A Fair Assessment of Evaluation Tools for the Murine Microbead Occlusion Model of Glaucoma
Abstract
1. Introduction
2. Results
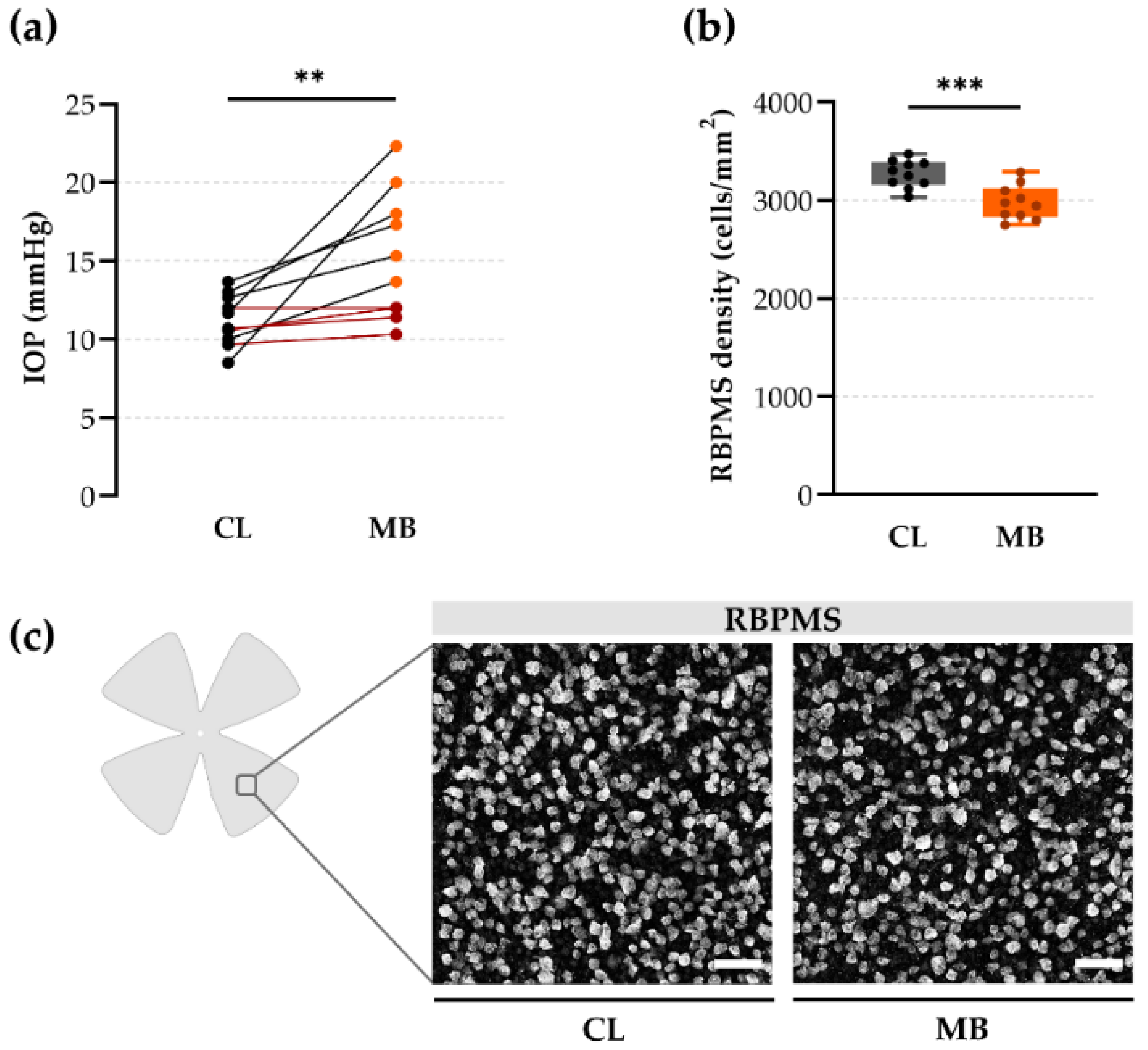
2.1. Gold Standard Evaluation Tools: Intraocular Pressure (IOP) and Retinal Ganglion Cell (RGC) Loss
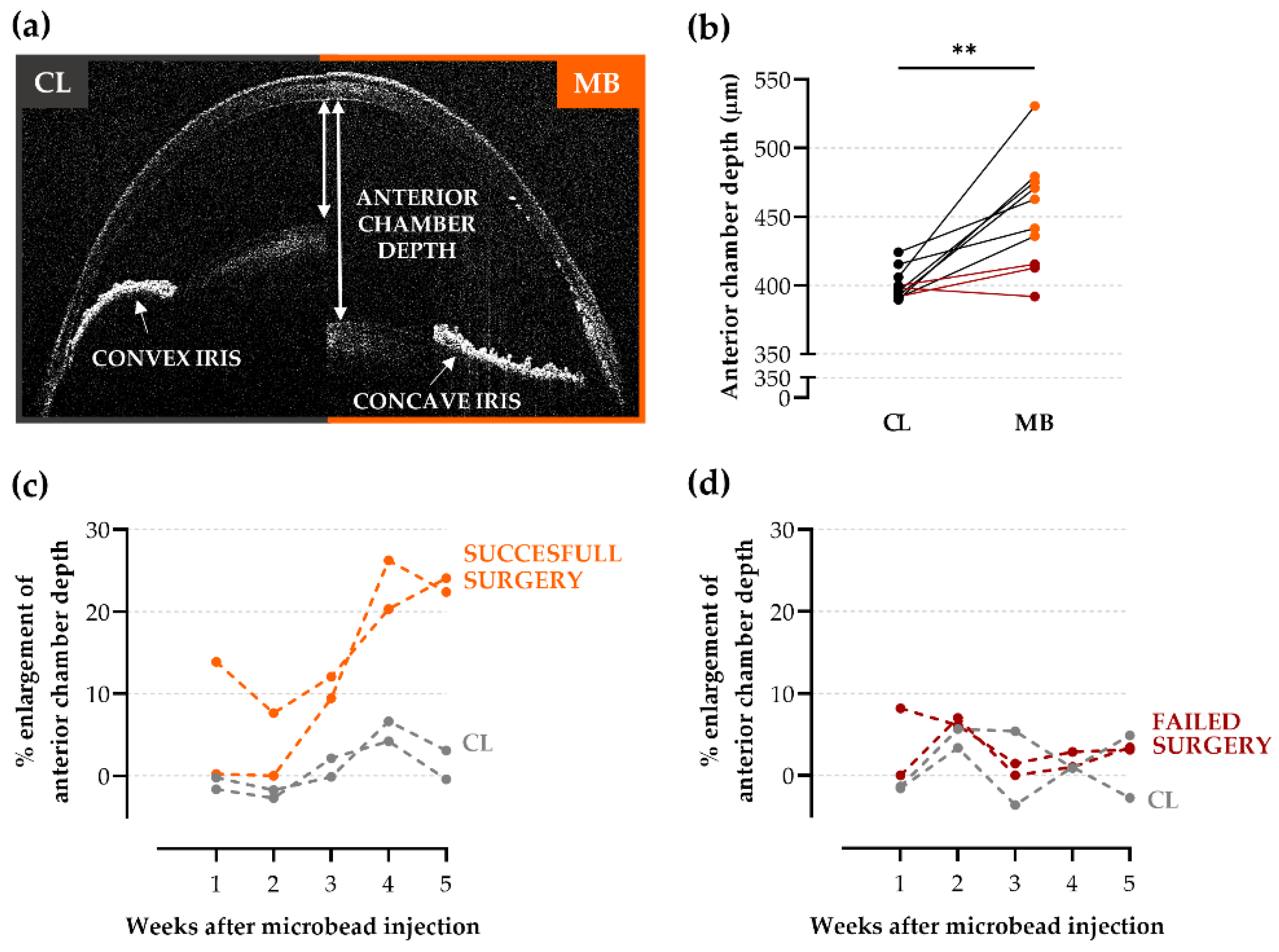
2.2. Anterior Chamber Depth as an Easy-to-Measure Parameter to Evaluate Model Induction
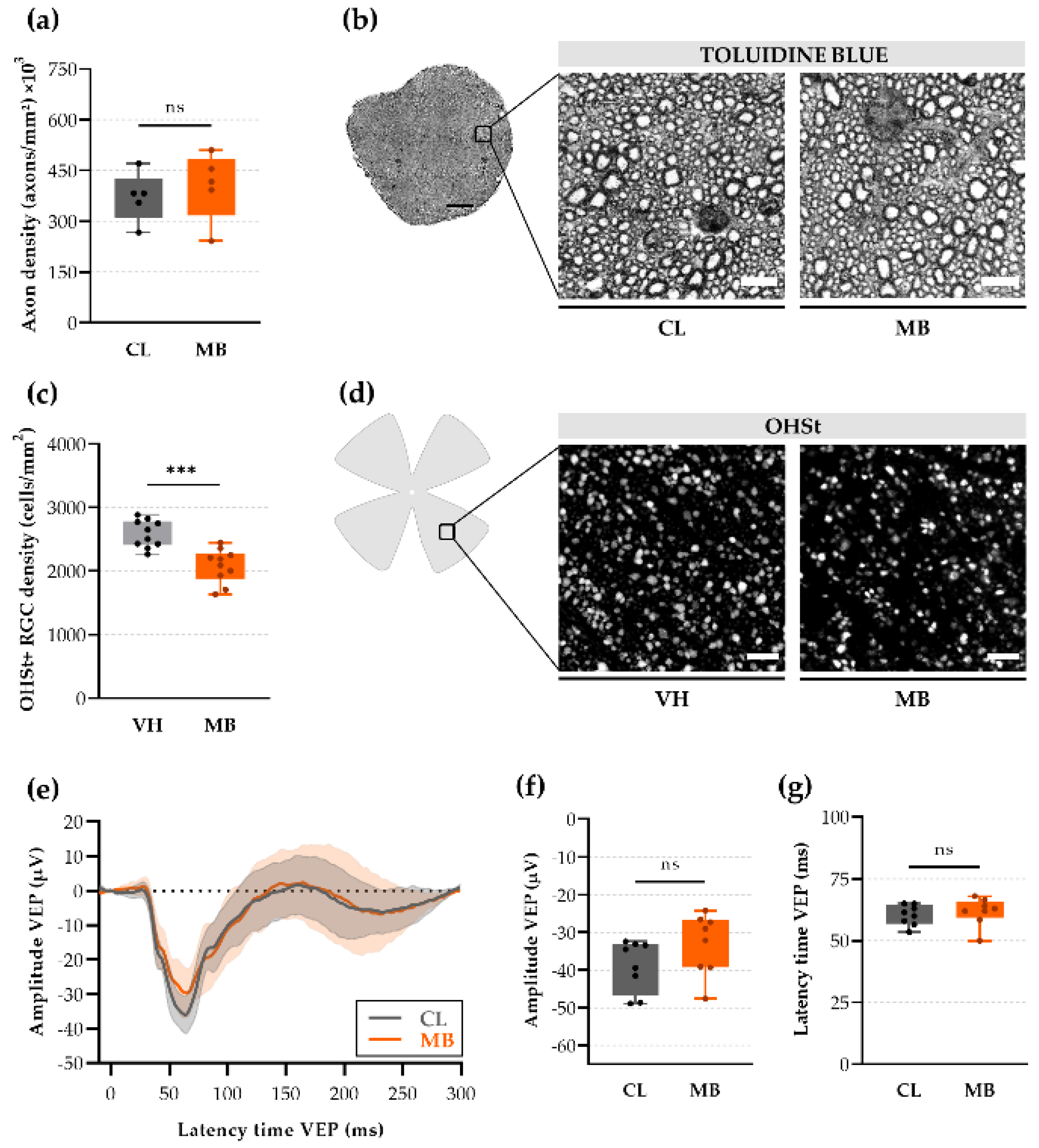
2.3. Axonal Transport Deficits yet Normal Signal Conduction after Ocular Hypertension
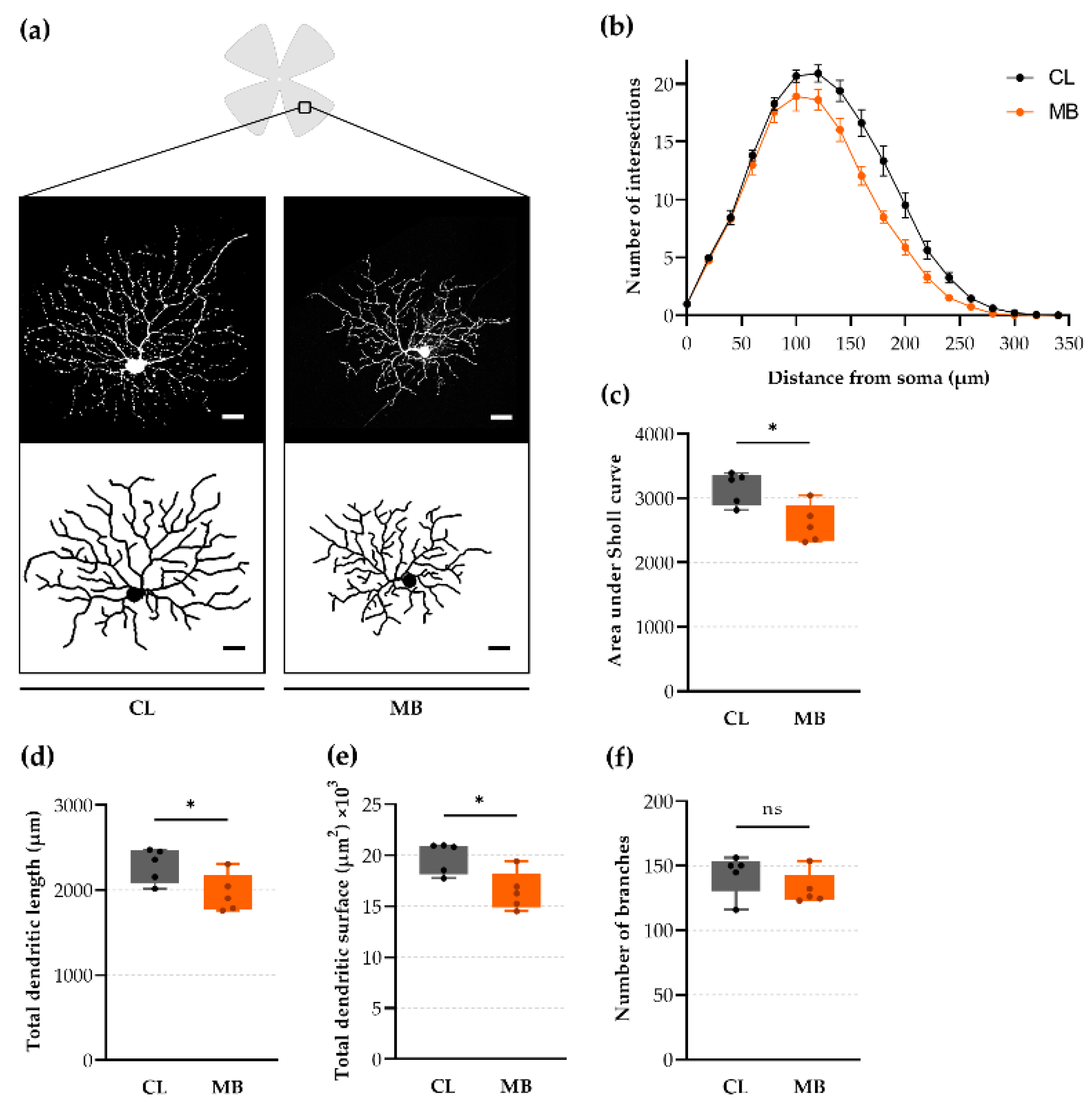
2.4. Rapid-Onset Changes in Dendritic Arborization after Microbead Injection
2.5. Changes in Retinal Ganglion Cell (RGC) Electrophysiology as a Precursor for RGC Degeneration
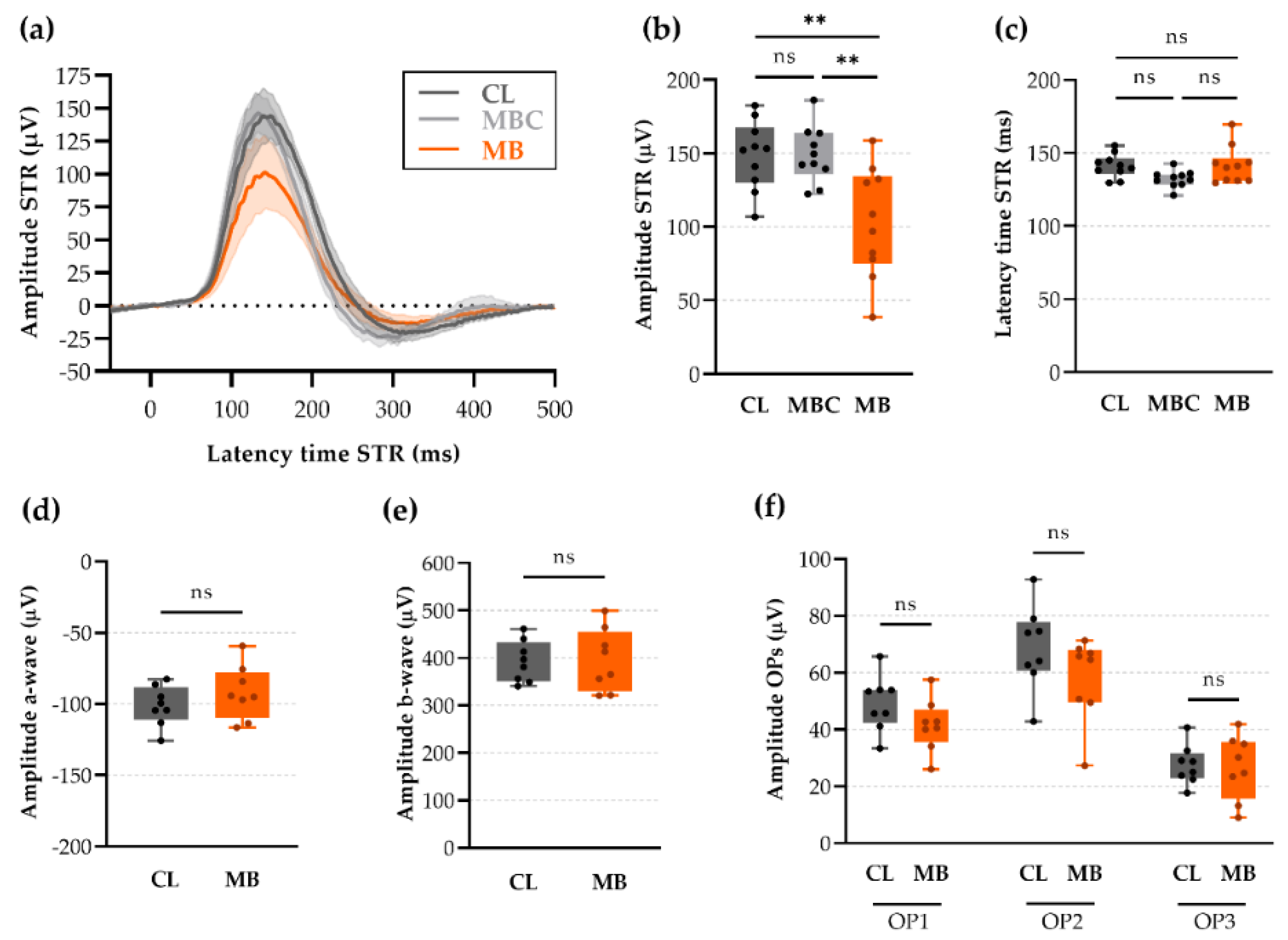
2.6. Anterior Chamber Depth and Scotopic Threshold Response (STR) as Easy, Alternative Measures to Assess Model Induction and Disease Progression In Vivo
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Microbead Occlusion Model
4.3. Intraocular Pressure (IOP) Measurements
4.4. Retrograde Tracing
4.5. Spectral Domain Optical Coherence Tomography (OCT)
4.6. Electroretinography (ERG)
4.7. Tissue Sampling and Processing
4.8. Analysis of Axonal Integrity on Semi-Thin Sections
4.9. Retinal Ganglion Cell (RGC) Soma Counts
4.10. Retinal Ganglion Cell (RGC) Dendritic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, I.-H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef]
- Evangelho, K.; Mastronardi, C.A.; De-la-Torre, A. Experimental Models of Glaucoma: A Powerful Translational Tool for the Future Development of New Therapies for Glaucoma in Humans—A Review of the Literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef]
