Structure–Function Relationship of Retinal Ganglion Cells in Multiple Sclerosis
Abstract
1. Introduction
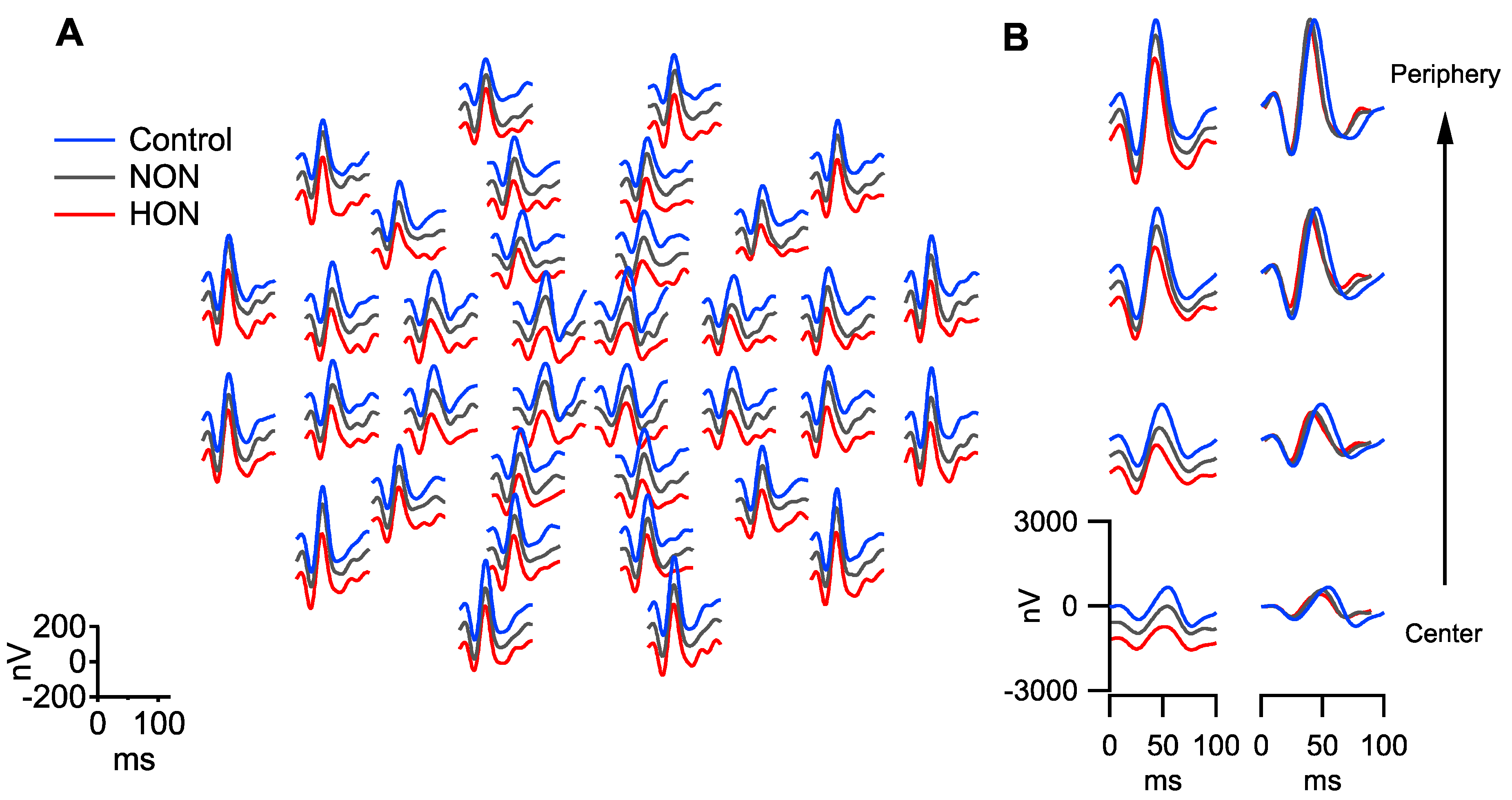
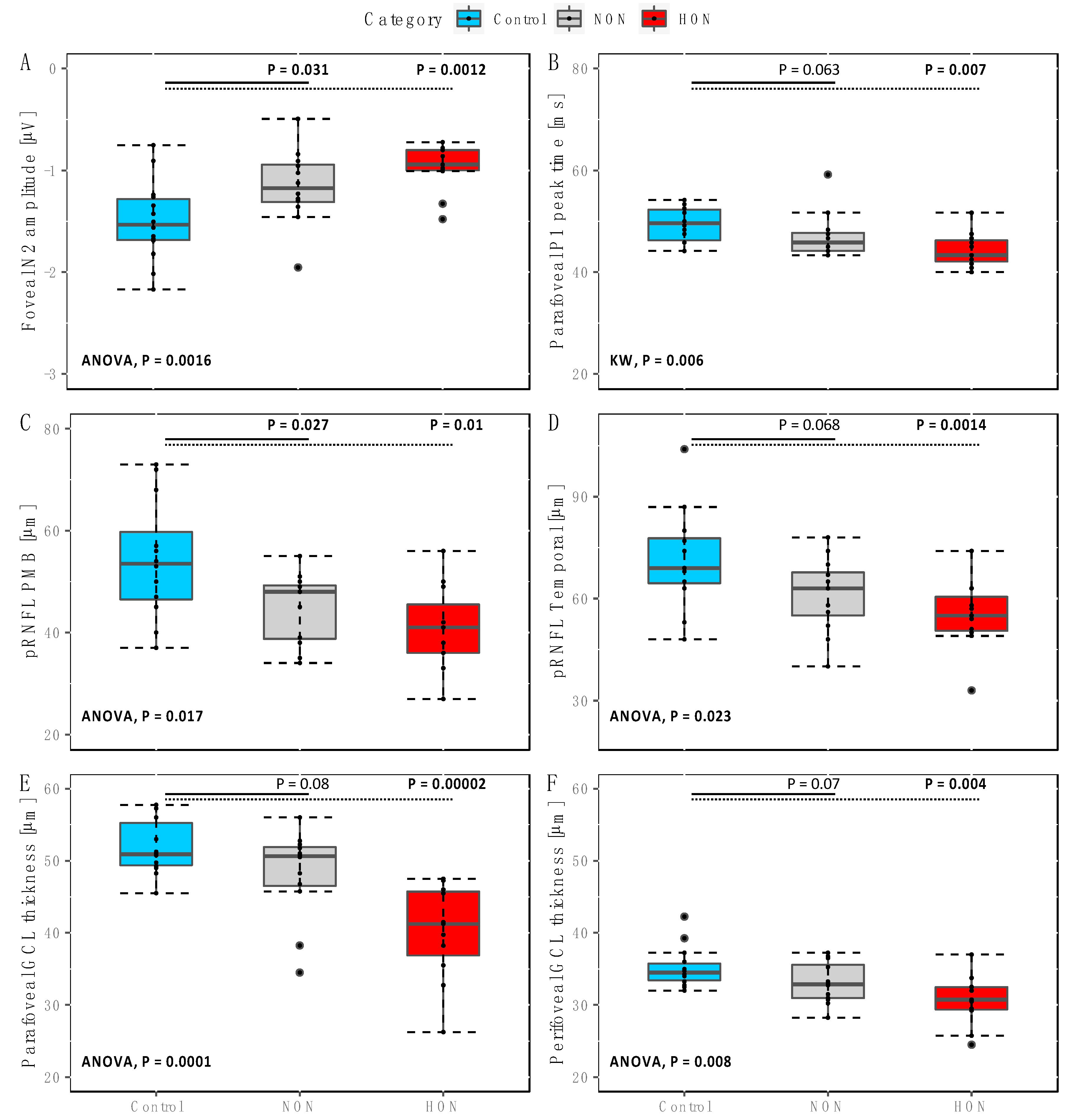
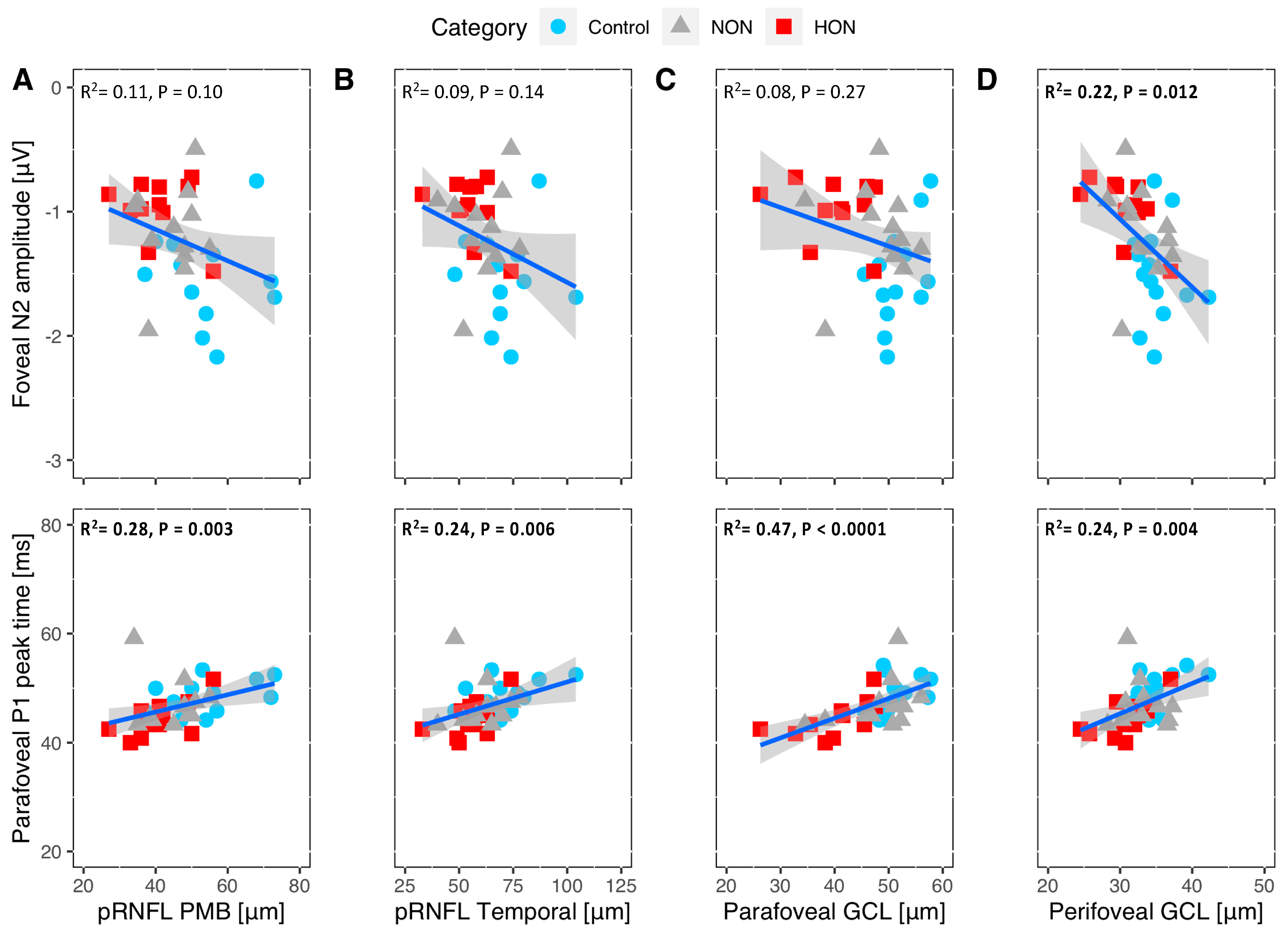
2. Results
2.1. Functional Changes in NON and HON
2.2. Structural Changes in NON and HON
2.3. Structural-Functional Correlation
3. Discussion
3.1. RGC Readout Using mfPERG-N2 vs. RGC and pRNFL Thickness
3.2. RGC Readout Using mfPERG-P1 vs. RGC and pRNFL Thickness
3.3. Outer Retinal Layers
3.4. Structure and Function Relationship of the RGC Measures
4. Materials and Methods
4.1. Participants
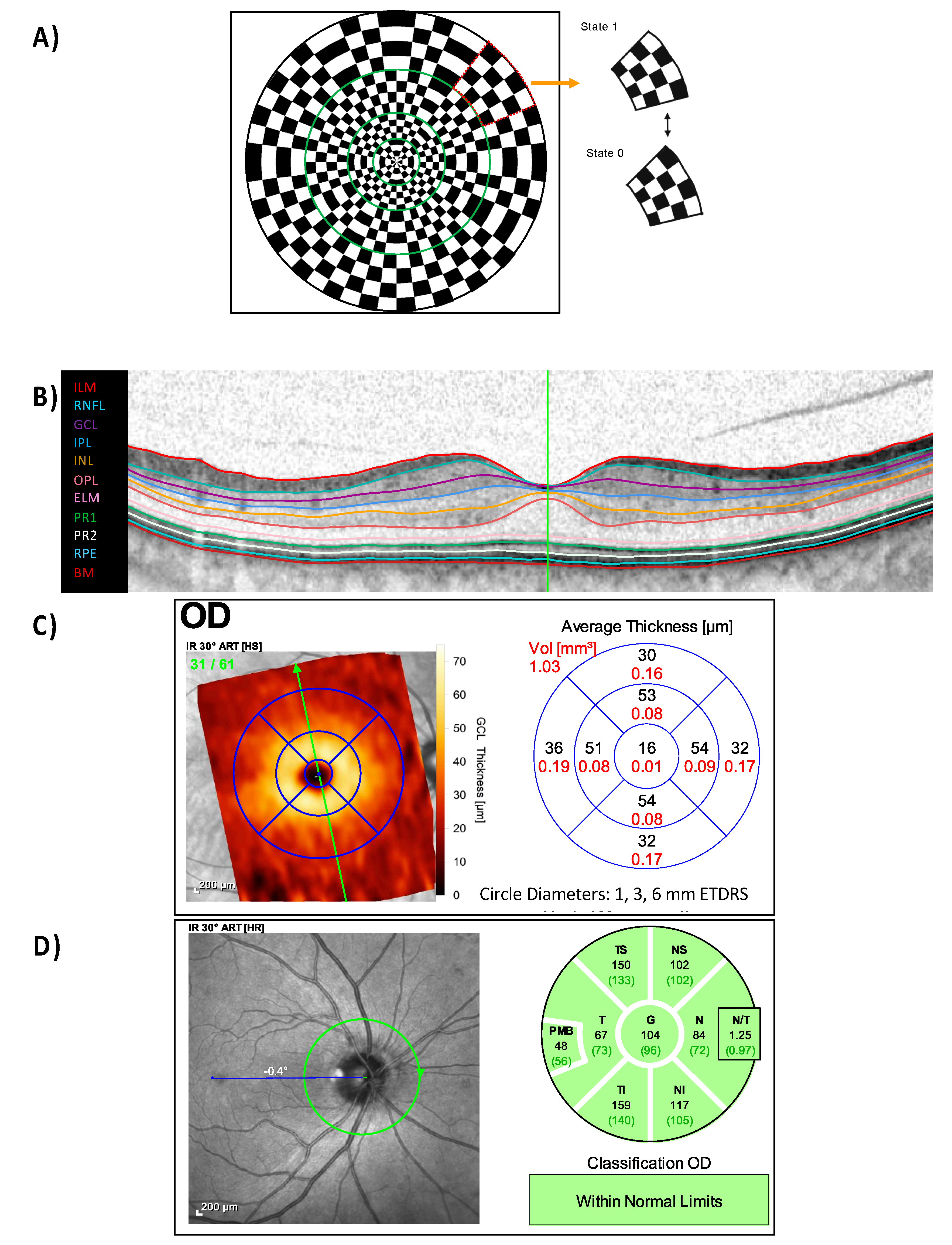
4.2. mfPERG stimuli, Procedure, Recordings and Analysis
4.3. Optical Coherence Tomography (OCT)
4.4. Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.G.; Feng, Y.F.; Xiang, Y.; Huang, J.H.; Savini, G.; Parisi, V.; Yang, W.J.; Fu, X.A. Retinal Nerve Fiber Layer Thickness Changes in Parkinson Disease: A Meta-Analysis. PLoS ONE 2014, 9, e85718. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Gonzalez-Moron, D.; Garcea, O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: A review. Mult. Scler. Relat. Disord. 2018, 22, 77–82. [Google Scholar] [CrossRef]
- McDonald, W.I.; Barnes, D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J. Neurol. Neurosurg. Psychiatry 1992, 55, 747–752. [Google Scholar] [CrossRef]
- Sriram, P.; Wang, C.; Yiannikas, C.; Garrick, R.; Barnett, M.; Parratt, J.; Graham, S.L.; Arvind, H.; Klistorner, A. Relationship between Optical Coherence Tomography and Electrophysiology of the Visual Pathway in Non-Optic Neuritis Eyes of Multiple Sclerosis Patients. PLoS ONE 2014, 9, e102546. [Google Scholar] [CrossRef]
