Yellow Passion Fruits. J. Agric. Food Chem. 1998, 46, 1076–1093. [Google Scholar] [CrossRef]
- Hiu, D.N.; Scheuer, P.J. The Volatile Constituents of Passion Fruit Juice. J. Food Sci. 1961, 26, 557–563. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Freitas, A.; Cruz, C.J.; Figueira, J.; Câmara, J.S. Profiling of passion fruit volatiles: An effective tool to discriminate between species and varieties. Food Res. Int. 2015, 77, 408–418. [Google Scholar] [CrossRef]
- Cercós, M.; Soler, G.; Iglesias, D.J.; Gadea, J.; Forment, J.; Talón, M. Global Analysis of Gene Expression During Development and Ripening of Citrus Fruit Flesh. A Proposed Mechanism for Citric Acid Utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar] [CrossRef]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase activity and expression during the development of lemon fruit. Physiol. Plant. 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Chen, L. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Ma, B.; Liao, L.; Fang, T.; Peng, Q.; Ogutu, C.; Zhou, H.; Ma, F.; Han, Y. A Ma10 gene encoding P-type ATPase is involved in fruit organic acid accumulation in apple. Plant Biotechnol. J. 2019, 17, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, S.; Xu, R.; Tu, S.; Tu, K. Interactions among chilling tolerance, sucrose degradation and organic acid metabolism in UV-C-irradiated peach fruit during postharvest cold storage. Acta Physiol. Plant. 2019, 41, 79. [Google Scholar] [CrossRef]
- Chan, H.T.; Chang, T.S.K.; Chenchin, E. Nonvolatile acids of passion fruit juice. J. Agric. Food Chem. 1972, 20, 110–112. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.B.; Zakaria, M.H.; Saupi, N. Nutritional, mineral and organic acid composition of passion fruit (Passiflora species). Food Res. 2018, 3, 231–240. [Google Scholar] [CrossRef]
- Perotti, V.E.; Figueroa, C.M.; Andreo, C.S.; Iglesias, A.A.; Podestá, F.E. Cloning, expression, purification and physical and kinetic characterization of the phosphoenolpyruvate carboxylase from orange (Citrus sinensis osbeck var. Valencia) fruit juice sacs. Plant Sci. 2010, 179, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-X.; Shi, C.-Y.; Liu, X.; Ning, D.-Y.; Jing, L.-F.; Yang, H.; Liu, Y.-Z. Citrate Accumulation-Related Gene Expression and/or Enzyme Activity Analysis Combined With Metabolomics Provide a Novel Insight for an Orange Mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef]
- Shiomi, S.; Wamocho, L.S.; Agong, S.G. Ripening characteristics of purple passion fruit on and off the vine. Postharvest Biol. Technol. 1996, 7, 161–170. [Google Scholar] [CrossRef]
- Patel, R.K.; Singh, A.; Prakash, J.; Nath, A.; Deka, B.C. Physico-biochemical changes during fruit growth, development and maturity in passion fruit genotypes. Indian J. Hortic. 2014, 71, 486–493. [Google Scholar]
- Parveen, K.; Khatkar, B.S. Physico-chemical properties and nutritional composition of aonla (Emblica officinalis) varieties. Int. Food Res. J. 2015, 22, 2358–2363. [Google Scholar]
- Sha, S.F.; Li, J.C.; Wu, J.; Zhang, S.L. Changes in the organic acid content and related metabolic enzyme activities in developing “Xinping” pear fruit. Afr. J. Agric. Res. 2011, 6, 3560–3567. [Google Scholar] [CrossRef][Green Version]