- Cone, F.E.; Gelman, S.E.; Son, J.L.; Pease, M.E.; Quigley, H.A. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp. Eye Res. 2010, 91, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Frankfort, B.J.; Khan, A.K.; Tse, D.Y.; Chung, I.; Pang, J.-J.; Yang, Z.; Gross, R.L.; Wu, S.M. Elevated Intraocular Pressure Causes Inner Retinal Dysfunction Before Cell Loss in a Mouse Model of Experimental Glaucoma. Investig. Opthalmol. Vis. Sci. 2013, 54, 762. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Seifert, P.; Jakobs, T.C. Astrocytes in the Optic Nerve Head of Glaucomatous Mice Display a Characteristic Reactive Phenotype. Investig. Opthalmol. Vis. Sci. 2017, 58, 924. [Google Scholar] [CrossRef]
- Van der Heijden, M.E.; Shah, P.; Cowan, C.S.; Yang, Z.; Wu, S.M.; Frankfort, B.J. Effects of Chronic and Acute Intraocular Pressure Elevation on Scotopic and Photopic Contrast Sensitivity in Mice. Investig. Opthalmol. Vis. Sci. 2016, 57, 3077. [Google Scholar] [CrossRef]
- Mukai, R.; Park, D.H.; Okunuki, Y.; Hasegawa, E.; Klokman, G.; Kim, C.B.; Krishnan, A.; Gregory-Ksander, M.; Husain, D.; Miller, J.W.; et al. Mouse model of ocular hypertension with retinal ganglion cell degeneration. PLoS ONE 2019, 14, e0208713. [Google Scholar] [CrossRef]
- Della Santina, L.; Ou, Y. Who’s lost first? Susceptibility of retinal ganglion cell types in experimental glaucoma. Exp. Eye Res. 2017, 158, 43–50. [Google Scholar] [CrossRef]
- Morquette, J.B.; Di Polo, A. Dendritic and synaptic protection: Is it enough to save the retinal ganglion cell body and axon? J. Neuro-Ophthalmol. 2008, 28, 144–154. [Google Scholar] [CrossRef]