- Petzold, A.; de Boer, J.F.; Schippling, S.; Vermersch, P.; Kardon, R.; Green, A.; Calabresi, P.A.; Polman, C. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 921–932. [Google Scholar] [CrossRef]
- Saidha, S.; Syc, S.B.; Ibrahim, M.A.; Eckstein, C.; Warner, C.V.; Farrell, S.K.; Oakley, J.D.; Durbin, M.K.; Meyer, S.A.; Balcer, L.J.; et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 2011, 134, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Wicki, C.A.; Hanson, J.V.M.; Schippling, S. Optical coherence tomography as a means to characterize visual pathway involvement in multiple sclerosis. Curr. Opin. Neurol. 2018, 31, 662–668. [Google Scholar] [CrossRef]
- Klistorner, A.; Sriram, P.; Vootakuru, N.; Wang, C.; Barnett, M.H.; Garrick, R.; Parratt, J.; Levin, N.; Raz, N.; Van der Walt, A.; et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology 2014, 82, 2165–2172. [Google Scholar] [CrossRef]
- Holder, G.E. The pattern electroretinogram in anterior visual pathway dysfunction and its relationship to the pattern visual evoked potential: A personal clinical review of 743 eyes. Eye 1997, 11, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Cuno, A.-K.; Hoffmann, M.B. Retinal conduction speed analysis reveals different origins of the P50 and N95 components of the (multifocal) pattern electroretinogram. Exp. Eye Res. 2018, 169, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Berninger, T.A.; Heider, W. Pattern electroretinograms in optic neuritis during the acute stage and after remission. