Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. The Protein Kinase C Family
3. Expression, Biological Role, and Prognosis of Protein Kinase C in NSCLC
4. Therapeutic Approaches Targeting PKCs in NSCLC
5. PKC-Mediated Resistance Acquisition and Drug Sensitivity in NSCLC
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Zheng, M. Classification and pathology of lung cancer. Surg. Oncol. Clin. 2016, 25, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris, M.G.; Johnson, B.E.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Aronson, S.L.; Engelman, J.A.; Shyr, Y.; Khuri, F.R.; Rudin, C.M.; et al. Identification of driver mutations in tumor specimens from 1000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC). J. Clin. Oncol. 2011, 29, CRA7506. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as firstline treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer: A multicentre, open-label, randomized, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Jackman, D.; Pao, W.; Riely, G.J.; Engelman, J.A.; Kris, M.G.; Jänne, P.A.; Lynch, T.; Johnson, B.E.; Miller, V.A. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 357–360. [Google Scholar] [CrossRef]
- Kang, J.H. Protein kinase C (PKC) isozymes and cancer. New J. Sci. 2014, 2014, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.M.; Cummings, E.; Pantos, C.; Singh, J. Protein kinase C and cardiac dysfunction: A review. Heart Fail. Rev. 2017, 22, 843–859. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, V.; Bogomolovas, J.; Ehler, E.; dos Remedios, C.G.; Yu, J.; Gao, C.; Lange, S. PKC and PKN in heart disease. J. Mol. Cell. Cardiol. 2019, 128, 212–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evcimen, N.D.; King, G.L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Scaini, G.; Bavaresco, D.V.; Leite, C.; Valvassoria, S.S.; Carvalho, A.F.; Quevedo, J. Role of protein kinase C in bipolar disorder: A review of the current literature. Mol. Neuropsychiatry 2017, 3, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.I.; Callender, J.A.; Hooli, B.; Antal, C.E.; Mullin, K.; Sherman, M.A.; Lesné, S.E.; Leitges, M.; Newton, A.; Tanzi, R.E.; et al. Gain-of function mutations in protein kinase Cα (PKCα) may promote synaptic defects in Alzheimer’s disease. Sci. Signal. 2016, 9, ra47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivunen, J.; Aaltonen, V.; Peltonen, J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006, 235, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C as a tumor suppressor. Semin. Cancer Biol. 2018, 48, 18–26. [Google Scholar] [CrossRef]
- Inoue, M.; Kishimoto, A.; Takai, Y.; Nishizuka, Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J. Biol. Chem. 1977, 252, 7610–7616. [Google Scholar] [CrossRef]
- Parker, P.J.; Coussens, L.; Totty, N.; Rhee, L.; Young, S.; Chen, E.; Stabel, S.; Waterfield, M.D.; Ullrich, A. The complete primary structure of protein kinase C—The major phorbol ester receptor. Science 1986, 233, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.; Parker, P.J.; Rhee, L.; Yang-Feng, T.L.; Chen, E.; Waterfield, M.D.; Francke, U.; Ullrich, A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science 1986, 233, 859–866. [Google Scholar] [CrossRef]