- Whitmore, A.V.; Libby, R.T.; John, S.W.M. Glaucoma: Thinking in new ways—A rôle for autonomous axonal self-destruction and other compartmentalised processes? Prog. Retin. Eye Res. 2005, 24, 639–662. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Ito, Y.A.; Belforte, N.; Cueva Vargas, J.L.; Di Polo, A. A Magnetic Microbead Occlusion Model to Induce Ocular Hypertension-Dependent Glaucoma in Mice. J. Vis. Exp. 2016, 109. [Google Scholar] [CrossRef] [PubMed]
- Masin, L.; Claes, M.; Bergmans, S.; Cools, L.; Andries, L.; Davis, B.M.; Moons, L.; De Groef, L. A novel retinal ganglion cell quantification tool based on deep learning. Sci. Rep. 2021, 11, 702. [Google Scholar] [CrossRef]
- Yazici, A.T.; Bozkurt, E.; Alagoz, C.; Alagoz, N.; Pekel, G.; Kaya, V.; Yilmaz, O.F. Central Corneal Thickness, Anterior Chamber Depth, and Pupil Diameter Measurements Using Visante OCT, Orbscan, and Pentacam. J. Refract. Surg. 2010, 26, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ritch, M.D.; Hannon, B.G.; Read, A.T.; Feola, A.J.; Cull, G.A.; Reynaud, J.; Morrison, J.C.; Burgoyne, C.F.; Pardue, M.T.; Ethier, C.R. AxoNet: A deep learning-based tool to count retinal ganglion cell axons. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.S.; Martin, K.R. Retinal ganglion cell dendrites and glaucoma: A case of missing the wood for the trees? Expert Rev. Ophthalmol. 2014, 9, 149–152. [Google Scholar] [CrossRef]