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 228, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.E. The incidence of abnormal pattern electroretinography in optic nerve demyelination. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 18–26. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G. The uniform field and pattern ERG in macaques with experimental glaucoma: Removal of spiking activity. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2797–2810. [Google Scholar]
- Wang, J.; Cheng, H.; Hu, Y.-S.; Tang, R.A.; Frishman, L.J. The Photopic Negative Response of the Flash Electroretinogram in Multiple Sclerosis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1315–1323. [Google Scholar] [CrossRef]
- Nakamura, H.; Miyamoto, K.; Yokota, S.; Ogino, K.; Yoshimura, N. Focal macular photopic negative response in patients with optic neuritis. Eye 2011, 25, 358–364. [Google Scholar] [CrossRef][Green Version]
- Hanson, J.V.M.; Hediger, M.; Manogaran, P.; Landau, K.; Hagenbuch, N.; Schippling, S.; Gerth-Kahlert, C. Outer Retinal Dysfunction in the Absence of Structural Abnormalities in Multiple Sclerosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 549–560. [Google Scholar] [CrossRef]
- Filgueiras, T.G.; Oyamada, M.K.; Preti, R.C.; Apóstolos-Pereira, S.L.; Callegaro, D.; Monteiro, M.L.R. Outer Retinal Dysfunction on Multifocal Electroretinography May Help Differentiating Multiple Sclerosis From Neuromyelitis Optica Spectrum Disorder. Front. Neurol. 2019, 10, 928. [Google Scholar] [CrossRef]