- Fischer, G.; Melgarejo, L.M.; Cutler, J. Pre-harvest factors that influence the quality of passion fruit: A review. Agron. Colomb. 2018, 36, 217–226. [Google Scholar] [CrossRef]
- Ali, M.M.; Yousef, A.F.; Li, B.; Chen, F. Effect of Environmental Factors on Growth and Development of Fruits. Trop. Plant Biol. 2021. [Google Scholar] [CrossRef]
- Durmaz, G.; Çam, M.; Kutlu, T.; HişiL, Y. Some Physical and Chemical Changes during Fruit Development of Five Common Apricot (Prunus armeniaca L.) Cultivars. Food Sci. Technol. Res. 2010, 16, 71–78. [Google Scholar] [CrossRef]
- Muhammad Jawad, U.; Gao, L.; Gebremeskel, H.; Safdar, L.B.; Yuan, P.; Zhao, S.; Xuqiang, L.; Nan, H.; Hongju, Z.; Liu, W. Expression pattern of sugars and organic acids regulatory genes during watermelon fruit development. Sci. Hortic. (Amsterdam) 2020, 265, 109102. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Fang, Z.-Z.; Zhou, D.-R.; Pan, S.-L.; Ye, X.-F. Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv. ‘Furongli’ (Prunus salicina Lindl). J. Sci. Food Agric. 2019, 99, 1010–1019. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Huo, Y.; Hu, H.; Peng, S.; Chen, Q. Contents and Changes of Organic Acid in Sand Pears from Different Germplasm Resources. Sci. Agric. Sin. 2009, 42, 216. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- de Oliveira, G.A.; de Castilhos, F.; Renard, C.M.-G.C.; Bureau, S. Comparison of NIR and MIR spectroscopic methods for determination of individual sugars, organic acids and carotenoids in passion fruit. Food Res. Int. 2014, 60, 154–162. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Q.; Jia, D.; Zhong, M.; Tao, J.; Liao, G.; Huang, C.; Xu, X. Characterization of Organic Acid Metabolism and Expression of Related Genes During Fruit Development of Actinidia eriantha ‘Ganmi 6’. Plants 2020, 9, 332. [Google Scholar] [CrossRef]
- YaLan, L.; ZhiFei, J.; Hong, C. Changes of the organic acid concentrations and the relative metabolic enzyme activities during the development of Prunus mume fruit. Acta Bot. Boreali Occident. Sin. 2017, 37, 130–137. [Google Scholar]
- Saradhuldhat, P.; Paull, R.E. Pineapple organic acid metabolism and accumulation during fruit development. Sci. Hortic. (Amsterdam) 2007, 112, 297–303. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X.; Lin, Q.; Chen, J.; Grierson, D.; Yin, X.; Sun, C.; Chen, K. Differential Expression of Organic Acid Degradation-Related Genes During Fruit Development of Navel Oranges (Citrus sinensis) in Two Habitats. Plant Mol. Biol. Rep. 2013, 31, 1131–1140. [Google Scholar] [CrossRef]
- Ronggao, G.; Wei, Y.; Zhihui, W.; Mingan, L.; Guolu, L. Study on The Sugar-Acid Ratio and Relevant Metabolizing Enzyme Activities in Navel Orange Fruits from Different Eco-Regions. Rev. Bras. Frutic. 2015, 37, 835–844. [Google Scholar] [CrossRef]
- Guillet, C.; Just, D.; Bénard, N.; Destrac-Irvine, A.; Baldet, P.; Hernould, M.; Causse, M.; Raymond, P.; Rothan, C. A fruit-specific phospho enol pyruvate carboxylase is related to rapid growth of tomato fruit. Planta 2002, 214, 717–726. [Google Scholar] [CrossRef]
- Yin, B.; Ma, H.; Li, Z. Differences in Organic Acids Contents and Citrate Acid Metabolism Enzyme Activities in Chunmei Peach Fruit inside and outside the Greenhouse. J. Henan Agric. Sci. 2020, 49, 103–110. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. Organic Acid Analysis in Different Citrus Juices under Reversed Phase Conditions. Not. Bot. Horti Agrobot. Cluj Napoca 2010, 38, 44–48. [Google Scholar] [CrossRef]
