Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils
Abstract
1. Introduction
2. ECS Tone and Its Health Implications
3. Crosstalk between Inflammatory and ECS Signaling Mediators
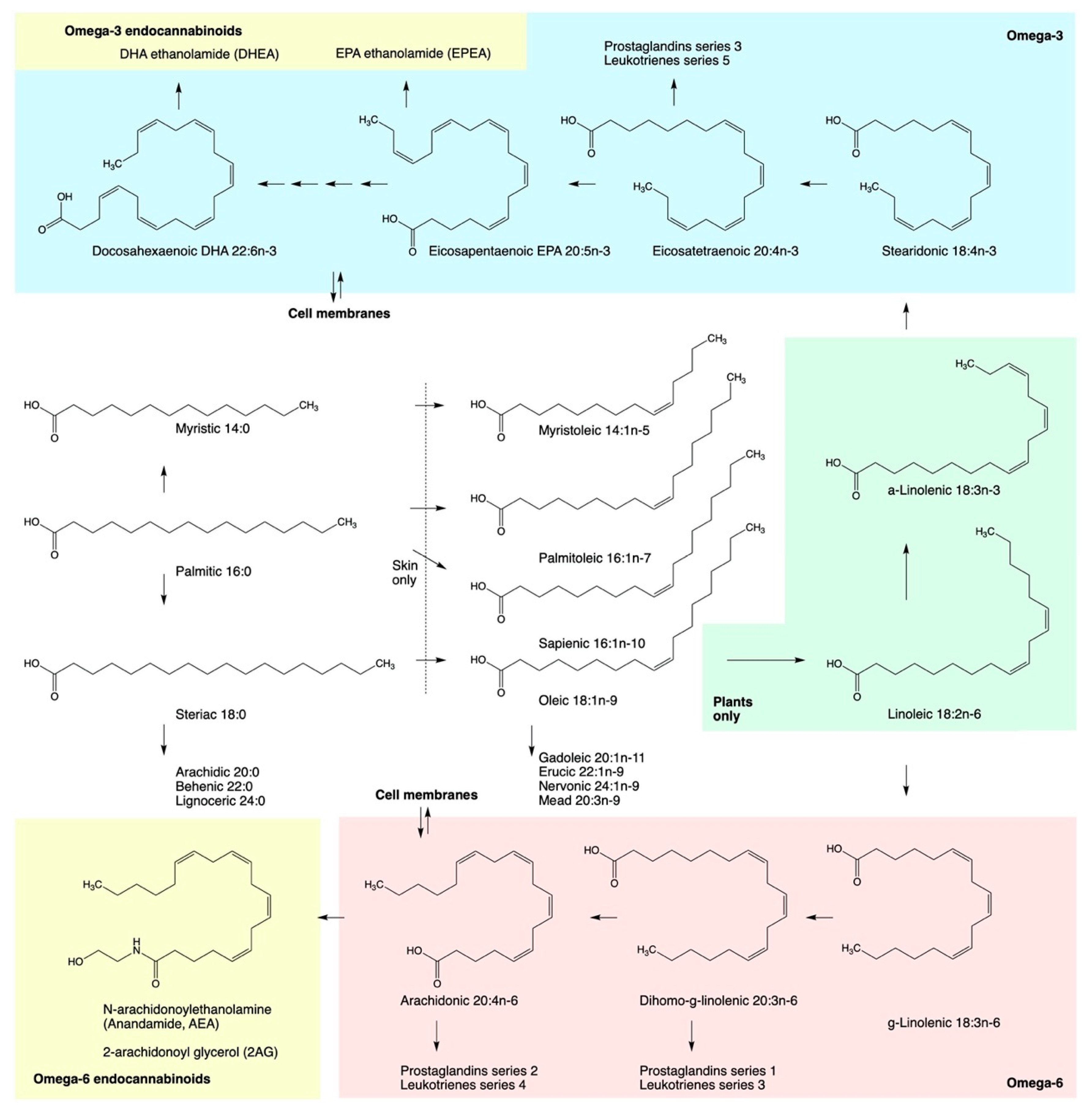
4. Direct Modulation of ECS by Fatty Acids
5. Hemp Oils as ECS Metabolic Modulators
6. Phytochemical Complexity of Hemp Oils
7. ECS and the Oxidative Stress: Role of Dietary Antioxidants
8. Other Dietary Interventions That Target ECS
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| AEA | N-arachidonoylethanolamine |
| ALA | α-linolenic acid |
| CBD | cannabidiol |
| CB | cannabinoid receptor |
| COX | cyclooxygenase |
| DHA | docosahexaenoic acid 22:6(n-3) |
| ECS | endocannabinoid system |
| EPA | eicosapentaenoic acid 20:5(n-3) |
| FA | fatty acid |
| FAAH | fatty acid amide hydrolase |
| HPLC | high-performance liquid chromatography |
| IL | interleukin |
| LOX | lipoxygenase |
| MAGL | monoacylglycerol lipase |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PPAR | peroxisome proliferator-activated receptor |
| PUFA | polyunsaturated omega fatty acid |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| THC | tetrahydrocannabinol |
| TNF-α | tumor necrosis factor-α |
| TRP | transient receptor potential channel s (PPARs) |
| SPM | specialized proresolving mediators |
References
- Gertsch, J. Cannabimimetic Phytochemicals in the Diet - an Evolutionary Link to Food Selection and Metabolic Stress Adaptation? Br. J. Pharmacol. 2017, 174, 1464–1483. [Google Scholar] [CrossRef]
- Di Marzo, V. “Endocannabinoids” and Other Fatty Acid Derivatives with Cannabimimetic Properties: Biochemistry and Possible Physiopathological Relevance. Biochim. Biophys. Acta 1998, 1392, 153–175. [Google Scholar] [CrossRef]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated Fatty Acids and Endocannabinoids in Health and Disease. Nutr. Neurosci. 2018, 21, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-A.; Wang, T.-Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.H.; Chen, Y.-R.; Druhan, L.J.; Zweier, J.L. S-Glutathionylation Uncouples ENOS and Regulates Its Cellular and Vascular Function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef]
- Han, Z.; Varadharaj, S.; Giedt, R.J.; Zweier, J.L.; Szeto, H.H.; Alevriadou, B.R. Mitochondria-Derived Reactive Oxygen Species Mediate Heme Oxygenase-1 Expression in Sheared Endothelial Cells. J. Pharmacol. Exp. Ther. 2009, 329, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, N.S.; Varadharaj, S.; Khanderao, G.D.; Davidson, C.J.; Kannan, S.; Firpo, M.A.; Zweier, J.L.; Benjamin, I.J. Sustained Activation of Nuclear Erythroid 2-Related Factor 2/Antioxidant Response Element Signaling Promotes Reductive Stress in the Human Mutant Protein Aggregation Cardiomyopathy in Mice. Antioxid. Redox Signal. 2011, 14, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated Fatty Acids and Their Effects on Cardiovascular Disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar]