- Hokazono, K.; Raza, A.S.; Oyamada, M.K.; Hood, D.C.; Monteiro, M.L.R. Pattern electroretinogram in neuromyelitis optica and multiple sclerosis with or without optic neuritis and its correlation with FD-OCT and perimetry. Doc. Ophthalmol. Adv. Ophthalmol. 2013, 127, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; You, Y.; Arunachalam, S.; Fontes, A.; Liu, S.; Gupta, V.; Parratt, J.; Wang, C.; Barnett, M.; Barton, J.; et al. Differing Structural and Functional Patterns of Optic Nerve Damage in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder. Ophthalmology 2019, 126, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.C.; Demirkaya, S.; Sobaci, G. Is Optical Coherence Tomography Really a New Biomarker Candidate in Multiple Sclerosis?—A Structural and Functional Evaluation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5773–5781. [Google Scholar] [CrossRef]
- Wilkins, L.W. Progressive inner nuclear layer dysfunction in non-optic neuritis eyes in MS. Neurol.—Neuroimmunol. Neuroinflamm. 2018, 5, e444. [Google Scholar] [CrossRef]
- Beykin, G.; Norcia, A.M.; Srinivasan, V.J.; Dubra, A.; Goldberg, J.L. Discovery and clinical translation of novel glaucoma biomarkers. Prog. Retin. Eye Res. 2021, 80, 100875. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Horbrügger, M.; Loewe, K.; Kaufmann, J.; Opfer, R.; Wagner, M.; Al-Nosairy, K.O.; Meuth, S.G.; Hoffmann, M.B.; Schippling, S. MS optic neuritis-induced long-term structural changes within the visual pathway. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e665. [Google Scholar] [CrossRef] [PubMed]