- Jaken, S.; Kiley, S.C. Purification and characterization of three types of protein kinase C from rabbit brain cytosol. Proc. Natl. Acad. Sci. USA 1987, 84, 4418–4422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, S.; Kawasaki, H.; Imajoh, S.; Suzuki, K.; Inagaki, M.; Yokokura, H.; Sakoh, T.; Hidaka, H. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature 1987, 325, 161. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Ohno, S.; Suzuki, K. Primary structures of human protein kinase C βI and βII differ only in their C-terminal sequences. FEBS Lett. 1987, 223, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Fuji, T.; Ogita, K.; Kikkawa, U.; Igarashi, K.; Nishizuka, Y. Identification of three additional members of rat protein kinase C family: δ-, ϵ- and ξ-subspecies. FEBS Lett. 1987, 226, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Osada, S.I.; Mizuno, K.; Saido, T.C.; Akita, Y.; Suzuki, K.; Kuroki, T.; Ohno, S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J. Biol. Chem. 1990, 265, 22434–22440. [Google Scholar] [CrossRef]
- Steinberg, S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008, 88, 1341–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, Y.; Kishimoto, A.; Kikkawa, U.; Mori, T.; Nishizuka, Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem. Biophys. Res. Commun. 1979, 91, 1218–1224. [Google Scholar] [CrossRef]
- Castagna, M.; Takai, Y.; Kaibuchi, K.; Sano, K.; Kikkawa, U.; Nishizuka, Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982, 257, 7847–7851. [Google Scholar] [CrossRef]
- Antal, C.E.; Hudson, A.M.; Kang, E.; Zanca, C.; Wirth, C.; Stephenson, N.L.; Trotter, E.W.; Gallegos, L.L.; Miller, C.J.; Furnari, F.B.; et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell 2015, 160, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef]
- Isakov, N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin. Cancer Biol. 2018, 48, 36–52. [Google Scholar] [CrossRef]
- Lahn, M.; Su, C.; Li, S.; Chedid, M.; Hanna, K.R.; Graff, J.R.; Sandusky, G.E.; Ma, D.; Niyikiza, C.; Sundell, K.L.; et al. Expression levels of protein kinase C-α in non-small-cell lung cancer. Clin. Lung Cancer 2004, 6, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; West, K.A.; Blumberg, P.M.; Dennis, P.A. Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKCδ promotes cellular survival and chemotherapeutic resistance. Cancer Res. 2003, 63, 780–786. [Google Scholar] [PubMed]
- Hirai, M.; Gamou, S.; Kobayashi, M.; Shimizu, N. Lung cancer cells often express high levels of protein kinase C activity. Jpn. J. Cancer Res. 1989, 80, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fu, Q.; Song, X.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; et al. HDGF and PRKCA upregulation is associated with a poor prognosis in patients with lung adenocarcinoma. Oncol. Lett. 2019, 18, 4936–4946. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Repasky, E.; Liu, H.T. Antisense inhibition of protein kinase Cα reverses the transformed phenotype in human lung carcinoma cells. Exp. Cell Res. 1999, 250, 253–263. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Liang, H.; Wang, T.; Yan, X.; Cao, M.; Wang, N.; Zhang, S.; Zen, K.; Zhang, C.; et al. miR-203 inhibits cell proliferation and migration of lung cancer cells by targeting PKCα. PLoS ONE 2013, 8, e73985. [Google Scholar] [CrossRef]
- Dean, N.; McKay, R.; Miraglia, L.; Howard, R.; Cooper, S.; Giddings, J.; Nicklin, P.; Meister, L.; Ziel, R.; Geiger, T.; et al. Inhibition of growth of human tumor cell lines in nude mice by an antisense oligonucleotide inhibitor of protein kinase C-α expression. Cancer Res. 1996, 56, 3499–3507. [Google Scholar]
