Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status
Abstract
1. Introduction
2. Molecular Targets of Tumour Angiogenesis
3. Indirect Targeting of Angiogenesis
3.1. [18F]-FDG
3.2. Hypoxia
3.3. Matrix Metalloproteinases (MMPs)
3.4. Prostate Specific Membrane Antigen (PSMA)
4. Direct Targeting of Angiogenesis
4.1. VEGF
4.2. Integrins
4.2.1. Fibronectin
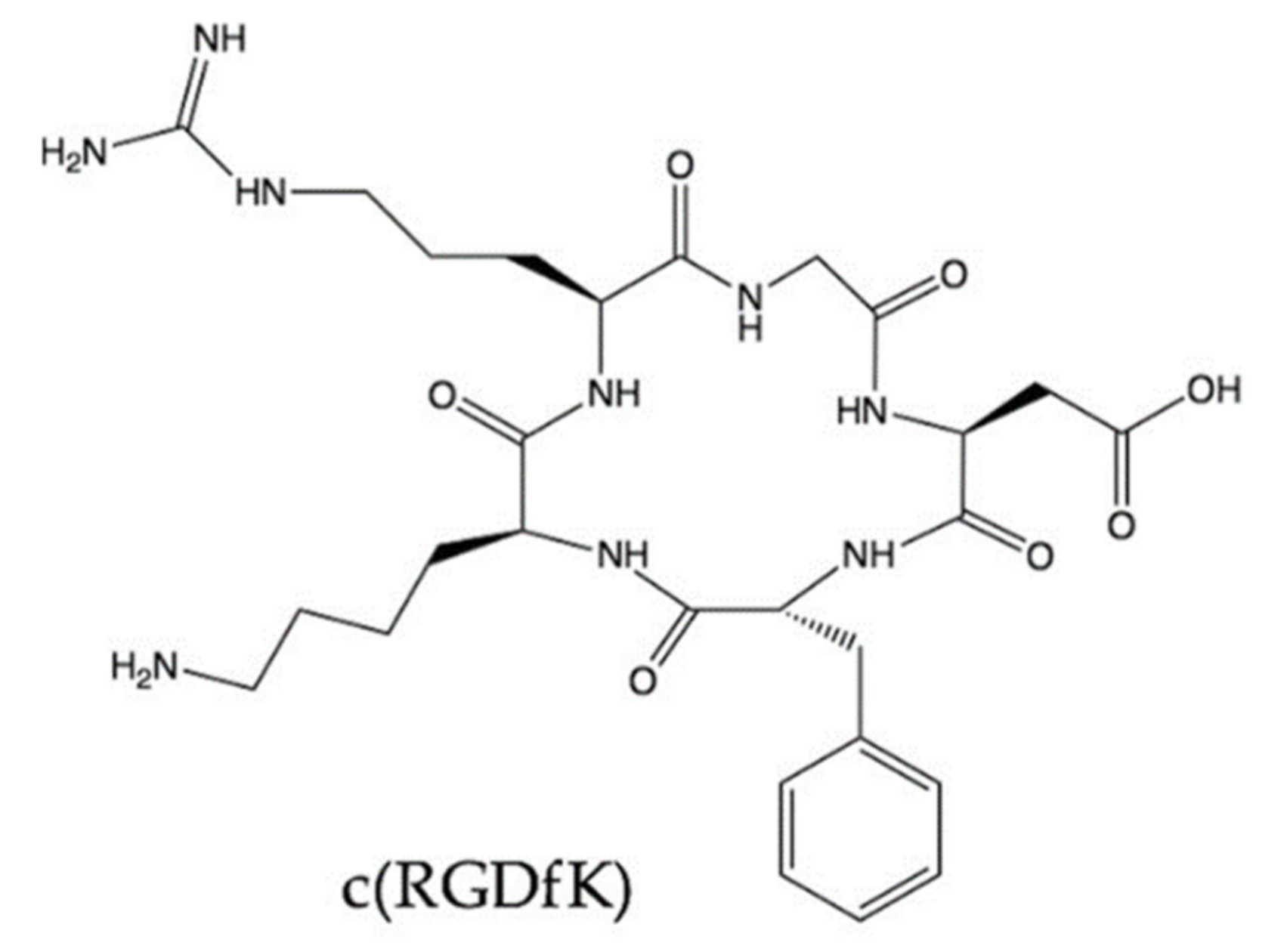
4.2.2. RGD-Motif
4.3. NGR
5. Beyond Imaging
6. Materials and Methods
- Selected reviews: PET imaging angiogenesis (353 results) and SPECT imaging angiogenesis (169 results).
- Original research: PET NGR (15 results), SPECT NGR (7 results), PET VEGF (413 results), SPECT VEGF (153 results), PET RGD (438 results), SPECT RGD (138 results), SPECT EGF (81 results), PET EGF (79 results), PET Fibroblast growth factor (132 results), SPECT Fibroblast growth factor (42 results), PET PDGF (39 results), SPECT PDGF (6 results), SPECT Angiopoietin-1 (3 results), PET Angiopoietin-1 (5 results), SPECT ephrin (2 results), PET ephrin (12 results), PET MMP angiogenesis (14 results) and SPECT MMP angiogenesis (6 results).