- Agostinone, J.; Di Polo, A. Retinal ganglion cell dendrite pathology and synapse loss: Implications for glaucoma. Prog. Brain Res. 2015, 220, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.R.; Noga, M.; Thakor, K.; Wang, Y.; Lemmon, V.P.; Park, K.K.; Tsoulfas, P. 3D Visualization of Individual Regenerating Retinal Ganglion Cell Axons Reveals Surprisingly Complex Growth Paths. Eneuro 2017, 4, ENEURO.0093-17.2017. [Google Scholar] [CrossRef]
- Krieger, B.; Qiao, M.; Rousso, D.L.; Sanes, J.R.; Meister, M. Four alpha ganglion cell types in mouse retina: Function, structure, and molecular signatures. PLoS ONE 2017, 12, e0180091. [Google Scholar] [CrossRef]
- Smith, M.A.; Plyler, E.S.; Dengler-Crish, C.M.; Meier, J.; Crish, S.D. Nodes of Ranvier in Glaucoma. Neuroscience 2018, 390, 104–118. [Google Scholar] [CrossRef]
- Bastian, C.; Brunet, S.; Baltan, S. Ex Vivo Studies of Optic Nerve Axon Electrophysiology. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020; pp. 169–177. [Google Scholar]
- Holder, G.E. Electrophysiological assessment of optic nerve disease. Eye 2004, 18, 1133–1143. [Google Scholar] [CrossRef]
- Ridder, W.H.; Nusinowitz, S. The visual evoked potential in the mouse—Origins and response characteristics. Vis. Res. 2006, 46, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.; Kumar, S.; Ramakrishnan, H.; Roy, K.; Viswanathan, S.; Bloomfield, S.A. Targeting neuronal gap junctions in mouse retina offers neuroprotection in glaucoma. J. Clin. Investig. 2017, 127, 2647–2661. [Google Scholar] [CrossRef]
- Akopian, A.; Kumar, S.; Ramakrishnan, H.; Viswanathan, S.; Bloomfield, S.A. Amacrine cells coupled to ganglion cells via gap junctions are highly vulnerable in glaucomatous mouse retinas. J. Comp. Neurol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Della Santina, L.; Inman, D.M.; Lupien, C.B.; Horner, P.J.; Wong, R.O.L. Differential Progression of Structural and Functional Alterations in Distinct Retinal Ganglion Cell Types in a Mouse Model of Glaucoma. J. Neurosci. 2013, 33, 17444–17457. [Google Scholar] [CrossRef]