- Scheel, M.; Finke, C.; Oberwahrenbrock, T.; Freing, A.; Pech, L.-M.; Schlichting, J.; Sömmer, C.; Wuerfel, J.; Paul, F.; Brandt, A.U. Retinal nerve fibre layer thickness correlates with brain white matter damage in multiple sclerosis: A combined optical coherence tomography and diffusion tensor imaging study. Mult. Scler. (Houndmills Basingstoke Engl.) 2014, 20, 1904–1907. [Google Scholar] [CrossRef]
- Klistorner, A.; Arvind, H.; Nguyen, T.; Garrick, R.; Paine, M.; Graham, S.; Yiannikas, C. Fellow eye changes in optic neuritis correlate with the risk of multiple sclerosis. Mult. Scler. (Houndmills Basingstoke Engl.) 2009, 15, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Klistorner, A.; Graham, E.C.; Yiannikas, C.; Barnett, M.; Parratt, J.; Garrick, R.; Wang, C.; You, Y.; Graham, S.L. Progression of retinal ganglion cell loss in multiple sclerosis is associated with new lesions in the optic radiations. Eur. J. Neurol. 2017, 24, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Oberwahrenbrock, T.; Ringelstein, M.; Jentschke, S.; Deuschle, K.; Klumbies, K.; Bellmann-Strobl, J.; Harmel, J.; Ruprecht, K.; Schippling, S.; Hartung, H.-P.; et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult. Scler. (Houndmills Basingstoke Engl.) 2013, 19, 1887–1895. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Hernandez, E.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Physiological significance of steady-state PERG losses in glaucoma: Clues from simulation of abnormalities in normal subjects. J. Glaucoma 2009, 18, 535–542. [Google Scholar] [CrossRef]