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Zetter, B.R. Angiogenesis and tumor metastasis. Annu. Rev. Med. 1998, 49, 407–424. [Google Scholar] [CrossRef]
- Chandra, A.; Rick, J.; Yagnik, G.; Aghi, M.K. Autophagy as a mechanism for anti-angiogenic therapy resistance. Semin. Cancer Biol. 2020, 66, 75–88. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Moawad, A.W.; Szklaruk, J.; Lall, C.; Blair, K.J.; O Kaseb, A.; Kamath, A.; A Rohren, S.; Elsayes, K.M. Angiogenesis in Hepatocellular Carcinoma; Pathophysiology, Targeted Therapy, and Role of Imaging. J. Hepatocell. Carcinoma 2020, 7, 77–89. [Google Scholar] [CrossRef]
- Reichardt, W.; von Elverfeldt, D. Preclinical Applications of Magnetic Resonance Imaging in Oncology. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2020; Volume 216, pp. 405–437. [Google Scholar]
- Nabavizadeh, S.A.; Ware, J.B.; Wolf, R.L. Emerging Techniques in Imaging of Glioma Microenvironment. Top. Magn. Reson. Imaging 2020, 29, 103–114. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nat. Cell Biol. 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Yunus, M.; Jansson, P.J.; Kovacevic, Z.; Kalinowski, D.S.; Richardson, D.R. Tumor-induced neoangiogenesis and receptor tyrosine kinases—Mechanisms and strategies for acquired resistance. Biochim. Biophys. Acta (BBA) - Gen. Subj. 2019, 1863, 1217–1225. [Google Scholar] [CrossRef]
- Loizzi, V.; Del Vecchio, V.; Gargano, G.; De Liso, M.; Kardashi, A.; Naglieri, E.; Resta, L.; Cicinelli, E.; Cormio, G. Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1967. [Google Scholar] [CrossRef]
- Adair, T.H.; Montani, J. Angiogenesis. Morgan Claypool Life Sci. 2010. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Heal. Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Ausprunk, D.H.; Folkman, J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 1977, 14, 53–65. [Google Scholar] [CrossRef]
- Döme, B.; Hendrix, M.J.; Paku, S.; Tóvári, J.; Tímár, J. Alternative Vascularization Mechanisms in Cancer: Pathology and Therapeutic Implications. Am. J. Pathol. 2007, 170, 1–15. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Brown, L.F.; Detmar, M.; Claffey, K.; Nagy, J.A.; Feng, D.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor:A multifunctional angiogenic cytokine. Galanin 1997, 79, 233–269. [Google Scholar] [CrossRef]
- Lopes, S.I.L.; Ferreira, S.; Caetano, M. PET/CT in the evaluation of hypoxia for radiotherapy planning in head and neck tumors: Systematic literature review. J. Nucl. Med. Technol. 2020, 120249540. [Google Scholar] [CrossRef]
- Lee, S.; Muralidharan, V.; Tebbutt, N.; Wong, P.; Fang, C.; Liu, Z.; Gan, H.; Sachinidis, J.; Pathmaraj, K.; Christophi, C.; et al. Prevalence of hypoxia and correlation with glycolytic metabolism and angiogenic biomarkers in metastatic colorectal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1585–1592. [Google Scholar] [CrossRef]
- Troost, E.G.; Laverman, P.; Kaanders, J.H.; Oyen, W.J.; Boerman, O.C.; Bussink, J. Intratumoral Spatial Distribution of Hypoxia and Angiogenesis Assessed by 18F-FAZA and 125I-Gluco-RGD Autoradiography. J. Nucl. Med. 2008, 49, 1732. [Google Scholar] [CrossRef][Green Version]
- Saksø, M.; Mortensen, L.S.; Primdahl, H.; Johansen, J.; Kallehauge, J.; Hansen, C.R.; Overgaard, J. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial (NCT01017224). Radiother. Oncol. 2020, 151, 126–133. [Google Scholar] [CrossRef]
- Furumoto, S.; Takashima, K.; Kubota, K.; Ido, T.; Iwata, R.; Fukuda, H. Tumor detection using 18F-labeled matrix metalloproteinase-2 inhibitor. Nucl. Med. Biol. 2003, 30, 119–125. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, D.; Cheng, C.; Zhang, A.; Ma, C.; Wang, L.; Zhang, D.; Liu, H.; Jiang, H.; Wang, T.; et al. Targeting of MMP2 activity in malignant tumors with a 68 Ga-labeled gelatinase inhibitor cyclic peptide. Nucl. Med. Biol. 2015, 42, 939–944. [Google Scholar] [CrossRef]
- Li, S.; Peck-Radosavljevic, M.; Kienast, O.; Preitfellner, J.; Hamilton, G.; Kurtaran, A.; Pirich, C.; Angelberger, P.; Dudczak, R. Imaging gastrointestinal tumours using vascular endothelial growth factor-165 (VEGF165) receptor scintigraphy. Ann. Oncol. 2003, 14, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, W.; Chen, K.; Li, Z.-B.; Kashefi, A.; He, L.; Chen, X. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2001–2010. [Google Scholar] [CrossRef]
- Backer, M.V.; Levashova, Z.; Patel, V.; Jehning, B.T.; Claffey, K.; Blankenberg, F.G.; Backer, J.M. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat. Med. 2007, 13, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, W.B.; De Vries, E.G.; Hospers, G.A.; Mulder, N.H.; De Jong, J.R.; Hollema, H.; Brouwers, A.H.; Van Dongen, G.A.; Perk, L.R.; Hooge, M.N.L.-D. In Vivo VEGF Imaging with Radiolabeled Bevacizumab in a Human Ovarian Tumor Xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Desar, I.M.; Stillebroer, A.B.; Oosterwijk, E.; Leenders, W.P.; Van Herpen, C.M.; Van Der Graaf, W.T.; Boerman, O.C.; Mulders, P.F.; Oyen, W.J.G. 111In-Bevacizumab Imaging of Renal Cell Cancer and Evaluation of Neoadjuvant Treatment with the Vascular Endothelial Growth Factor Receptor Inhibitor Sorafenib. J. Nucl. Med. 2010, 51, 1707–1715. [Google Scholar] [CrossRef]