- Weitlauf, C.; Ward, N.J.; Lambert, W.S.; Sidorova, T.N.; Ho, K.W.; Sappington, R.M.; Calkins, D.J. Short-term increases in transient receptor potential vanilloid-1 mediate stress-induced enhancement of neuronal excitation. J. Neurosci. 2014, 34, 15369–15381. [Google Scholar] [CrossRef]
- Ward, N.J.; Ho, K.W.; Lambert, W.S.; Weitlauf, C.; Calkins, D.J. Absence of Transient Receptor Potential Vanilloid-1 Accelerates Stress-Induced Axonopathy in the Optic Projection. J. Neurosci. 2014, 34, 3161–3170. [Google Scholar] [CrossRef]
- Morquette, B.; Morquette, P.; Agostinone, J.; Feinstein, E.; McKinney, R.A.; Kolta, A.; Di Polo, A. REDD2-mediated inhibition of mTOR promotes dendrite retraction induced by axonal injury. Cell Death Differ. 2015, 22, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, Y.; Yoshida, M.; Chen, H.; Yang, J.F.; Kim, T.S.; Cang, J.; Troy, J.B.; Liu, X. Sustained Ocular Hypertension Induces Dendritic Degeneration of Mouse Retinal Ganglion Cells That Depends on Cell Type and Location. Investig. Opthalmol. Vis. Sci. 2013, 54, 1106. [Google Scholar] [CrossRef]
- Tran, N.M.; Shekhar, K.; Whitney, I.E.; Jacobi, A.; Benhar, I.; Hong, G.; Yan, W.; Adiconis, X.; Arnold, M.E.; Lee, J.M.; et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 2019, 104, 1039–1055.e12. [Google Scholar] [CrossRef]
- Agostinone, J.; Alarcon-Martinez, L.; Gamlin, C.; Yu, W.-Q.; Wong, R.O.L.; Di Polo, A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141, 1963–1980. [Google Scholar] [CrossRef]