- Burdge, G.C.; Calder, P.C. Conversion of Alpha-Linolenic Acid to Longer-Chain Polyunsaturated Fatty Acids in Human Adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Maccarrone, M. Endocannabinoid Signaling and Its Regulation by Nutrients. Biofactors 2014, 40, 373–380. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. COX-2-Derived Endocannabinoid Metabolites as Novel Inflammatory Mediators. Trends Pharmacol. Sci. 2014, 35, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Chiu, C.-Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 Fatty Acids and Their Lipid Mediators: Towards an Understanding of Resolvin and Protectin Formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging Class of Omega-3 Fatty Acid Endocannabinoids & Their Derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2020, 64, 1900824. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and Endocannabinoid-Related Mediators: Targets, Metabolism and Role in Neurological Disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Elphick, M.R. The Evolution and Comparative Neurobiology of Endocannabinoid Signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early Phytocannabinoid Chemistry to Endocannabinoids and Beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Ross, R.A. Cannabinoid Receptors and Their Ligands. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 101–121. [Google Scholar] [CrossRef]
- Ross, R.A. Anandamide and Vanilloid TRPV1 Receptors. Br. J. Pharmacol. 2003, 140, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.H.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Spedding, M.; Peters, J.A.; Harmar, A.J.; CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br. J. Pharmacol. 2013, 170, 1459–1581. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid Receptors: Where They Are and What They Do. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 10–14. [Google Scholar] [CrossRef]
- Járai, Z.; Wagner, J.A.; Varga, K.; Lake, K.D.; Compton, D.R.; Martin, B.R.; Zimmer, A.M.; Bonner, T.I.; Buckley, N.E.; Mezey, E.; et al. Cannabinoid-Induced Mesenteric Vasodilation through an Endothelial Site Distinct from CB1 or CB2 Receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 14136–14141. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Suplita, R.L. Endocannabinoid Mechanisms of Pain Modulation. AAPS J. 2006, 8, E693–E708. [Google Scholar] [CrossRef]
- Malan, T.P.; Ibrahim, M.M.; Vanderah, T.W.; Makriyannis, A.; Porreca, F. Inhibition of Pain Responses by Activation of CB2 Cannabinoid Receptors. Chem. Phys. Lipids 2002, 121, 191–200. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.; Larsen, K.; Lu, L.; Perkins, I.; Nong, L.; Friedman, H. The Cannabinoid System and Immune Modulation. J. Leukoc. Biol. 2003, 74, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Mechoulam, R. Is Lipid Signaling through Cannabinoid 2 Receptors Part of a Protective System? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kunos, G. Modulating the Endocannabinoid System in Human Health and Disease: Successes and Failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Canseco-Alba, A.; Zhang, H.-Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid Type 2 Receptors in Dopamine Neurons Inhibits Psychomotor Behaviors, Alters Anxiety, Depression and Alcohol Preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Jurado-Barba, R.; Rubio, G.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Roques, B.P.; Fournié-Zaluski, M.-C.; Wurm, M. Inhibiting the Breakdown of Endogenous Opioids and Cannabinoids to Alleviate Pain. Nat. Rev. Drug Discov. 2012, 11, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, G.; Alapafuja, S.O.; Bátkai, S.; Nikas, S.P.; Cinar, R.; Offertáler, L.; Osei-Hyiaman, D.; Liu, J.; Mukhopadhyay, B.; Harvey-White, J.; et al. Inhibitor of Fatty Acid Amide Hydrolase Normalizes Cardiovascular Function in Hypertension without Adverse Metabolic Effects. Chem. Biol. 2010, 17, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Mulvihill, M.M.; Mukhopadhyay, P.; Xu, H.; Erdélyi, K.; Hao, E.; Holovac, E.; Haskó, G.; Cravatt, B.F.; Nomura, D.K.; et al. Monoacylglycerol Lipase Controls Endocannabinoid and Eicosanoid Signaling and Hepatic Injury in Mice. Gastroenterology 2013, 144, 808–817.e15. [Google Scholar] [CrossRef]