- Berndorff, D.; Borkowski, S.; Moosmayer, D.; Viti, F.; Müller-Tiemann, B.; Sieger, S.; Friebe, M.; Hilger, C.S.; Zardi, L.; Neri, D.; et al. Imaging of tumor angiogenesis using 99mTc-labeled human recombinant anti-ED-B fibronectin antibody fragments. J. Nucl. Med. 2006, 47, 1707–1716. [Google Scholar]
- Santimaria, M.; Moscatelli, G.; Viale, G.L.; Giovannoni, L.; Neri, G.; Viti, F.; Leprini, A.; Borsi, L.; Castellani, P.; Zardi, L.; et al. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin. Cancer Res. 2003, 9, 571–579. [Google Scholar]
- Tijink, B.M.; Perk, L.R.; Budde, M.; Walsum, M.S.-V.; Visser, G.W.M.; Kloet, R.W.; Dinkelborg, L.M.; Leemans, C.R.; Neri, D.; Van Dongen, G.A.M.S. 124I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of 131I-L19-SIP radioimmunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Berndorff, D.; Friebe, M.; Dinkelborg, L.M.; Welch, M.J. Small-Animal PET of Tumor Angiogenesis Using a 76Br-Labeled Human Recombinant Antibody Fragment to the ED-B Domain of Fibronectin. J. Nucl. Med. 2007, 48, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.J.; Haubner, R.; Sarbia, M.; Goebel, M.; Luderschmidt, S.; Grosu, A.L.; Schnell, O.; Niemeyer, M.; Kessler, H.; Wester, H.-J.; et al. Positron Emission Tomography Using [18F]Galacto-RGD Identifies the Level of Integrin αvβ3 Expression in Man. Clin. Cancer Res. 2006, 12, 3942–3949. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Valls, P.O.; Inglese, M.; Dubash, S.; Chen, M.; Gabra, H.; Montes, A.; Challapalli, A.; Arshad, M.; Tharakan, G.; et al. [18F]Fluciclatide PET as a biomarker of response to combination therapy of pazopanib and paclitaxel in platinum-resistant/refractory ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; Kolb, H.C.; Zhang, J.J.; Bélanger, M.-J.; Stubbs, J.B.; Stabin, M.G.; Hostetler, E.D.; Alpaugh, R.K.; Von Mehren, M.; Walsh, J.C.; et al. Biodistribution and Radiation Dosimetry of the Integrin Marker 18F-RGD-K5 Determined from Whole-Body PET/CT in Monkeys and Humans. J. Nucl. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Park, R.; Hou, Y.; Khankaldyyan, V.; Gonzales-Gomez, I.; Tohme, M.; Bading, J.R.; Laug, W.E.; Conti, P.S. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1081–1089. [Google Scholar] [CrossRef]
- Durante, S.; Dunet, V.; Gorostidi, F.; Mitsakis, P.; Schaefer, N.; Delage, J.; Prior, J.O. Head and neck tumors angiogenesis imaging with 68Ga-NODAGA-RGD in comparison to 18F-FDG PET/CT: A pilot study. EJNMMI Res. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ma, W.; Wang, Z.; Yang, W.; Ma, X.; Kang, F.; Wang, J. Biodistribution and SPECT Imaging Study of99mTc Labeling NGR Peptide in Nude Mice Bearing Human HepG2 Hepatoma. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Kis, A.; Szabó, J.P.; Dénes, N.; Vágner, A.; Nagy, G.; Garai, I.; Fekete, A.; Szikra, D.; Hajdu, I.; Matolay, O.; et al. In Vivo Imaging of Hypoxia and Neoangiogenesis in Experimental Syngeneic Hepatocellular Carcinoma Tumor Model Using Positron Emission Tomography. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Zong, S.; Wang, J.; Conti, P.S.; Chen, K. MicroPET Imaging of CD13 Expression Using a 64Cu-Labeled Dimeric NGR Peptide Based on Sarcophagine Cage. Mol. Pharm. 2014, 11, 3938–3946. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Duro-Castano, A.; Gallon, E.; Decker, C.; Vicent, M.J. Modulating angiogenesis with integrin-targeted nanomedicines. Adv. Drug Deliv. Rev. 2017, 119, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Brooks, P.; Clark, R.; Cheresh, D.; Huang, A.; Golumbek, P.; Ahmadzadeh, M.; Jaffee, E.; Pardoll, D.; Levitsky, H. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef]
- Quail, D.F.; A Joyce, J. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial–mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial–mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. OncoTargets Ther. 2017, 10, 4719–4729. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Busk, M.; Overgaard, J.; Horsman, M.R. Imaging of Tumor Hypoxia for Radiotherapy: Current Status and Future Directions. Semin. Nucl. Med. 2020, 50, 562–583. [Google Scholar] [CrossRef]
- Pedersen, M.W.; Holm, S.; Lund, E.L.; Hojgaard, L.; Kristjansen, P.E. Coregulation of Glucose Uptake and Vascular Endothelial Growth Factor (VEGF) in Two Small-Cell Lung Cancer (SCLC) Sublines In Vivo and In Vitro. Neoplasia 2001, 3, 80–87. [Google Scholar] [CrossRef]
- Airley, R.E.; Mobasheri, A. Hypoxic Regulation of Glucose Transport, Anaerobic Metabolism and Angiogenesis in Cancer: Novel Pathways and Targets for Anticancer Therapeutics. Chemotherapy 2007, 53, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, P.; Noto, B.; Avramovic, N.; Grubert, L.S.; Huss, S.; Bögemann, M.; Stegger, L.; Weckesser, M.; Schäfers, M.; Rahbar, K. Targeting PSMA by radioligands in non-prostate disease—current status and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Mirus, M.; Tokalov, S.V.; Abramyuk, A.; Heinold, J.; Prochnow, V.; Zöphel, K.; Kotzerke, J.; Abolmaali, N. Noninvasive assessment and quantification of tumor vascularization using [18F]FDG-PET/CT and CE-CT in a tumor model with modifiable angiogenesis—An animal experimental prospective cohort study. EJNMMI Res. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.G.; Koczan, D.; Klippel, S.; Pan, L.; Cheng, C.; Willis, S.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Impact of Angiogenesis-Related Gene Expression on the Tracer Kinetics of 18F-FDG in Colorectal Tumors. J. Nucl. Med. 2008, 49, 1238–1244. [Google Scholar] [CrossRef]