- Deeds, L.; Teodorescu, S.; Chu, M.; Yu, Q.; Chen, C.Y. A p53-independent G1 cell cycle checkpoint induced by the suppression of protein kinase C α and θ isoforms. J. Biol. Chem. 2003, 278, 39782–39793. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.S.; Wickramarachchi, D.; Singhal, J.; Yadav, S.; Awasthi, Y.C.; Awasthi, S. Determinants of differential doxorubicin sensitivity between SCLC and NSCLC. FEBS Lett. 2006, 580, 2258–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
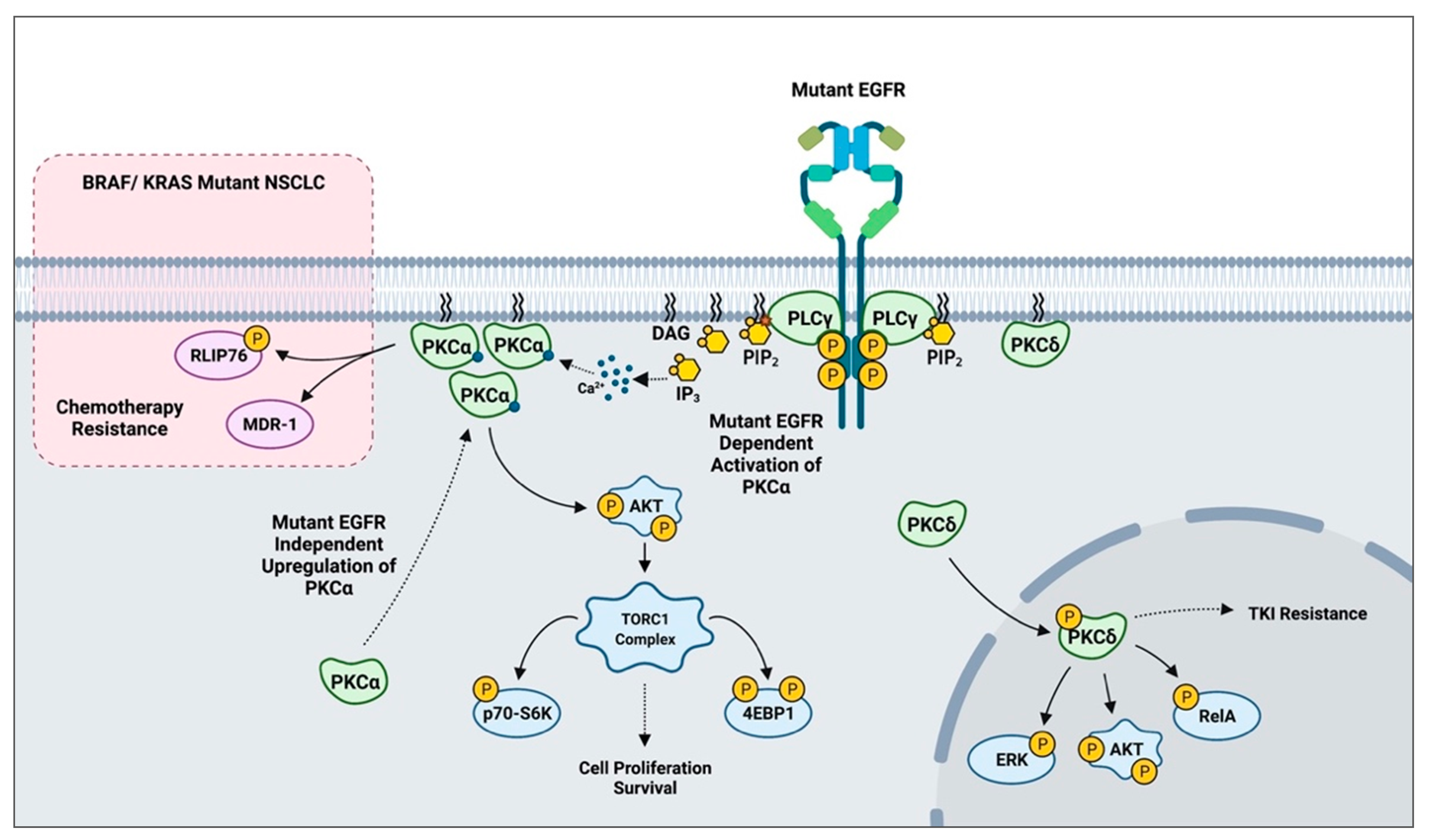
- Singhal, S.S.; Yadav, S.; Singhal, J.; Awasthi, Y.C.; Awasthi, S. Mitogenic and drug-resistance mediating effects of PKCα require RLIP76. Biochem. Biophys. Res. Commun. 2006, 348, 722–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Liu, H.T. Antisense expression of protein kinase C alpha improved sensitivity to anticancer drugs in human lung cancer LTEPa-2 cells. Acta Pharmacol. Sin. 1998, 19, 265–268. [Google Scholar]
- Gao, Z.; Tang, L.; Su, B.; Sha, H.; Han, B. Effects of protein kinase C inhibitor, chelerythrine chloride, on drug-sensitivity of NSCLC cell lines. Chin. J. Lung Cancer 2007, 1, 455–460. [Google Scholar]
- Sonnemann, J.; Gekeler, V.; Ahlbrecht, K.; Brischwein, K.; Liu, C.; Bader, P.; Müller, C.; Niethammer, D.; Beck, J.F. Down-regulation of protein kinase Cη by antisense oligonucleotides sensitizes A549 lung cancer cells to vincristine and paclitaxel. Cancer Lett. 2004, 209, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Mori, T.; Kitazaki, H.; Niidome, T.; Takayama, K.; Nakanishi, Y.; Katayama, Y. Kinase activity of protein kinase cα in serum as a diagnostic biomarker of human lung cancer. Anticancer Res. 2013, 33, 485–488. [Google Scholar]
- Symonds, J.M.; Ohm, A.M.; Carter, C.J.; Heasley, L.E.; Boyle, T.A.; Franklin, W.A.; Reyland, M.E. Protein kinase C δ is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res. 2011, 71, 2087–2097. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Lee, H.J.; Lee, D.H.; Bae, S.; Soh, J.W.; Jeoung, D.; Kim, J.; Cho, C.K.; Lee, Y.J.; Lee, Y.S. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res. 2007, 67, 6333–6341. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.M.; Wang, H.; Jiang, G.; Chen, M.G.; Lu, L.; Xiao, L. Protein Kinase Cε Is Overexpressed in Primary Human Non-Small Cell Lung Cancers and Functionally Required for Proliferation of Non-Small Cell Lung Cancer Cells in a p21/Cip1-Dependent Manner. Cancer Res. 2007, 67, 6053–6063. [Google Scholar] [CrossRef] [Green Version]
- Totoń, E.; Ignatowicz, E.; Skrzeczkowska, K.; Rybczyńska, M. Protein kinase Cε as a cancer marker and target for anticancer therapy. Pharmacol. Rep. 2011, 63, 19–29. [Google Scholar] [CrossRef]
- Caino, M.C.; Lopez-Haber, C.; Kim, J.; Mochly-Rosen, D.; Kazanietz, M.G. Protein kinase Cɛ is required for non-small cell lung carcinoma growth and regulates the expression of apoptotic genes. Oncogene 2012, 31, 2593–2600. [Google Scholar] [CrossRef] [Green Version]
- Caino, M.C.; Lopez-Haber, C.; Kissil, J.L.; Kazanietz, M.G. Non-small cell lung carcinoma cell motility, rac activation and metastatic dissemination are mediated by protein kinase C epsilon. PLoS ONE 2012, 7, e31714. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Su, Y.; Xu, L. Targeting PKCε by miR-143 regulates cell apoptosis in lung cancer. FEBS Lett. 2013, 587, 3661–3667. [Google Scholar] [CrossRef] [Green Version]