- Ribani, M.; Bottoli, C.B.G.; Collins, C.H.; Jardim, I.C.S.F.; Melo, L.F.C. Validation for chromatographic and electrophoretic methods. Quim. Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]
- Hu, M.; Du, J.; Du, L.; Luo, Q.; Xiong, J. Anti-fatigue activity of purified anthocyanins prepared from purple passion fruit (P. edulis Sim) epicarp in mice. J. Funct. Foods 2020, 65, 103725. [Google Scholar] [CrossRef]
- Munhoz, C.F.; Santos, A.A.; Arenhart, R.A.; Santini, L.; Monteiro-Vitorello, C.B.; Vieira, M.L.C. Analysis of plant gene expression during passion fruit- Xanthomonas axonopodis interaction implicates lipoxygenase 2 in host defence. Ann. Appl. Biol. 2015, 167, 135–155. [Google Scholar] [CrossRef]




| Acid | Linearity (r2) | Slope (y) | Response (Sy) | Sy/y | LOD * (µg·mL−1) | LOQ ** (µg·mL−1) |
|---|---|---|---|---|---|---|
| Tartaric | 0.9992 | 5034.58 | 9870.7 | 1.96 | 6.46 | 19.6 |
| Ascorbic | 0.9998 | 8025.61 | 6625.4 | 0.82 | 2.72 | 8.25 |
| Citric | 0.9998 | 34,189.52 | 32,649.86 | 0.95 | 3.15 | 9.54 |
| Malic | 0.9998 | 6714 | 5509.95 | 0.82 | 2.7 | 8.2 |
| Lactic | 0.9996 | 4746.69 | 6666.9 | 1.4 | 4.63 | 14.04 |
| Aconitic | 0.9999 | 2918.31 | 2134.37 | 0.73 | 2.41 | 7.31 |
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| PEPC1 | GCTGGGATCGAGGATATGGC | CGAACTCTGTGGTGGGTCTC |
| PEPC2 | CTGGTAAAGATGCAGGGCGA | CCTCTTCCAACAGTCCCACC |
| Aco1 | GCTGAAACACTTGGCTTGACT | GTCTGTGACGACTGCAACATC |
| Aco2 | TACGCATGGGAACCCACATC | TCCGAAGTTGAGCAAGCAGT |
| Aco3 | AGGTCCACTGCTTTTGGGAG | CACATCCTCACCAGGCTTGA |
| Aco4 | TGGCACGGTTGACATTGACT | CACGCTTGATTGCACAACCT |
| Aco5 | TTGGCAGACAGAGCTACCAT | CTCTCTCAATCTGGGGCTCAC |
| CS1 | TCACTGTTCTTTTTGGCGTGT | AGCCACTCCATCGTTACACT |
| CS2 | GTTGCTTTGGAGAAGGCTGC | GTGATTCTCGCCAATGTGCC |
| IDH1 | GCTGAAGCAGCTCATGGAAC | TCCCCGATTCAACGACTCTG |
| IDH2 | GTTTGAGGCCGCTGGGATA | TCACTTTGCACGTCCCCATC |
| IDH3 | CCACAGGGCAAAGTTGGATGA | TGCTCTCAGTTCATCAGCGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wei, X.; Ali, M.M.; Rizwan, H.M.; Li, B.; Li, H.; Jia, K.; Yang, X.; Ma, S.; Li, S.; et al. Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits. Int. J. Mol. Sci. 2021, 22, 5765. https://doi.org/10.3390/ijms22115765
Zhang X, Wei X, Ali MM, Rizwan HM, Li B, Li H, Jia K, Yang X, Ma S, Li S, et al. Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits. International Journal of Molecular Sciences. 2021; 22(11):5765. https://doi.org/10.3390/ijms22115765
Chicago/Turabian StyleZhang, Xiaoxue, Xiaoxia Wei, Muhammad Moaaz Ali, Hafiz Muhammad Rizwan, Binqi Li, Han Li, Kaijie Jia, Xuelian Yang, Songfeng Ma, Shaojia Li, and et al. 2021. "Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits" International Journal of Molecular Sciences 22, no. 11: 5765. https://doi.org/10.3390/ijms22115765
APA StyleZhang, X., Wei, X., Ali, M. M., Rizwan, H. M., Li, B., Li, H., Jia, K., Yang, X., Ma, S., Li, S., & Chen, F. (2021). Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. flavicarpa) and Purple (Passiflora edulis f. edulis) Passion Fruits. International Journal of Molecular Sciences, 22(11), 5765. https://doi.org/10.3390/ijms22115765