- Groves, A.M.; Shastry, M.; Rodriguez-Justo, M.; Malhotra, A.; Endozo, R.; Davidson, T.; Kelleher, T.; Miles, K.A.; Ell, P.J.; Keshtgar, M.R. 18F-FDG PET and biomarkers for tumour angiogenesis in early breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 38, 46–52. [Google Scholar] [CrossRef]
- Provost, C.; Rozenblum-Beddok, L.; Nataf, V.; Merabtene, F.; Prignon, A.; Talbot, J.-N. [68Ga]RGD Versus [18F]FDG PET Imaging in Monitoring Treatment Response of a Mouse Model of Human Glioblastoma Tumor with Bevacizumab and/or Temozolomide. Mol. Imaging Biol. 2019, 21, 297–305. [Google Scholar] [CrossRef]
- Toriihara, A.; Duan, H.; Thompson, H.M.; Park, S.; Hatami, N.; Baratto, L.; Fan, A.C.; Iagaru, A. 18F-FPPRGD2 PET/CT in patients with metastatic renal cell cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1518–1523. [Google Scholar] [CrossRef]
- Vatsa, R.; Ashwathanarayana, A.G.; Singh, G.; Kavanal, A.J.; Kumar, S.; Rana, N.; Shukla, J.; Mittal, B.R. A Comparison of Angiogenesis and Glycolytic Imaging in Patients With Clinical Suspected Locally Advanced Breast Cancer. Clin. Nucl. Med. 2019, 44, e479–e483. [Google Scholar] [CrossRef]
- Guo, J.; Higashi, K.; Ueda, Y.; Oguchi, M.; Takegami, T.; Toga, H.; Sakuma, T.; Yokota, H.; Katsuda, S.; Tonami, H.; et al. Microvessel density: Correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J. Nucl. Med. 2006, 47, 419–425. [Google Scholar]
- Colavolpe, C.; Chinot, O.; Metellus, P.; Mancini, J.; Barrie, M.; Bequet-Boucard, C.; Tabouret, E.; Mundler, O.; Figarella-Branger, D.; Guedj, E. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro-Oncology 2012, 14, 649–657. [Google Scholar] [CrossRef]
- De Bruyne, S.; Van Damme, N.; Smeets, P.A.M.; Ferdinande, L.; Ceelen, W.; Mertens, J.C.; Van De Wiele, C.; Troisi, R.; Libbrecht, L.; Laurent, S.; et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br. J. Cancer 2012, 106, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Cho, A.; Yun, M.; Choi, Y.D.; Rha, S.Y.; Kang, W.J. Prognostic Value of Pretreatment Metabolic Tumor Volume and Total Lesion Glycolysis Using 18F-FDG PET/CT in Patients With Metastatic Renal Cell Carcinoma Treated With Anti–Vascular Endothelial Growth Factor–Targeted Agents. Clin. Nucl. Med. 2017, 42, e235–e241. [Google Scholar] [CrossRef]
- Rajendran, J.G.; Krohn, K.A. Imaging hypoxia and angiogenesis in tumors. Radiol. Clin. North Am. 2005, 43, 169–187. [Google Scholar] [CrossRef]
- Lee, S.T.; Scott, A.M. Hypoxia Positron Emission Tomography Imaging With 18F-Fluoromisonidazole. Semin. Nucl. Med. 2007, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.G.; Krohn, K.A. F-18 Fluoromisonidazole for Imaging Tumor Hypoxia: Imaging the Microenvironment for Personalized Cancer Therapy. Semin. Nucl. Med. 2015, 45, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Saeki, T.; Osaki, A.; Yamane, T.; Kuji, I. Bevacizumab Induces Acute Hypoxia and Cancer Progression in Patients with Refractory Breast Cancer: Multimodal Functional Imaging and Multiplex Cytokine Analysis. Clin. Cancer Res. 2017, 23, 5769–5778. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.-M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Machein, M.R.; Plate, K.H. VEGF in Brain Tumors. J. Neuro-Oncol. 2000, 50, 109–120. [Google Scholar] [CrossRef]
- Parliament, M.B.; Allalunis-Turner, M.J.; Franko, A.J.; Olive, P.L.; Mandyam, R.; Santos, C.; Wolokoff, B. Vascular endothelial growth factor expression is independent of hypoxia in human malignant glioma spheroids and tumours. Br. J. Cancer 2000, 82, 635–641. [Google Scholar] [CrossRef][Green Version]
- Sorace, A.G.; Elkassem, A.A.; Galgano, S.J.; Lapi, S.E.; Larimer, B.M.; Partridge, S.C.; Quarles, C.C.; Reeves, K.; Napier, T.S.; Song, P.N.; et al. Imaging for Response Assessment in Cancer Clinical Trials. Semin. Nucl. Med. 2020, 50, 488–504. [Google Scholar] [CrossRef]
- Hirata, K.; Yamaguchi, S.; Shiga, T.; Kuge, Y.; Tamaki, N. The Roles of Hypoxia Imaging Using 18F-Fluoromisonidazole Positron Emission Tomography in Glioma Treatment. J. Clin. Med. 2019, 8, 1088. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Shimizu, Y.; Yoshioka, T.; Nishijima, K.-I.; Zhao, S.; Higashino, K.; Numata, Y.; Tamaki, N.; Kuge, Y. FMISO accumulation in tumor is dependent on glutathione conjugation capacity in addition to hypoxic state. Ann. Nucl. Med. 2017, 31, 596–604. [Google Scholar] [CrossRef]
- Dearling, J.L.; Packard, A.B. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010, 37, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, M.; Rajerison, H.; Guerard, F.; Mougin-Degraef, M.; Barbet, J.; Michel, N.; Cherel, M.; Faivre-Chauvet, A. Contribution of [64Cu]-ATSM PET in molecular imaging of tumour hypoxia compared to classical [18F]-MISO—A selected review. Nucl. Med. Rev. 2011, 14, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Yoshimoto, M.; Matsumoto, H.; Furukawa, T.; Zhang, M.-R.; Inubushi, M.; Tsuji, A.B.; Fujibayashi, Y.; Higashi, T.; Saga, T. 64Cu-ATSM internal radiotherapy to treat tumors with bevacizumab-induced vascular decrease and hypoxia in human colon carcinoma xenografts. Oncotarget 2017, 8, 88815–88826. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N. The Validation Path of Hypoxia PET Imaging: Focus on Brain Tumours. Curr. Med. Chem. 2018, 25, 3074–3095. [Google Scholar] [CrossRef]