- Zurgil, U.; Ben-Ari, A.; Rotem-Dai, N.; Karp, G.; Krasnitsky, E.; Frost, S.A.; Livneh, E. PKCη is an anti-apoptotic kinase that predicts poor prognosis in breast and lung cancer. Biochem. Soc. Trans. 2014, 42, 1519–1523. [Google Scholar] [CrossRef]
- Krasnitsky, E.; Baumfeld, Y.; Freedman, J.; Sion-Vardy, N.; Ariad, S.; Novack, V.; Livneh, E. PKCη is a novel prognostic marker in non-small cell lung cancer. Anticancer Res. 2012, 32, 1507–1513. [Google Scholar]
- Basu, A. The Enigmatic Protein Kinase C-eta. Cancers 2019, 11, 214. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Tang, L.; Lin, H.; Huang, J.; Zhang, T.; Liu, Y.; Wang, J.; Zhan, P.; Yin, X.; Su, X.; et al. The oncogenic role of PKCiota gene amplification and overexpression in Chinese non-small cell lung cancer. Lung Cancer 2014, 84, 190–195. [Google Scholar] [CrossRef]
- Regala, R.P.; Weems, C.; Jamieson, L.; Khoor, A.; Edell, E.S.; Lohse, C.M.; Fields, A.P. Atypical protein kinase Cι is an oncogene in human non-small cell lung cancer. Cancer Res. 2005, 65, 8905–8911. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.P.; Regala, R.P. Protein kinase Cι: Human oncogene, prognostic marker and therapeutic target. Pharmacol. Res. 2007, 55, 487–497. [Google Scholar] [CrossRef] [Green Version]
- BommaReddy, R.R.; Patel, R.; Smalley, T.; Acevedo-Duncan, M. Effects of atypical protein kinase C inhibitor (DNDA) on lung cancer proliferation and migration by PKC-ι/FAK ubiquitination through the Cbl-b pathway. OncoTargets Ther. 2020, 13, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Chung, C.; Kim, J.M.; Lee, D.; Cho, S.Y.; Lee, T.H.; Cho, H.J.; Yeo, M.K. Clinical significance of atypical protein kinase C (PKCι and PKCζ) and its relationship with yes-associated protein in lung adenocarcinoma. BMC Cancer 2019, 19, 804. [Google Scholar] [CrossRef] [Green Version]
- Regala, R.P.; Weems, C.; Jamieson, L.; Copland, J.A.; Thompson, E.A.; Fields, A.P. Atypical protein kinase Cι plays a critical role in human lung cancer cell growth and tumorigenicity. J. Biol. Chem. 2005, 280, 31109–31115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, N.; Horikoshi, Y.; Matsuzaki, T.; Toriumi, K.; Kitatani, K.; Ogura, G.; Masuda, R.; Nakamura, N.; Takekoshi, S.; Iwazaki, M. Localization of aPKC lambda/iota and its interacting protein, Lgl2, is significantly associated with lung adenocarcinoma progression. Tokai J. Exp. Clin. Med. 2013, 38, 146–158. [Google Scholar]
- Liu, L.; Lei, B.; Wang, L.; Chang, C.; Yang, H.; Liu, J.; Huang, G.; Xie, W. Protein kinase C-iota-mediated glycolysis promotes non-small-cell lung cancer progression. OncoTargets Ther. 2019, 12, 5835–5848. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, E.; Klee, E.W.; Thompson, E.A.; Fields, A.P. Meta-analysis of oncogenic protein kinase Cι signaling in lung adenocarcinoma. Clin. Cancer Res. 2009, 15, 1527–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justilien, V.; Jameison, L.; Der, C.J.; Rossman, K.L.; Fields, A.P. Oncogenic activity of Ect2 is regulated through protein kinase Cι-mediated phosphorylation. J. Biol. Chem. 2011, 286, 8149–8157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, J.C.; Vu, N.; Shultz, M.D.; Mba, M.U.; Shapiro, B.A.; Chalfant, C.E. The Proto-oncogene PKCι regulates the alternative splicing of Bcl-x pre-mRNA. Mol. Cancer Res. 2012, 10, 660–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.A.; Justilien, V.; Jamieson, L.; Murray, N.R.; Fields, A.P. Protein