- Kreuz, A.C.; de Moraes, C.G.; Hatanaka, M.; Oyamada, M.K.; Monteiro, M.L.R. Macular and Multifocal PERG and FD-OCT in Preperimetric and Hemifield Loss Glaucoma. J. Glaucoma 2018, 27, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ganekal, S.; Dorairaj, S.; Jhanji, V. Pattern Electroretinography Changes in Patients with Established or Suspected Primary Open Angle Glaucoma. J. Curr. Glaucoma Pract. 2013, 7, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Frishman, L.J.; Saszik, S.; Viswanathan, S. Retinal Origins of the Primate Multifocal ERG: Implications for the Human Response. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1673–1685. [Google Scholar]
- Ikeda, H.; Franchi, A.; Turner, G.; Shilling, J.; Graham, E. Electroretinography and electro-oculography to localize abnormalities in early-stage inflammatory eye disease. Doc. Ophthalmol. 1989, 73, 387–394. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Hoffmann, M.B.; Flechner, J.-J. Slow pattern-reversal stimulation facilitates the assessment of retinal function with multifocal recordings. Clin. Neurophysiol. 2008, 119, 409–417. [Google Scholar] [CrossRef]
- Dawson, W.W.; Trick, G.L.; Litzkow, C.A. Improved electrode for electroretinography. Investig. Ophthalmol. Vis. Sci. 1979, 18, 988–991. [Google Scholar]
- R Core Team. R: The R Project for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.r-project.org/ (accessed on 21 March 2021).