- Li, F.; Joergensen, J.T.; E Hansen, A.; Kjaer, A. Kinetic modeling in PET imaging of hypoxia. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 490–506. [Google Scholar]
- Stieb, S.; Eleftheriou, A.; Warnock, G.; Guckenberger, M.; Riesterer, O. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2201–2217. [Google Scholar] [CrossRef]
- Sanduleanu, S.; Van Der Wiel, A.M.; Lieverse, R.I.; Marcus, D.; Ibrahim, A.; Primakov, S.; Wu, G.; Theys, J.; Yaromina, A.; Dubois, L.; et al. Hypoxia PET Imaging with [18F]-HX4—A Promising Next-Generation Tracer. Cancers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Zegers, C.M.; Van Elmpt, W.; Reymen, B.; Even, A.J.; Troost, E.; Ollers, M.C.; Hoebers, F.J.P.; Houben, R.M.; Eriksson, J.; Windhorst, A.D.; et al. In Vivo Quantification of Hypoxic and Metabolic Status of NSCLC Tumors Using [18F]HX4 and [18F]FDG-PET/CT Imaging. Clin. Cancer Res. 2014, 20, 6389–6397. [Google Scholar] [CrossRef]
- Van Elmpt, W.; Zegers, C.M.L.; Reymen, B.; Even, A.J.G.; Dingemans, A.-M.C.; Oellers, M.; Wildberger, J.E.; Mottaghy, F.M.; Das, M.; Troost, E.; et al. Multiparametric imaging of patient and tumour heterogeneity in non-small-cell lung cancer: Quantification of tumour hypoxia, metabolism and perfusion. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Zegers, C.M.L.; Hoebers, F.J.P.; Van Elmpt, W.; Bons, J.A.; Öllers, M.C.; Troost, E.; Eekers, D.; Balmaekers, L.; Arts-Pechtold, M.; Mottaghy, F.M.; et al. Evaluation of tumour hypoxia during radiotherapy using [18F]HX4 PET imaging and blood biomarkers in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2010, 278, 16–27. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- Conway, R.E.; Joiner, K.; Patterson, A.; Bourgeois, D.; Rampp, R.; Hannah, B.C.; McReynolds, S.; Elder, J.M.; Gilfilen, H.; Shapiro, L.H. Prostate specific membrane antigen produces pro-angiogenic laminin peptides downstream of matrix metalloprotease-2. Angiogenesis 2013, 16, 847–860. [Google Scholar] [CrossRef]
- Heesch, A.; Maurer, J.; Stickeler, E.; Beheshti, M.; Mottaghy, F.M.; Morgenroth, A. Development of Radiotracers for Breast Cancer—The Tumor Microenvironment as an Emerging Target. Cells 2020, 9, 2334. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, H.; Li, L.; Feng, M.; Chen, X.; Hao, B.; Lv, Z.; Zhou, X.; Cao, Y. Prostate-Specific Membrane Antigen (PSMA) Promotes Angiogenesis of Glioblastoma Through Interacting With ITGB4 and Regulating NF-κB Signaling Pathway. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Kadambi, A.; Carreira, C.M.; O Yun, C.; Padera, T.P.; E Dolmans, D.; Carmeliet, P.; Fukumura, D.; Jain, R.K. Vascular endothelial growth factor (VEGF)-C differentially affects tumor vascular function and leukocyte recruitment: Role of VEGF-receptor 2 and host VEGF-A. Cancer Res. 2001, 61, 2404–2408. [Google Scholar]
- Tsuzuki, Y.; Fukumura, D.; Oosthuyse, B.; Koike, C.; Carmeliet, P.; Jain, R.K. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha→ hypoxia response element→ VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000, 60, 6248–6252. [Google Scholar]
- Duda, D.G.; Batchelor, T.T.; Willett, C.G.; Jain, R.K. VEGF-targeted cancer therapy strategies: Current progress, hurdles and future prospects. Trends Mol. Med. 2007, 13, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Soltau, J.; Drevs, J. Mode of action and clinical impact of VEGF signaling inhibitors. Expert Rev. Anticancer Ther. 2009, 9, 649–662. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G. VEGF Receptor Signaling in Tumor Angiogenesis. Oncology 2000, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Asabella, A.N.; Di Palo, A.; Altini, C.; Ferrari, C.; Rubini, G. Multimodality Imaging in Tumor Angiogenesis: Present Status and Perspectives. Int. J. Mol. Sci. 2017, 18, 1864. [Google Scholar] [CrossRef] [PubMed]
- Rudlowski, C.; Pickart, A.-K.; Fuhljahn, C.; Friepoertner, T.; Schlehe, B.; Biesterfeld, S.; Schroeder, W. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: A long-term follow-up. Int. J. Gynecol. Cancer 2006, 16, 183–189. [Google Scholar] [CrossRef]
- Bergsland, E.K. Update on Clinical Trials Targeting Vascular Endothelial Growth Factor in Cancer. Am. J. Health Pharm. 2004, 61, S12–S20. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Li, S.; Peck-Radosavljevic, M.; Koller, E.; Koller, F.; Kaserer, K.; Kreil, A.; Kapiotis, S.; Hamwi, A.; Weich, H.A.; Valent, P.; et al. Characterization of 123I-vascular endothelial growth factor-binding sites expressed on human tumour cells: Possible implication for tumour scintigraphy. Int. J. Cancer 2001, 91, 789–796. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Kinuya, S.; Kawashima, A.; Nishii, R.; Yokoyama, K.; Kawai, K. Radioiodinated VEGF to image tumor angiogenesis in a LS180 tumor xenograft model. Nucl. Med. Biol. 2006, 33, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, F.G.; Mandl, S.; Cao, Y.-A.; O’Connell-Rodwell, C.; Contag, C.; Mari, C.; I Gaynutdinov, T.; Vanderheyden, J.-L.; Backer, M.V.; Backer, J.M. Tumor imaging using a standardized radiolabeled adapter protein docked to vascular endothelial growth factor. J. Nucl. Med. 2004, 45, 1373–1380. [Google Scholar] [PubMed]
- Backer, M.V.; Patel, V.; Jehning, B.T.; Claffey, K.P.; Backer, J.M. Surface immobilization of active vascular endothelial growth factor via a cysteine-containing tag. Biomaterials 2006, 27, 5452–5458. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, J.; Chan, S.; Wang, H.; Wang, C.; Soong, Y. Vascular Endothelial Growth Factor (VEGF) System Expression in Human Fallopian Tube with Ectopic Pregnancy. Fertil. Steril. 2005, 84, S436. [Google Scholar] [CrossRef]