kinase Cι drives a NOTCH3-dependent stem-like phenotype in mutant KRAS lung adenocarcinoma. Cancer Cell 2016, 29, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.P.; Ali, S.A.; Justilien, V.; Murray, N.R. Targeting oncogenic protein kinase Cι for treatment of mutant KRAS LADC. Small GTPases 2017, 8, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Codony-Servat, C.; Codony-Servat, J.; Lligé, D.; Chaib, I.; Sun, X.; Miao, J.; Sun, R.; Cai, X.; Verlicchi, A.; et al. Targeting PKCι-PAK1 signaling pathways in EGFR and KRAS mutant adenocarcinoma and lung squamous cell carcinoma. Cell Commun. Signal. 2019, 17, 137. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Liu, Y.; Khoor, A.; Wang, X.; Thompson, E.A.; Leitges, M.; Justilien, V.; Weems, C.; Murray, N.R.; Fields, A.P. Protein Kinase Cι and Wnt/β-Catenin Signaling: Alternative Pathways to Kras/Trp53-Driven Lung Adenocarcinoma. Cancer Cell 2019, 36, 156–167. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Wang, J.; Wan, W.; Sun, R.; Zhao, Y.; Zhang, N. Down-regulation of PKCζ expression inhibits chemotaxis signal transduction in human lung cancer cells. Lung Cancer 2009, 63, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Menon, K.; Alvarez, E.; Shih, C.; Faul, M.M. Antiangiogenic and antitumor effects of a protein kinase Cβ inhibitor in human breast cancer and ovarian cancer xenografts. Investig. New Drugs 2002, 20, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Carmo, A.D.; Balça-Silva, J.; Matias, D.; Lopes, M. PKC signaling in glioblastoma. Cancer Biol. Ther. 2013, 14, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, Y.L.; Vallentin, A.; Hunrichs, B.S.; Hellerstein, M.K.; Peehl, D.M.; Mochly-Rosen, D. Centrosomal PKCβII and pericentrin are critical for human prostate cancer growth and angiogenesis. Cancer Res. 2008, 68, 6831–6839. [Google Scholar] [CrossRef] [Green Version]
- Kazi, J.U.; Kabir, N.N.; Rönnstrand, L. Protein kinase C (PKC) as a drug target in chronic lymphocytic leukemia. Med. Oncol. 2013, 30, 757. [Google Scholar] [CrossRef] [Green Version]
- Zum Bueschenfelde, C.M.; Wagner, M.; Lutzny, G.; Oelsner, M.; Feuerstacke, Y.; Decker, T.; Bogner, C.; Peschel, C.; Ringshausen, I. Recruitment of PKC-βII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia 2010, 24, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Murray, N.R.; Baumgardner, G.P.; Burns, D.J.; Fields, A.P. Protein kinase C isotypes in human erythroleukemia (K562) cell proliferation and differentiation. Evidence that beta II protein kinase C is required for proliferation. J. Biol. Chem. 1993, 268, 15847–15853. [Google Scholar] [CrossRef]
- Lutzny, G.; Kocher, T.; Schmidt-Supprian, M.; Rudelius, M.; Klein-Hitpass, L.; Finch, A.J.; Dürig, J.; Wagner, M.; Haferlach, C.; Kohlmann, A.; et al. Protein kinase c-β-dependent activation of NF-κB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell 2013, 23, 77–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetsel, W.C.; Khan, W.A.; Merchenthaler, I.; Rivera, H.; Halpern, A.E.; Phung, H.M.; Negro-Vilar, A.; Hannun, Y.A. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J. Cell. Biol. 1992, 117, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Brezar, V.; Tu, W.J.; Seddiki, N. PKC-theta in regulatory and effector T-cell functions. Front. Immunol. 2015, 6, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baier, G.; Telford, D.; Giampa, L.; Coggeshall, K.M.; Baier-Bitterlich, G.; Isakov, N.; Altman, A. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J. Biol. Chem. 1993, 268, 4997–5004. [Google Scholar] [CrossRef]