- Huggins, J.P.; Smart, T.S.; Langman, S.; Taylor, L.; Young, T. An Efficient Randomised, Placebo-Controlled Clinical Trial with the Irreversible Fatty Acid Amide Hydrolase-1 Inhibitor PF-04457845, Which Modulates Endocannabinoids but Fails to Induce Effective Analgesia in Patients with Pain Due to Osteoarthritis of the Knee. Pain 2012, 153, 1837–1846. [Google Scholar] [CrossRef]
- Nomura, D.K.; Morrison, B.E.; Blankman, J.L.; Long, J.Z.; Kinsey, S.G.; Marcondes, M.C.G.; Ward, A.M.; Hahn, Y.K.; Lichtman, A.H.; Conti, B.; et al. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science 2011, 334, 809–813. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid Oxygenation by Cyclooxygenases, Lipoxygenases, and Cytochromes P450: Cross-Talk between the Eicosanoid and Endocannabinoid Signaling Pathways. Chem. Rev. 2011, 111, 5899–5921. [Google Scholar] [CrossRef]
- Forsell, P.K.A.; Brunnström, A.; Johannesson, M.; Claesson, H.-E. Metabolism of Anandamide into Eoxamides by 15-Lipoxygenase-1 and Glutathione Transferases. Lipids 2012, 47, 781–791. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The Inflammatory Response to Cell Death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid Mediator Class Switching during Acute Inflammation: Signals in Resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel Lipid Mediators and Resolution Mechanisms in Acute Inflammation: To Resolve or Not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Mirakaj, V.; Serhan, C.N. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front. Immunol. 2012, 3, 81. [Google Scholar] [CrossRef]
- DeLuca, P.; Rothman, D.; Zurier, R.B. Marine and Botanical Lipids as Immunomodulatory and Therapeutic Agents in the Treatment of Rheumatoid Arthritis. Rheum. Dis. Clin. North Am. 1995, 21, 759–777. [Google Scholar] [PubMed]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Salem, N.; Pawlosky, R.; Wegher, B.; Hibbeln, J. In Vivo Conversion of Linoleic Acid to Arachidonic Acid in Human Adults. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 407–410. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Hargrove, R.L.; Zhao, G.; Etherton, T.D. Polyunsaturated Fatty Acids in the Food Chain in the United States. Am. J. Clin. Nutr. 2000, 71, 179S–188S. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in Consumption of Omega-3 and Omega-6 Fatty Acids in the United States during the 20th Century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Bosma-den Boer, M.M.; van Wetten, M.-L.; Pruimboom, L. Chronic Inflammatory Diseases Are Stimulated by Current Lifestyle: How Diet, Stress Levels and Medication Prevent Our Body from Recovering. Nutr. Metab. 2012, 9, 32. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The Effects of Diet on Inflammation: Emphasis on the Metabolic Syndrome. J. Am. Coll. Cardiol. 2006. [Google Scholar] [CrossRef]