- Kalesnykas, G.; Oglesby, E.N.; Zack, D.J.; Cone, F.E.; Steinhart, M.R.; Tian, J.; Pease, M.E.; Quigley, H.A. Retinal Ganglion Cell Morphology after Optic Nerve Crush and Experimental Glaucoma. Investig. Opthalmol. Vis. Sci. 2012, 53, 3847. [Google Scholar] [CrossRef]
- Leung, C.K.; Weinreb, R.N.; Li, Z.W.; Liu, S.; Lindsey, J.D.; Choi, N.; Liu, L.; Cheung, C.Y.; Ye, C.; Qiu, K.; et al. Long-Term In Vivo Imaging and Measurement of Dendritic Shrinkage of Retinal Ganglion Cells. Investig. Opthalmol. Vis. Sci. 2011, 52, 1539. [Google Scholar] [CrossRef] [PubMed]
- Mak, H.K.; Ng, S.H.; Ren, T.; Ye, C.; Leung, C.K. Impact of PTEN/SOCS3 deletion on amelioration of dendritic shrinkage of retinal ganglion cells after optic nerve injury. Exp. Eye Res. 2020, 192, 107938. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, T.C.; Libby, R.T.; Ben, Y.; John, S.W.M.; Masland, R.H. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J. Cell Biol. 2005, 171, 313–325. [Google Scholar] [CrossRef] [PubMed]
- El-Danaf, R.N.; Huberman, A.D. Characteristic Patterns of Dendritic Remodeling in Early-Stage Glaucoma: Evidence from Genetically Identified Retinal Ganglion Cell Types. J. Neurosci. 2015, 35, 2329–2343. [Google Scholar] [CrossRef]
- Bhandari, A.; Smith, J.C.; Zhang, Y.; Jensen, A.A.; Reid, L.; Goeser, T.; Fan, S.; Ghate, D.; Van Hook, M.J. Early-Stage Ocular Hypertension Alters Retinal Ganglion Cell Synaptic Transmission in the Visual Thalamus. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Chou, T.