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
| Control (N = 14) | NON (N = 12) | HON (N = 11) | p-Value | |
|---|---|---|---|---|
| Age (y) | 41.0 [12.6] (20–60) | 42.0 [10.9] (27–61) | 39.0 [9.8] (25–52) | 0.803 |
| Female N (%) | 7 [50] | 8 [66.7] | 8 [72.7] | 0.384 |
| Disease duration (y) | - | 6.4 [4.6] (1–13) | 10.3 [8.0] (1–26) | 0.179 |
| BCVA (logMAR) | −0.02 (−0.1–0.00) | −0.02 (−0.1–0.1) | 0.00 (−0.1–0.1) | 0.54 |
| Median EDSS | - | 2.0 (1–7) | 1.5 (0–4.5) | 0.332 |
| N1 | P1 | N2 | |||||
| Amplitude (µV) | Group | Mean [SD] | ANOVA F(2,34) | Mean [SD] | ANOVA F(2,34) | Mean [SD] | ANOVA F(2,34) |
| Summed | Control | −5.36 [1.38] | 1.2 | 11.96 [3.05] | 0.8 | −10.55 [2.48] | 1.2 |
| NON | −5.05 [1.6] | 11.46 [3.73] | −9.82 [3.26] | ||||
| HON | −4.5 [1.08] | 10.44 [2.29] | −8.86 [2.16] | ||||
| Ring 1 | Control | −0.5 [0.16] | 2 | 1.24 [0.37] | 3.6 | −1.5 [0.39] | 7.8 ** |
| NON | −0.41 [0.11] | 1.10 [0.24] | −1.16 [0.36] | ||||
| HON | −0.4 [0.16] | 0.92 [0.23] | −0.97 [0.24] | ||||
| Ring 2 | Control | −1.05 [0.28] | 1.3 | −2.06 [0.44] | 2.4 | −2.06 [0.44] | 4 |
| NON | −1.01 [0.38] | −1.77 [0.64] | −1.77 [0.64] | ||||
| HON | −0.86 [0.24] | −1.51 [0.33] | −1.51 [0.33] | ||||
| Ring 3 | Control | −1.84 [0.57] | 2.3 | 4.09 [1.0] | 1.4 | −3.4 [0.68] | 2.2 |
| NON | −1.69 [0.53] | 3.89 [1.34] | −3.21 [1.15] | ||||
| HON | −1.40 [0.41] | 3.4 [0.67] | −2.7 [0.59] | ||||
| Ring 4 | Control | −2.02 [0.63] | 0.2 | 5.02 [1.49] | 0.2 | −4.52 [1.24] | 0.3 |
| NON | −2.00 [0.69] | 4.94 [1.58] | −4.35 [1.3] | ||||
| HON | −1.88 [0.53] | 4.62 [1.27] | −4.1 [1.29] | ||||
| Peak Time (ms) | Group | Median (Range) | KW | Median (Range) | KW | Median (Range) | KW |
| Summed | Control | 25 (5.0) | 4.9 | 45.42 (8.33) | 5 | 73.33 (9.17) | 0.1 |
| NON | 25 (7.5) | 43.33 (13.33) | 72.92 (10.0) | ||||
| HON | 24.17 (4.17) | 42.5 (6.67) | 73.33 (6.67) | ||||
| Ring 1 | Control | 27.08 (10.83) | 5.3 | 54.58 (11.67) | 3.2 | 75.83 (18.33) | 0.4 |
| NON | 25.83 (16.67) | 53.33 (19.17) | 75.42 (15.83) | ||||
| HON | 25.83 (9.17) | 52.5 (13.33) | 76.67 (16.67) | ||||
| Ring 2 | Control | 25.83 (6.67) | 7.8 | 49.58 (10.0) | 10.2 * | 73.33 (19.17) | 0.7 |
| NON | 25.42 (14.17) | 45.83 (15.83) | 72.92 (10.0) | ||||
| HON | 24.17 (4.17) | 43.33 (11.67) | 72.5 (20.0) | ||||
| Ring 3 | Control | 25 (5.0) | 5.9 | 44.58 (8.33) | 6.6 | 72.92 (15.83) | 0.4 |
| NON | 24.58 (7.5) | 43.33 (11.67) | 71.67 (15.0) | ||||
| HON | 23.33 (5.0) | 41.67 (8.33) | 73.33 (22.5) | ||||
| Ring 4 | Control | 25 (5.0) | 3 | 43.33 (9.17) | 2.4 | 70.42 (20.83) | 1.4 |
| NON | 24.17 (5.0) | 42.5 (9.17) | 70.83 (14.17) | ||||
| HON | 24.17 (4.17) | 41.67 (7.5) | 72.5 (11.67) |
| Macula | Center | Parafoveal | Perifoveal | ||||
| Thickness (µm) | Group | Mean [SD] | ANOVA F(2,34) | Mean [SD] | ANOVA F(2,34) | Mean [SD] | ANOVA F(2,34) |
| Total | Control | 279.71 [25.63] | 0.2 | 344.43 [13.55] | 3.8 | 298.89 [9.66] | 1.4 |
| NON | 282.67 [27.77] | 337.48 [18.45] | 293.52 [13.32] | ||||