- Abera, M.B.; Kazanietz, M.G. Protein kinase Cα mediates erlotinib resistance in lung cancer cells. Mol. Pharmacol. 2015, 87, 832–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Yang, Z.; Le Zhang, C.S.; Li, Y.; Zhang, X. Additive effects of cherlerythrine chloride combination with erlotinib in human non-small cell lung cancer cells. PLoS ONE 2017, 12, e0175466. [Google Scholar]
- Lee, P.C.; Fang, Y.F.; Yamaguchi, H.; Wang, W.J.; Chen, T.C.; Hong, X.; Ke, B.; Xia, W.; Wei, Y.; Zha, Z.; et al. Targeting PKCδ as a therapeutic strategy against heterogeneous mechanisms of EGFR inhibitor resistance in EGFR-mutant lung cancer. Cancer Cell 2018, 34, 954–969. [Google Scholar] [CrossRef] [Green Version]
- Graff, J.R.; McNulty, A.M.; Hanna, K.R.; Konicek, B.W.; Lynch, R.L.; Bailey, S.N.; Banks, C.; Capen, A.; Goode, R.; Lewis, J.E.; et al. The protein kinase Cβ–selective inhibitor, enzastaurin (LY317615. HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005, 65, 7462–7469. [Google Scholar] [CrossRef] [Green Version]
- Hanauske, A.R.; Oberschmidt, O.; Hanauske-Abel, H.; Lahn, M.M.; Eismann, U. Antitumor activity of enzastaurin (LY317615. HCl) against human cancer cell lines and freshly explanted tumors investigated in vitro soft-agar cloning experiments. Investig. New Drugs 2007, 25, 205–210. [Google Scholar] [CrossRef]
- Mukohara, T.; Nagai, S.; Koshiji, M.; Yoshizawa, K.; Minami, H. Phase I dose escalation and pharmacokinetic study of oral enzastaurin (LY317615) in advanced solid tumors. Cancer Sci. 2010, 101, 2193–2199. [Google Scholar] [CrossRef]
- Carducci, M.A.; Musib, L.; Kies, M.S.; Pili, R.; Truong, M.; Brahmer, J.R.; Cole, P.; Sullivan, R.; Riddle, J.; Schimidt, J.; et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J. Clin. Oncol. 2006, 24, 4092–4099. [Google Scholar] [CrossRef]
- Oh, Y.; Herbst, R.S.; Burris, H.; Cleverly, A.; Musib, L.; Lahn, M.; Bepler, G. Enzastaurin, an Oral Serine/Threonine Kinase Inhibitor, As Second- or Third-Line Therapy of Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 1135–1141. [Google Scholar] [CrossRef]
- Tekle, C.; Giovannetti, E.; Sigmond, J.; Graff, J.R.; Smid, K.; Peters, G.J. Molecular pathways involved in the synergistic interaction of the PKCβ inhibitor enzastaurin with the antifolate pemetrexed in non-small cell lung cancer cells. Br. J. Cancer 2008, 99, 750–759. [Google Scholar] [CrossRef]
- Tanai, C.; Yamamoto, N.; Ohe, Y.; Takahashi, T.; Kunitoh, H.; Murakami, H.; Yamamoto, N.; Nakamura, Y.; Nokihara, H.; Shukuya, T.; et al. A phase I study of enzastaurin combined with pemetrexed in advanced non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1068–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappori, A.; Bepler, G.; Barlesi, F.; Soria, J.C.; Reck, M.; Bearz, A.; Barata, F.; Scagliotti, G.; Park, K.; Wagle, A.; et al. Phase II, double-blinded, randomized study of enzastaurin plus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Vansteenkiste, J.; Ramlau, R.; Von Pawel, J.; San Antonio, B.; Eschbach, C.; Szczesna, A.; Kennedy, L.; Visseren-Grul, C.; Chouaki, N.; Reck, M. A phase II randomized study of cisplatin-pemetrexed plus either enzastaurin or placebo in chemonaive patients with advanced non-small cell lung cancer. Oncology 2012, 82, 25–29. [Google Scholar] [CrossRef]