-H.; Kocaoglu, O.P.; Borja, D.; Ruggeri, M.; Uhlhorn, S.R.; Manns, F.; Porciatti, V. Postnatal Elongation of Eye Size in DBA/2J Mice Compared with C57BL/6J Mice: In Vivo Analysis with Whole-Eye OCT. Investig. Opthalmol. Vis. Sci. 2011, 52, 3604. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Two methods to trace retinal ganglion cells with fluorogold: From the intact optic nerve or by stereotactic injection into the optic tract. Exp. Eye Res. 2015, 131, 12–19. [Google Scholar] [CrossRef][Green Version]
- Sergeys, J.; Etienne, I.; Van Hove, I.; Lefevere, E.; Stalmans, I.; Feyen, J.H.M.; Moons, L.; Van Bergen, T. Longitudinal In Vivo Characterization of the Streptozotocin-Induced Diabetic Mouse Model: Focus on Early Inner Retinal Responses. Investig. Opthalmol. Vis. Sci. 2019, 60, 807. [Google Scholar] [CrossRef]
- Crish, S.D.; Sappington, R.M.; Inman, D.M.; Horner, P.J.; Calkins, D.J. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 5196–5201. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.; Li, C.; Do, Q.; Burke, E.G.; Heynderickx, S.; Farrow, K. A projection specific logic to sampling visual inputs in mouse superior colliculus. eLife 2019, 8. [Google Scholar] [CrossRef]
- Cuntz, H.; Forstner, F.; Borst, A.; Häusser, M. One Rule to Grow Them All: A General Theory of Neuronal Branching and Its Practical Application. PLoS Comput. Biol. 2010, 6, e1000877. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 11 April 2021).
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Tridico, F. Glaucoma: Biological Trabecular and Neuroretinal Pathology with Perspectives of Therapy Innovation and Preventive Diagnosis. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]