| HON | 286.73 [28.63] | 326.32 [17.1] | 290.75 [13.94] | ||||
| mRNFL | Control | 12.79 [2.75] | 0.3 | 21.95 [1.58] | 0.1 | 36.14 [2.65] | 3 |
| NON | 12.33 [3.06] | 22.04 [2.39] | 36.54 [5.9] | ||||
| HON | 13.18 [2.79] | 21.64 [2.13] | 32.05 [5.86] | ||||
| GCL | Control | 16.00 [5.11] | 0.2 | 51.75 [3.7] | 13.8 *** | 35.16 [2.8] | 6.5 ** |
| NON | 17.00 [8.5] | 48.21 [6.2] | 33.04 [2.9] | ||||
| HON | 18.09 [11.17] | 40.14 [6.7] | 30.73 [3.5] | ||||
| IPL | Control | 21.43 [4.3] | 0.2 | 41.84 [2.3] | 8.2 ** | 28.88 [1.9] | 3 |
| NON | 22.92 [6.9] | 39.46 [4.3] | 27.38 [2.8] | ||||
| HON | 22.73 [6.4] | 36.16 [3.8] | 26.55 [2.6] | ||||
| Control | 18.71 [4.63] | 0.2 | 46.79 [2.9] | 12 *** | 32.02 [2.32] | 4.9 * | |
| GCIPL | NON | 19.96 [7.65] | 43.83 [5.2] | 30.21 [2.76] | |||
| HON | 20.41 [8.72] | 38.15 [5.04] | 28.64 [3.02] | ||||
| INL | Control | 20.29 [6.91] | 1.3 | 40.82 [4.02] | 0.4 | 32.52 [1.88] | 0.4 |
| NON | 21.42 [7.33] | 39.6 [2.98] | 32.06 [2.66] | ||||
| HON | 25.64 [10.85] | 40.2 [2.4] | 32.84 [1.94] | ||||
| ONL | Control | 92.71 [8.84] | 0.4 | 71.25 [5.83] | 0.6 | 59.95 [5.37] | 1.6 |
| NON | 94.5 [15.22] | 68.23 [8.43] | 56.19 [5.74] | ||||
| HON | 89.18 [19.95] | 70.0 [6.16] | 58.25 [4.63] | ||||
| ORL | Control | 90.86 [2.28] | 0.5 | 81.77 [1.25] | 2.3 | 78.21 [1.28] | 2.7 |
| NON | 91.17 [3.35] | 83.38 [2.56] | 79.69 [1.75] | ||||
| HON | 89.55 [6.01] | 82.91 [2.0] | 79.48 [2.28] | ||||
| Optic disc | G | PMB | T | ||||
| Group | Mean [SD] | F(2,32) | Mean [SD] | F(2,32) | Mean [SD] | F(2,32) | |
| pRNFL | Control | 94.64 [5.84] | 2.4 | 54.33 [11.77] | 6.5 * | 71.42 [14.89] | 5.2 * |
| NON | 92.92 [11.41] | 45.0 [6.81] | 61.17 [10.97] | ||||
| HON | 86.18 [12.02] | 40.82 [8.3] | 55.18 [10.31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nosairy, K.O.; Horbrügger, M.; Schippling, S.; Wagner, M.; Haghikia, A.; Pawlitzki, M.; Hoffmann, M.B. Structure–Function Relationship of Retinal Ganglion Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 3419. https://doi.org/10.3390/ijms22073419
Al-Nosairy KO, Horbrügger M, Schippling S, Wagner M, Haghikia A, Pawlitzki M, Hoffmann MB. Structure–Function Relationship of Retinal Ganglion Cells in Multiple Sclerosis. International Journal of Molecular Sciences. 2021; 22(7):3419. https://doi.org/10.3390/ijms22073419
Chicago/Turabian StyleAl-Nosairy, Khaldoon O., Marc Horbrügger, Sven Schippling, Markus Wagner, Aiden Haghikia, Marc Pawlitzki, and Michael B. Hoffmann. 2021. "Structure–Function Relationship of Retinal Ganglion Cells in Multiple Sclerosis" International Journal of Molecular Sciences 22, no. 7: 3419. https://doi.org/10.3390/ijms22073419
APA StyleAl-Nosairy, K. O., Horbrügger, M., Schippling, S., Wagner, M., Haghikia, A., Pawlitzki, M., & Hoffmann, M. B. (2021). Structure–Function Relationship of Retinal Ganglion Cells in Multiple Sclerosis. International Journal of Molecular Sciences, 22(7), 3419. https://doi.org/10.3390/ijms22073419