- Cai, W.; Chen, K.; Mohamedali, K.A.; Cao, Q.; Gambhir, S.S.; Rosenblum, M.G.; Chen, X. PET of vascular endothelial growth factor receptor expression. J. Nucl. Med. 2006, 47, 2048–2056. [Google Scholar]
- Li, S.; Peck-Radosavljevic, M.; Kienast, O.; Preitfellner, J.; Havlik, E.; Schima, W.; Traub-Weidinger, T.; Graf, S.; Beheshti, M.; Schmid, M.; et al. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. QJ Nucl. Med. Mol. Imaging 2004, 48, 198–206. [Google Scholar]
- Rainer, E.; Wang, H.; Traub-Weidinger, T.; Widhalm, G.; Fueger, B.; Chang, J.; Zhu, Z.; Marosi, C.; Haug, A.; Hacker, M.; et al. The prognostic value of [123I]-vascular endothelial growth factor ([123I]-VEGF) in glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2396–2403. [Google Scholar] [CrossRef]
- Oosting, S.F.; Brouwers, A.H.; Van Es, S.C.; Nagengast, W.B.; Munnink, T.H.O.; Hooge, M.N.L.-D.; Hollema, H.; De Jong, J.R.; De Jong, I.J.; De Haas, S.; et al. 89Zr-Bevacizumab PET Visualizes Heterogeneous Tracer Accumulation in Tumor Lesions of Renal Cell Carcinoma Patients and Differential Effects of Antiangiogenic Treatment. J. Nucl. Med. 2014, 56, 63–69. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Jansen, M.H.; Van Zanten, S.E.V.; Van Vuurden, D.G.; Huisman, M.C.; Vugts, D.J.; Hoekstra, O.S.; Van Dongen, G.A.; Kaspers, G.-J.L. Molecular Drug Imaging:89Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2016, 58, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 1–9. [Google Scholar] [CrossRef]
- Tarli, L.; Balza, E.; Viti, F.; Borsi, L.; Castellani, P.; Berndorff, D.; Dinkelborg, L.; Neri, D.; Zardi, L. A High-Affinity Human Antibody That Targets Tumoral Blood Vessels. Blood 1999, 94, 192–198. [Google Scholar] [CrossRef]
- Bogdanowich-Knipp, S.J.; Chakrabarti, S.; Siahaan, T.J.; Williams, T.D.; Dillman, R.K. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. 1999, 53, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Buonaguro, F.M. New Insights in the Design of Bioactive Peptides and Chelating Agents for Imaging and Therapy in Oncology. Molecules 2017, 22, 1282. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef]
- Decristoforo, C.; Santos, I.; Pietzsch, H.J.; Kuenstler, J.U.; Duatti, A.; Smith, C.J.; Rey, A.; Alberto, R.; Von Guggenberg, E.; Haubner, R. Comparison of in vitro and in vivo properties of [99mTc]cRGD peptides labeled using different novel Tc-cores. QJ Nucl. Med. Mol. Imaging 2007, 51, 33–41. [Google Scholar]
- Axelsson, R.; Bach-Gansmo, T.; Castell-Conesa, J.; McParland, B.J. An open-label, multicenter, phase 2a study to assess the feasibility of imaging metastases in late-stage cancer patients with the αvβ3-selective angiogenesis imaging agent 99mTc-NC100692. Acta Radiol. 2010, 51, 40–46. [Google Scholar] [CrossRef]
- Haubner, R.; A Weber, W.; Beer, A.J.; Vabuliene, E.; Reim, D.; Sarbia, M.; Becker, K.-F.; Goebel, M.; Hein, R.; Wester, H.-J.; et al. Noninvasive Visualization of the Activated αvβ3 Integrin in Cancer Patients by Positron Emission Tomography and [18F]Galacto-RGD. PLoS Med. 2005, 2, e70. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.-B.; Cai, W.; He, L.; Chin, F.T.; Li, F.; Chen, X. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): Synthesis and microPET imaging of αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1823–1831. [Google Scholar] [CrossRef]
- Gao, S.; Wu, H.; Li, W.; Zhao, S.; Teng, X.; Lu, H.; Hu, X.; Wang, S.; Yu, J.; Yuan, S. A pilot study imaging integrin αvβ3 with RGD PET/CT in suspected lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2029–2037. [Google Scholar] [CrossRef]
- Withofs, N.; Signolle, N.; Somja, J.; Lovinfosse, P.; Nzaramba, E.M.; Mievis, F.; Giacomelli, F.; Waltregny, D.; Cataldo, D.; Gambhir, S.S.; et al. 18F-FPRGD2 PET/CT Imaging of Integrin v 3 in Renal Carcinomas: Correlation with Histopathology. J. Nucl. Med. 2015, 56, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Withofs, N.; Charlier, E.; Simoni, P.; Alvarez-Miezentseva, V.; Mievis, F.; Giacomelli, F.; Mella, C.; Gambhir, S.S.; Malaise, O.; De Seny, D.; et al. 18F-FPRGD2 PET/CT imaging of musculoskeletal disorders. Ann. Nucl. Med. 2015, 29, 839–847. [Google Scholar] [CrossRef]
- Mittra, E.S.; Goris, M.L.; Iagaru, A.H.; Kardan, A.; Burton, L.; Berganos, R.; Chang, E.; Liu, S.; Shen, B.; Chin, F.T.; et al. Pilot Pharmacokinetic and Dosimetric Studies of18F-FPPRGD2: A PET Radiopharmaceutical Agent for Imaging αvβ3Integrin Levels. Radiology 2011, 260, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Jamali, M.; Barkhodari, A.; Mosci, C.; Mittra, E.; Shen, B.; Chin, F.; Gambhir, S.S.; Iagaru, A. Biodistribution of the 18F-FPPRGD2 PET radiopharmaceutical in cancer patients: An atlas of SUV measurements. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Iagaru, A.; Mosci, C.; Mittra, E.; Zaharchuk, G.; Fischbein, N.; Harsh, G.; Li, G.; Nagpal, S.; Recht, L.; Gambhir, S.S. Glioblastoma Multiforme Recurrence: An Exploratory Study of18F FPPRGD2PET/CT. Radiology 2015, 277, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Z.-B.; Chen, K.; Cai, W.; He, L.; Chin, F.T.; Li, F.; Chen, X. microPET of Tumor Integrin v 3 Expression Using 18F-Labeled PEGylated Tetrameric RGD Peptide (18F-FPRGD4). J. Nucl. Med. 2007, 48, 1536–1544. [Google Scholar] [CrossRef]