| Variable | Odds Ratio | CI95% | p-Value |
|---|---|---|---|
| IOP | 4.97 | 1.87–18.87 | 0.006 |
| Anterior chamber depth | 37.64 | 4.82–1462.22 | 0.009 |
| RBPMS+ RGC counts | 0.48 | 0.19–1.01 | 0.076 |
| STR | 0.41 | 0.19–0.78 | 0.010 |
| Variable | Odds Ratio | CI95% | p-Value |
|---|---|---|---|
| RBPMS+ RGC counts | 0.52 | 0.19–1.16 | 0.141 |
| STR | 0.35 | 0.12–0.80 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claes, M.; Santos, J.R.F.; Masin, L.; Cools, L.; Davis, B.M.; Arckens, L.; Farrow, K.; De Groef, L.; Moons, L. A Fair Assessment of Evaluation Tools for the Murine Microbead Occlusion Model of Glaucoma. Int. J. Mol. Sci. 2021, 22, 5633. https://doi.org/10.3390/ijms22115633
Claes M, Santos JRF, Masin L, Cools L, Davis BM, Arckens L, Farrow K, De Groef L, Moons L. A Fair Assessment of Evaluation Tools for the Murine Microbead Occlusion Model of Glaucoma. International Journal of Molecular Sciences. 2021; 22(11):5633. https://doi.org/10.3390/ijms22115633
Chicago/Turabian StyleClaes, Marie, Joana R. F. Santos, Luca Masin, Lien Cools, Benjamin M. Davis, Lutgarde Arckens, Karl Farrow, Lies De Groef, and Lieve Moons. 2021. "A Fair Assessment of Evaluation Tools for the Murine Microbead Occlusion Model of Glaucoma" International Journal of Molecular Sciences 22, no. 11: 5633. https://doi.org/10.3390/ijms22115633
APA StyleClaes, M., Santos, J. R. F., Masin, L., Cools, L., Davis, B. M., Arckens, L., Farrow, K., De Groef, L., & Moons, L. (2021). A Fair Assessment of Evaluation Tools for the Murine Microbead Occlusion Model of Glaucoma. International Journal of Molecular Sciences, 22(11), 5633. https://doi.org/10.3390/ijms22115633