- Fichna, J.; Bawa, M.; Thakur, G.A.; Tichkule, R.; Makriyannis, A.; McCafferty, D.-M.; Sharkey, K.A.; Storr, M. Cannabinoids Alleviate Experimentally Induced Intestinal Inflammation by Acting at Central and Peripheral Receptors. PLoS ONE 2014, 9, e109115. [Google Scholar] [CrossRef] [PubMed]
- Gatta-Cherifi, B.; Cota, D. New Insights on the Role of the Endocannabinoid System in the Regulation of Energy Balance. Int. J. Obes. 2016, 40, 210–219. [Google Scholar] [CrossRef]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid System Acts as a Regulator of Immune Homeostasis in the Gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef]
- Horn, H.; Böhme, B.; Dietrich, L.; Koch, M. Endocannabinoids in Body Weight Control. Pharmaceuticals 2018, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating Levels of Endocannabinoids and Oxylipins Altered by Dietary Lipids in Older Women Are Likely Associated with Previously Identified Gene Targets. Biochim. Biophys. Acta 2016, 1861, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Arachidonic Acid as a Bioactive Molecule. J. Clin. Invest. 2001, 107, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Bordicchia, M.; Marcucci, P.; Bedetta, S.; Santini, S.; Giovagnoli, A.; Scappini, L.; Minardi, D.; Muzzonigro, G.; Dessì-Fulgheri, P.; et al. Altered Pattern of Cannabinoid Type 1 Receptor Expression in Adipose Tissue of Dysmetabolic and Overweight Patients. Metab. Clin. Exp. 2009, 58, 361–367. [Google Scholar] [CrossRef]
- Gatta-Cherifi, B.; Matias, I.; Vallée, M.; Tabarin, A.; Marsicano, G.; Piazza, P.V.; Cota, D. Simultaneous Postprandial Deregulation of the Orexigenic Endocannabinoid Anandamide and the Anorexigenic Peptide YY in Obesity. Int. J. Obes. 2012, 36, 880–885. [Google Scholar] [CrossRef]
- Balvers, M.G.J.; Verhoeckx, K.C.M.; Meijerink, J.; Bijlsma, S.; Rubingh, C.M.; Wortelboer, H.M.; Witkamp, R.F. Time-Dependent Effect of in Vivo Inflammation on Eicosanoid and Endocannabinoid Levels in Plasma, Liver, Ileum and Adipose Tissue in C57BL/6 Mice Fed a Fish-Oil Diet. Int. Immunopharmacol. 2012, 13, 204–214. [Google Scholar] [CrossRef]
- Hutchins, H.L.; Li, Y.; Hannon, K.; Watkins, B.A. Eicosapentaenoic Acid Decreases Expression of Anandamide Synthesis Enzyme and Cannabinoid Receptor 2 in Osteoblast-like Cells. J. Nutr. Biochem. 2011, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Rathmell, J.C. Metabolic Pathways in T Cell Fate and Function. Trends Immunol. 2012, 33, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.; Smith, K.N.; Rajnarayanan, R. Analysis of Natural Product Regulation of Cannabinoid Receptors in the Treatment of Human Disease. Pharmacol. Ther. 2017, 180, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Breivogel, C.S.; Childers, S.R.; Deadwyler, S.A.; Hampson, R.E.; Porrino, L.J. Cannabinoid Physiology and Pharmacology: 30 Years of Progress. Neuropharmacology 2004, 47 (Suppl. 1), 345–358. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Compton, D.R.; Rice, K.C.; De Costa, B.R.; Razdan, R.K.; Melvin, L.S.; Johnson, M.R.; Martin, B.R. Cannabinoid Structure-Activity Relationships: Correlation of Receptor Binding and in Vivo Activities. J. Pharmacol. Exp. Ther. 1993, 265, 218–226. [Google Scholar] [PubMed]
- Tramèr, M.R.; Carroll, D.; Campbell, F.A.; Reynolds, D.J.; Moore, R.A.; McQuay, H.J. Cannabinoids for Control of Chemotherapy Induced Nausea and Vomiting: Quantitative Systematic Review. BMJ 2001, 323, 16–21. [Google Scholar] [CrossRef]