- Socinski, M.A.; Raju, R.N.; Stinchcombe, T.; Kocs, D.M.; Couch, L.S.; Barrera, D.; Rousey, S.R.; Choksi, J.K.; Jotte, R.; Patt, D.A.; et al. Randomized, phase II trial of pemetrexed and carboplatin with or without enzastaurin versus docetaxel and carboplatin as first-line treatment of patients with stage IIIB/IV non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1963–1969. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.L.; Cao, F.F.; Wang, Y.; Meng, F.L.; Zhang, Y.; Zhong, D.S.; Zhou, Q.H. The protein kinase C (PKC) inhibitors combined with chemotherapy in the treatment of advanced non-small cell lung cancer: Meta-analysis of randomized controlled trials. Clin. Transl. Oncol. 2015, 17, 371–377. [Google Scholar] [CrossRef]
- Newton, A.C.; Brognard, J. Reversing the Paradigm: Protein Kinase C as a Tumor Suppressor. Trends Pharmacol. Sci. 2017, 38, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Liu, D.; Hornia, A.; Devonish, W.; Pagano, M.; Foster, D.A. Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 1998, 18, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Young, S.; Parker, P.J.; Ullrich, A.; Stabel, S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem. J. 1987, 244, 775–779. [Google Scholar] [CrossRef] [Green Version]
- Gescher, A.; Reed, D.J. Characterization of the growth inhibition induced by tumor-promoting phorbol esters and of their receptor binding in A549 human lung carcinoma cells. Cancer Res. 1985, 45, 4315–4321. [Google Scholar]
- Dale, I.L.; Gescher, A. Effects of activators of protein kinase C, including bryostatins 1 and 2, on the growth of A549 human lung carcinoma cells. Int. J. Cancer 1989, 43, 158–163. [Google Scholar] [CrossRef]
- Dale, I.L.; Bradshaw, T.D.; Gescher, A.; Pettit, G.R. Comparison of effects of bryostatins 1 and 2 and 12-O-tetradecanoylphorbol-13-acetate on protein kinase C activity in A549 human lung carcinoma cells. Cancer Res. 1989, 49, 3242–3245. [Google Scholar] [PubMed]
- Bradshaw, T.D.; Gescher, A.; Pettit, G.R. Modulation by staurosporine of phorbol-ester-induced effects on growth and protein kinase C localization in A549 human lung-carcinoma cells. Int. J. Cancer 1992, 51, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tanwell, C.S.; Gescher, A.; Bradshaw, T.D.; Pettit, G.R. The role of protein kinase C isoenzymes in the growth inhibition caused by bryostatin 1 in human A549 lung and MCF-7 breast carcinoma cells. Int. J. Cancer 1994, 56, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Tahara, E.; Kadara, H.; Lacroix, L.; Lotan, D.; Lotan, R. Activation of protein kinase C by phorbol 12-myristate 13-acetate suppresses the growth of lung cancer cells through KLF6 induction. Cancer Biol. Ther. 2009, 8, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Caino, M.C.; von Burstin, V.A.; Oliva, J.L.; Kazanietz, M.G. Phorbol Ester-Induced Apoptosis and Senescence in Cancer Cell Models. Methods Enzymol. 2008, 446, 123–139. [Google Scholar]
- Hornung, R.L.; Pearson, J.W.; Beckwith, M.; Longo, D.L. Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: In vitro versus in vivo activity. Cancer Res. 1992, 52, 101–107. [Google Scholar]
- Winegarden, J.D.; Mauer, A.M.; Gajewski, T.F.; Hoffman, P.C.; Krauss, S.; Rudin, C.M.; Vokes, E.E. A phase II study of bryostatin-1 and paclitaxel in patients with advanced non-small cell lung cancer. Lung Cancer 2003, 39, 191–196. [Google Scholar] [CrossRef]