- Lobeek, D.; Rijpkema, M.; Terry, S.Y.A.; Molkenboer-Kuenen, J.D.M.; Joosten, L.; Van Genugten, E.A.J.; Grunsven, A.C.H.V.E.-V.; Kaanders, J.H.A.M.; Pegge, S.A.H.; Boerman, O.C.; et al. Imaging angiogenesis in patients with head and neck squamous cell carcinomas by [68Ga]Ga-DOTA-E-[c(RGDfK)]2 PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2647–2655. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Kim, Y.-S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving Tumor Uptake and Excretion Kinetics of 99mTc-Labeled Cyclic Arginine-Glycine-Aspartic (RGD) Dimers with Triglycine Linkers. J. Med. Chem. 2008, 51, 7980–7990. [Google Scholar] [CrossRef]
- Wang, J.; Kim, Y.-S.; Liu, S. 99mTc-Labeling of HYNIC-Conjugated Cyclic RGDfK Dimer and Tetramer Using EDDA as Coligand. Bioconjugate Chem. 2008, 19, 634–642. [Google Scholar] [CrossRef]
- Dijkgraaf, I.; Liu, S.; Kruijtzer, J.A.; Soede, A.C.; Oyen, W.J.; Liskamp, R.M.; Corstens, F.H.; Boerman, O.C. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD peptide. Nucl. Med. Biol. 2007, 34, 29–35. [Google Scholar] [CrossRef]
- Li, Z.-B.; Cai, W.; Cao, Q.; Chen, K.; Wu, Z.; Elhendy, A.; Chen, X. 64Cu-Labeled Tetrameric and Octameric RGD Peptides for Small-Animal PET of Tumor v 3 Integrin Expression. J. Nucl. Med. 2007, 48, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tohme, M.; Park, R.; Hou, Y.; Bading, J.R.; Conti, P.S. Micro-PET Imaging of αvβ3-Integrin Expression with 18F-Labeled Dimeric RGD Peptide. Mol. Imaging 2004, 3, 96–104. [Google Scholar] [CrossRef]
- Shi, J.; Kim, Y.-S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving Tumor Uptake and Pharmacokinetics of64Cu-Labeled Cyclic RGD Peptide Dimers with Gly3and PEG4Linkers. Bioconjugate Chem. 2009, 20, 750–759. [Google Scholar] [CrossRef]
- Dijkgraaf, I.; Kruijtzer, J.A.W.; Liu, S.; Soede, A.C.; Oyen, W.J.; Corstens, F.H.M.; Liskamp, R.M.J.; Boerman, O.C. Improved targeting of the αvβ3 integrin by multimerisation of RGD peptides. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, N.; Abdi, K.; Beiki, D.; Emami, F.; Arab, S.S.; Sabzevari, O.; Pakdin-Parizi, Z.; Geramifar, P. Radiosynthesis, Biological Evaluation, and Preclinical Study of a 68Ga-Labeled Cyclic RGD Peptide as an Early Diagnostic Agent for Overexpressed αvβ3 Integrin Receptors in Non-Small-Cell Lung Cancer. Contrast Media Mol. Imaging 2020, 2020. [Google Scholar] [CrossRef]
- Zhang-Yin, J.; Provost, C.; Cancel-Tassin, G.; Rusu, T.; Penent, M.; Radulescu, C.; Comperat, E.; Cussenot, O.; Montravers, F.; Renard-Penna, R.; et al. A comparative study of peptide-based imaging agents [68Ga]Ga-PSMA-11, [68Ga]Ga-AMBA, [68Ga]Ga-NODAGA-RGD and [68Ga]Ga-DOTA-NT-20.3 in preclinical prostate tumour models. Nucl. Med. Biol. 2020, 84-85, 88–95. [Google Scholar] [CrossRef]
- Novy, Z.; Stepankova, J.; Hola, M.; Flasarova, D.; Popper, M.; Petrik, M. Preclinical Evaluation of Radiolabeled Peptides for PET Imaging of Glioblastoma Multiforme. Molecules 2019, 24, 2496. [Google Scholar] [CrossRef] [PubMed]
- Isal, S.; Pierson, J.; Imbert, L.; Clement, A.; Collet, C.; Pinel, S.; Veran, N.; Reinhard, A.; Poussier, S.; Gauchotte, G.; et al. PET imaging of 68Ga-NODAGA-RGD, as compared with 18F-fluorodeoxyglucose, in experimental rodent models of engrafted glioblastoma. EJNMMI Res. 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fu, H.; Wu, H.; Huang, J.; Yao, L.; Zhang, X.; Li, Y. Syntheses and Preliminary Evaluation of Dual Target PET Probe [18F]-NOTA-Gly3- E (2PEG4-RGD-WH701) for PET Imaging of Breast Cancer. Anti-Cancer Agents Med. Chem. 2020, 20, 1548–1557. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Shang, N.; Liu, Z.; Yu, Q.; Wang, S.; Teng, X.; Zhang, Q.; Hu, X.; Zhao, W.; et al. Pretreatment PET/CT imaging of angiogenesis based on 18F-RGD tracer uptake may predict antiangiogenic response. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 940–947. [Google Scholar] [CrossRef]
- Van Der Gucht, A.; Pomoni, A.; Jreige, M.; Allemann, P.; Prior, J.O. 68Ga-NODAGA-RGDyK PET/CT Imaging in Esophageal Cancer. Clin. Nucl. Med. 2016, 41, e491–e492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Zhao, W.; Sun, X.; Liu, N.; Zhou, Y.; Luan, X.; Gao, S.; Zhao, S.; Yu, J.; Yuan, S. 18F-RGD PET/CT imaging reveals characteristics of angiogenesis in non-small cell lung cancer. Transl. Lung Cancer Res. 2020, 9, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.M.; Keam, B.; Kim, Y.J.; Paeng, J.C.; Moon, K.C.; Kim, D.; Heo, D.S. A Phase II Trial of Pazopanib in Patients with Metastatic Alveolar Soft Part Sarcoma. Oncologist 2018, 24, 20–e29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, Z.; Huang, C.; Chen, Y.; Miao, W. [99mTc]3PRGD2 for integrin receptor imaging of esophageal cancer: A comparative study with [18F]FDG PET/CT. Ann. Nucl. Med. 2018, 33, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Ji, N.; Zhao, X.; Zheng, K.; Qiao, Z.; Li, F.; Lang, L.; Iagaru, A.; Niu, G.; et al. Combined 68Ga-NOTA-PRGD2 and 18F-FDG PET/CT Can Discriminate Uncommon Meningioma Mimicking High-Grade Glioma. Clin. Nucl. Med. 2018, 43, 648–654. [Google Scholar] [CrossRef]
- Ikeda, N.; Nakajima, Y.; Tokuhara, T.; Hattori, N.; Sho, M.; Kanehiro, H.; Miyake, M. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin. Cancer Res. 2003, 9, 1503–1508. [Google Scholar]