- Aagaard, L.; Hallgreen, C.E.; Hansen, E.H. Serious Adverse Events Reported for Antiobesity Medicines: Postmarketing Experiences from the EU Adverse Event Reporting System EudraVigilance. Int. J. Obes. 2016, 40, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Cocchetto, D.M.; Cook, L.F.; Cato, A.E. A Critical Review of the Safety and Antiemetic Efficacy of Delta-9-Tetrahydrocannabinol. Drug Intell. Clin. Pharm. 1981, 15, 867–875. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ9-Tetrahydrocannabinol, Cannabidiol and Δ9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are Cannabidiol and Δ(9) -Tetrahydrocannabivarin Negative Modulators of the Endocannabinoid System? A Systematic Review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty Acid-Binding Proteins (FABPs) Are Intracellular Carriers for Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an Equilibrative Nucleoside Transporter by Cannabidiol: A Mechanism of Cannabinoid Immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Corrêa, F.M.; Guimarães, F.S. 5-HT1A Receptors Are Involved in the Cannabidiol-Induced Attenuation of Behavioural and Cardiovascular Responses to Acute Restraint Stress in Rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Nemo, L. The Race to Relearn Hemp Farming. Available online: https://www.scientificamerican.com/article/the-race-to-relearn-hemp-farming/ (accessed on 11 January 2021).
- Pate, D.W. Chemical Ecology of Cannabis. J. Int. Hemp Assoc. 1994, 2, 32–37. [Google Scholar]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Potter, D.J. A Review of the Cultivation and Processing of Cannabis (Cannabis sativa L.) for Production of Prescription Medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Clifford, B. The Quantitative Determination of Phytocannabinoids in Hemp Oils Using HPLC with UV Detection. Cannabis Sci. Technol. 2018, 1, 38–43. [Google Scholar]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Zaccara, G.; Cagnetti, C.; Del Giovane, C.; Silvestrini, M. Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs 2018, 78, 1791–1804. [Google Scholar] [CrossRef]
- Varadharaj, S.; Kelly, O.J.; Khayat, R.N.; Kumar, P.S.; Ahmed, N.; Zweier, J.L. Role of Dietary Antioxidants in the Preservation of Vascular Function and the Modulation of Health and Disease. Front. Cardiovasc. Med. 2017, 4, 64. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Janefjord, E.; Mååg, J.L.V.; Harvey, B.S.; Smid, S.D. Cannabinoid Effects on β Amyloid Fibril and Aggregate Formation, Neuronal and Microglial-Activated Neurotoxicity in Vitro. Cell. Mol. Neurobiol. 2014, 34, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mnich, K.; Finn, D.P.; Dowd, E.; Gorman, A.M. Inhibition by Anandamide of 6-Hydroxydopamine-Induced Cell Death in PC12 Cells. Int. J. Cell Biol. 2010, 2010, 818497. [Google Scholar] [CrossRef] [PubMed]
- Casarejos, M.J.; Perucho, J.; Gomez, A.; Muñoz, M.P.; Fernandez-Estevez, M.; Sagredo, O.; Fernandez Ruiz, J.; Guzman, M.; de Yebenes, J.G.; Mena, M.A. Natural Cannabinoids Improve Dopamine Neurotransmission and Tau and Amyloid Pathology in a Mouse Model of Tauopathy. J. Alzheimers Dis. 2013, 35, 525–539. [Google Scholar] [CrossRef]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.-W.; Kim, Y.; Kang, J.-H.; Kang, S.-S.; Ahn, Y.K.; Park, C.-S.; Kim, J.J. CB1 and CB2 Cannabinoid Receptors Differentially Regulate the Production of Reactive Oxygen Species by Macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Wang, M.; Abais, J.M.; Meng, N.; Zhang, Y.; Ritter, J.K.; Li, P.