- Geiger, T.; Müller, M.; Dean, N.M.; Fabbro, D. Antitumor activity of a PKC-alpha antisense oligonucleotide in combination with standard chemotherapeutic agents against various human tumors transplanted into nude mice. Anticancer Drug Des. 1998, 13, 35–45. [Google Scholar]
- Villalona-Calero, M.A.; Ritch, P.; Figueroa, J.A.; Otterson, G.A.; Belt, R.; Dow, E.; George, S.; Leonardo, J.; McCachren, S.; Miller, G.L.; et al. A Phase I/II study of LY900003, an antisense inhibitor of protein kinase C-α, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2004, 10, 6086–6093. [Google Scholar] [CrossRef] [Green Version]
- Ritch, P.; Rudin, C.M.; Bitran, J.D.; Edelman, M.J.; Makalinao, A.; Irwin, D.; Lilenbaum, R.; Peterson, P.; John, W.J. Phase II study of PKC-α antisense oligonucleotide aprinocarsen in combination with gemcitabine and carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer 2006, 52, 173–180. [Google Scholar] [CrossRef]
- Lynch, T.J.; Raju, R.; Lind, M.; Riviere, A.; Gatzemeier, U.; Dorr, A.; Holmund, J.; Yuen, A.; Sikic, B. Randomized phase III trial of chemotherapy and antisense oligonucleotide LY900003 (ISIS 3521) in patients with advanced NSCLC. Lung Cancer 2003, 41, S35. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Douillard, J.Y.; Koralewski, P.; Manegold, C.; Smit, E.F.; Reyes, J.M.; Chang, G.; John, W.J.; Peterson, P.M.; Obasaju, C.K.; et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.F.; Liu, M.; Clarke, C.J.; Espaillat, M.P.; Haley, J.D.; Jin, T.; Wang, D.; Obeid, L.M.; Hannun, Y.A. PKCα is required for Akt-mTORC1 activation in non-small cell lung carcinoma (NSCLC) with EGFR mutation. Oncogene 2019, 38, 7311–7328. [Google Scholar] [CrossRef]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef] [Green Version]
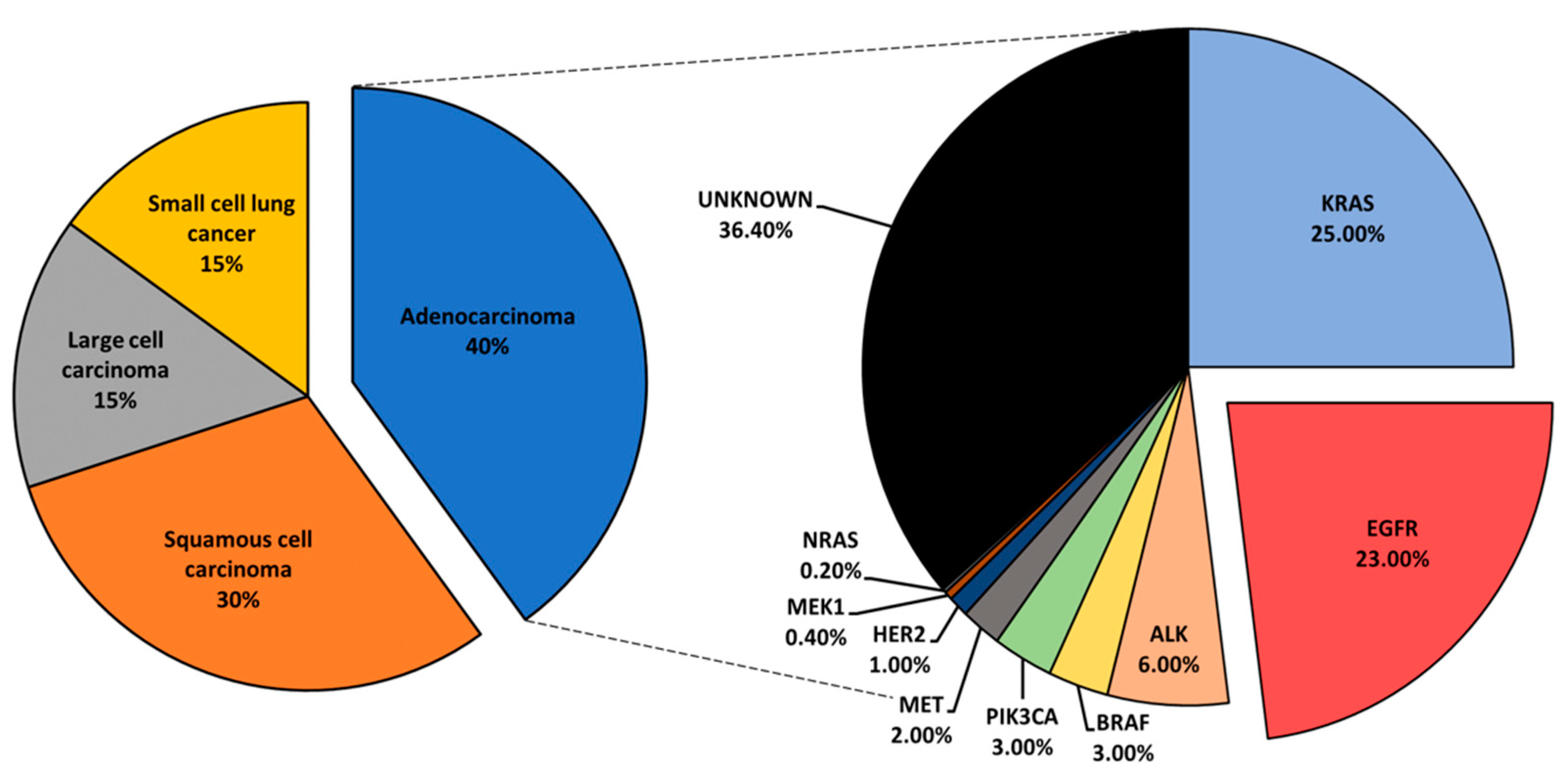

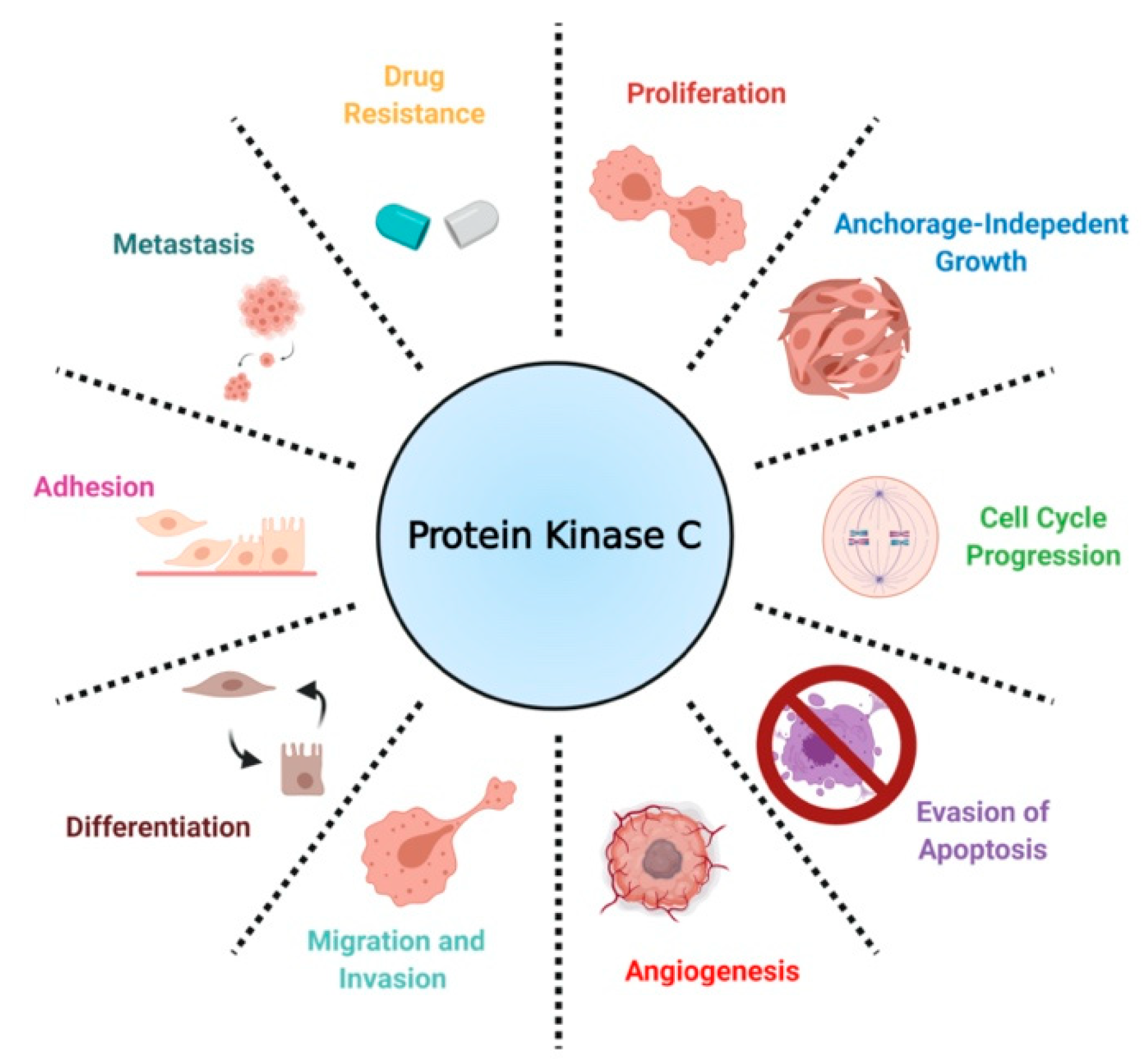
| Isozyme | Biological Roles | References |
|---|---|---|
| PKC α | Promotes proliferation, invasion, migration, cell cycle progression, evasion of apoptosis, drug resistance | [36,37,38,39,40,41,42,82,83] |
| PKC β1, β2 | Unknown | |
| PKC γ | Not expressed | |
| PKC δ | Mediates drug sensitivity, invasion, cell survival | [33,46,47,82,84] |
| PKC ε | Promotes proliferation, invasion, migration, cell cycle progression, anchorage-independent growth, evasion of apoptosis | [48,50,51,52] |
| PKC η | Mediates drug sensitivity | [44] |
| PKC θ | Not expressed | |
| PKC ζ | Chemotaxis | [71] |
| PKC ι | Promotes proliferation, invasion, migration, anchorage-independent growth, evasion of apoptosis, stemness, glucose metabolism | [57,61,62,63,64,65,66,67,68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi, M.M.; Salama, M.F.; Hannun, Y.A. Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 5527. https://doi.org/10.3390/ijms22115527
Sadeghi MM, Salama MF, Hannun YA. Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer. International Journal of Molecular Sciences. 2021; 22(11):5527. https://doi.org/10.3390/ijms22115527
Chicago/Turabian StyleSadeghi, Mohammad Mojtaba, Mohamed F. Salama, and Yusuf A. Hannun. 2021. "Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer" International Journal of Molecular Sciences 22, no. 11: 5527. https://doi.org/10.3390/ijms22115527
APA StyleSadeghi, M. M., Salama, M. F., & Hannun, Y. A. (2021). Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer. International Journal of Molecular Sciences, 22(11), 5527. https://doi.org/10.3390/ijms22115527