- Pang, L.; Zhang, N.; Xiangwei, M.; Wang, D.; Wang, G.; Meng, X. Serum APN/CD13 as a novel diagnostic and prognostic biomarker of pancreatic cancer. Oncotarget 2016, 7, 77854–77864. [Google Scholar] [CrossRef]
- Shimizu, T.; Tani, K.; Hase, K.; Ogawa, H.; Huang, L.; Shinomiya, F.; Sone, S. CD13/aminopeptidase N-induced lymphocyte involvement in inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 2002, 46, 2330–2338. [Google Scholar] [CrossRef]
- Ma, W.; Kang, F.; Wang, Z.; Yang, W.; Li, G.; Ma, X.; Li, G.; Chen, K.; Zhang, Y.; Wang, J. 99mTc-labeled monomeric and dimeric NGR peptides for SPECT imaging of CD13 receptor in tumor-bearing mice. Amino Acids 2013, 44, 1337–1345. [Google Scholar] [CrossRef]
- Persigehl, T.; Ring, J.; Bremer, C.; Heindel, W.; Holtmeier, R.; Stypmann, J.; Claesener, M.; Hermann, S.; Schäfers, M.; Zerbst, C.; et al. Non-invasive monitoring of tumor-vessel infarction by retargeted truncated tissue factor tTF–NGR using multi-modal imaging. Angiogenesis 2013, 17, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Jószai, I.; Enyedi, K.N.; Lakatos, S.; Garai, I.; Mező, G.; Kertész, I.; et al. In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging. Int. J. Pharm. 2020, 589, 119881. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Z.; Ma, X.; Ma, W.; Zhao, M.; Fu, T.; Li, G.; Wang, S.; Wang, Z.; Yang, W.; et al. The uptake exploration of 68Ga-labeled NGR in well-differentiated hepatocellular carcinoma xenografts: Indication for the new clinical translational of a tracer based on NGR. Oncol. Rep. 2017, 38, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Li, X.; Kang, F.; Ma, X.; Yang, W.; Ma, W.; Wang, J. A Uniquely Modified DKL-based Peptide Probe for Positron Emission Tomography Imaging. Curr. Pharm. Des. 2019, 25, 96–103. [Google Scholar] [CrossRef]
- Gai, Y.; Jiang, Y.; Long, Y.; Sun, L.; Liu, Q.; Qin, C.; Zhang, Y.; Zeng, D.; Lan, X. Evaluation of an Integrin αvβ3 and Aminopeptidase N Dual-Receptor Targeting Tracer for Breast Cancer Imaging. Mol. Pharm. 2019, 17, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Shao, Y.; Yang, W.; Li, G.; Zhang, Y.; Zhang, M.; Zuo, C.; Chen, K.; Wang, J. Evaluation of 188Re-labeled NGR–VEGI protein for radioimaging and radiotherapy in mice bearing human fibrosarcoma HT-1080 xenografts. Tumor Biol. 2016, 37, 9121–9129. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Guo, Z.; Fu, K.; Yao, L.; Fu, L.; Guo, W.; Wen, X.; Jacobson, O.; Zhang, X.; et al. Targeted Radionuclide Therapy in Patient-Derived Xenografts Using 177Lu-EB-RGD. Mol. Cancer Ther. 2020, 19, 2034–2043. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Fu, K.; Lin, Q.; Wen, X.; Jacobson, O.; Sun, L.; Wu, H.; Zhang, X.; Guo, Z.; et al. Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 2019, 9, 7948–7960. [Google Scholar] [CrossRef]

| Imaging Method | Target | Tracer Name | Modality | Stage |
|---|---|---|---|---|
| Indirect targeting of angiogenesis | Glucose metabolism | [18F]-FDG | PET | accepted for clinical use |
| Hypoxia | [18F]-HX4 | PET | clinical trial [19] | |
| [18F]-FMISO | PET | clinical trial [20] | ||
| [18F]-FAZA | PET | clinical trial [21], [22] | ||
| MMPs | [18F]-SAV03 | PET | in vivo preclinical stage [23] | |
| [68Ga]-NOTA-C6 | PET | in vivo preclinical stage [24] | ||
| Direct targeting of angiogenesis | VEGF | [123I]-VEGF165 | SPECT | clinical trial [25] |
| [64Cu]-DOTA-scVEGF | PET | in vivo preclinical stage [26] | ||
| [99mTc]-HYNIC-scVEGF | SPECT | in vivo preclinical stage [27] | ||
| [89Zr]-Bevacizumab | PET | in vivo preclinical stage [28] | ||
| [111In]-Bevacizumab | SPECT | clinical trial [29] | ||
| Integrins | 99mTc-labelled anti-ED-B fibronectin antibody | SPECT | in vivo preclinical stage [30] | |
| 123I-labelled anti-ED-B fibronectin antibody | SPECT | in vivo preclinical stage [31] | ||
| 124I-labelled anti-ED-B fibronectin antibody | SPECT | in vivo preclinical stage [32] | ||
| 76Br-labelled anti-ED-B fibronectin antibody | PET | in vivo preclinical stage [33] | ||
| [123I]-L19(scFv) | SPECT | clinical trial [31] | ||
| [18F]-Galacto-RGD | PET | clinical trial [34] | ||
| [18F]-Fluciclatide | PET | clinical trial [35] | ||
| [18F]-RGD-K5 | PET | clinical trial [36] | ||
| [18F]-FB-RGD | PET | in vivo preclinical stage [37] | ||
| [18F]-PEG-RGD2 | PET | in vivo preclinical stage [37] | ||
| [68Ga] Ga-NODAGA-RGD | PET | clinical trial [38] | ||
| NGR | 99mTc-labelled NGR | SPECT | in vivo preclinical stage [39] | |
| [68Ga]-NOTA-c(NGR) | PET | in vivo preclinical stage [40] | ||
| 64Cu-labelled NGR | PET | in vivo preclinical stage [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, A.; Mottaghy, F.M.; Bauwens, M. Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status. Int. J. Mol. Sci. 2021, 22, 5544. https://doi.org/10.3390/ijms22115544
Florea A, Mottaghy FM, Bauwens M. Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status. International Journal of Molecular Sciences. 2021; 22(11):5544. https://doi.org/10.3390/ijms22115544
Chicago/Turabian StyleFlorea, Alexandru, Felix M. Mottaghy, and Matthias Bauwens. 2021. "Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status" International Journal of Molecular Sciences 22, no. 11: 5544. https://doi.org/10.3390/ijms22115544
APA StyleFlorea, A., Mottaghy, F. M., & Bauwens, M. (2021). Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status. International Journal of Molecular Sciences, 22(11), 5544. https://doi.org/10.3390/ijms22115544