-L.; Tang, W.-X. Upregulation of Cannabinoid Receptor-1 and Fibrotic Activation of Mouse Hepatic Stellate Cells during Schistosoma J. Infection: Role of NADPH Oxidase. Free Radic. Biol. Med. 2014, 71, 109–120. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Wang, Q.; Hu, W.; Wang, D.; Li, X.; Su, T.; Qin, X.; Zhang, X.; Ma, K.; et al. Effects of Cannabinoid Receptor Type 2 on Endogenous Myocardial Regeneration by Activating Cardiac Progenitor Cells in Mouse Infarcted Heart. Sci. China Life Sci. 2014, 57, 201–208. [Google Scholar] [CrossRef]
- Vacek, J.; Vostalova, J.; Papouskova, B.; Skarupova, D.; Kos, M.; Kabelac, M.; Storch, J. Antioxidant Function of Phytocannabinoids: Molecular Basis of Their Stability and Cytoprotective Properties under UV-Irradiation. Free Radic. Biol. Med. 2021, 164, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Activation in the Treatment of Neurodegenerative Diseases: A Focus on Its Role in Mitochondrial Bioenergetics and Function. Biol. Chem. 2016, 397, 383–400. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 Cell Defence Pathway: Keap1-Dependent and -Independent Mechanisms of Regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of Humans by Plant Glucosinolates: Efficiency of Conversion of Glucosinolates to Isothiocyanates by the Gastrointestinal Microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of Alkylamides in Echinacea Plant Materials and Dietary Supplements by Ultrafast Liquid Chromatography with Diode Array and Mass Spectrometric Detection. J. Agric. Food Chem. 2011, 59, 8086–8094. [Google Scholar] [CrossRef] [PubMed]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea Are a New Class of Cannabinomimetics. Cannabinoid Type 2 Receptor-Dependent and -Independent Immunomodulatory Effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, Z.; Nicolussi, S.; Rau, M.; Lorántfy, L.; Forgo, P.; Hohmann, J.; Csupor, D.; Gertsch, J. Identification of Endocannabinoid System-Modulating N-Alkylamides from Heliopsis Helianthoides Var. Scabra and Lepidium Meyenii. J. Nat. Prod 2014, 77, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Villano, R.; Allarà, M.; Ujváry, I.; Di Marzo, V. Kavalactones and the Endocannabinoid System: The Plant-Derived Yangonin Is a Novel CB1 Receptor Ligand. Pharmacol. Res. 2012, 66, 163–169. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L.; Raduner, S.; Cottiglia, F.; Floris, C.; Altmann, K.-H.; Gertsch, J. Falcarinol Is a Covalent Cannabinoid CB1 Receptor Antagonist and Induces Pro-Allergic Effects in Skin. Biochem. Pharmacol. 2010, 79, 1815–1826. [Google Scholar] [CrossRef]
- Keppel Hesselink, J.M.; de Boer, T.; Witkamp, R.F. Palmitoylethanolamide: A Natural Body-Own Anti-Inflammatory Agent, Effective and Safe against Influenza and Common Cold. Int. J. Inflam. 2013, 2013, 151028. [Google Scholar] [CrossRef] [PubMed]
- Wośko, S.; Serefko, A.; Szopa, A.; Wlaź, P.; Wróbel, A.; Wlaź, A.; Górska, J.; Poleszak, E. CB1 Cannabinoid Receptor Ligands Augment the Antidepressant-like Activity of Biometals (Magnesium and Zinc) in the Behavioural Tests. J. Pharm. Pharmacol. 2018, 70, 566–575. [Google Scholar] [CrossRef]
- Simon, V.; Cota, D. MECHANISMS IN ENDOCRINOLOGY: Endocannabinoids and Metabolism: Past, Present and Future. Eur. J. Endocrinol. 2017, 176, R309–R324. [Google Scholar] [CrossRef]
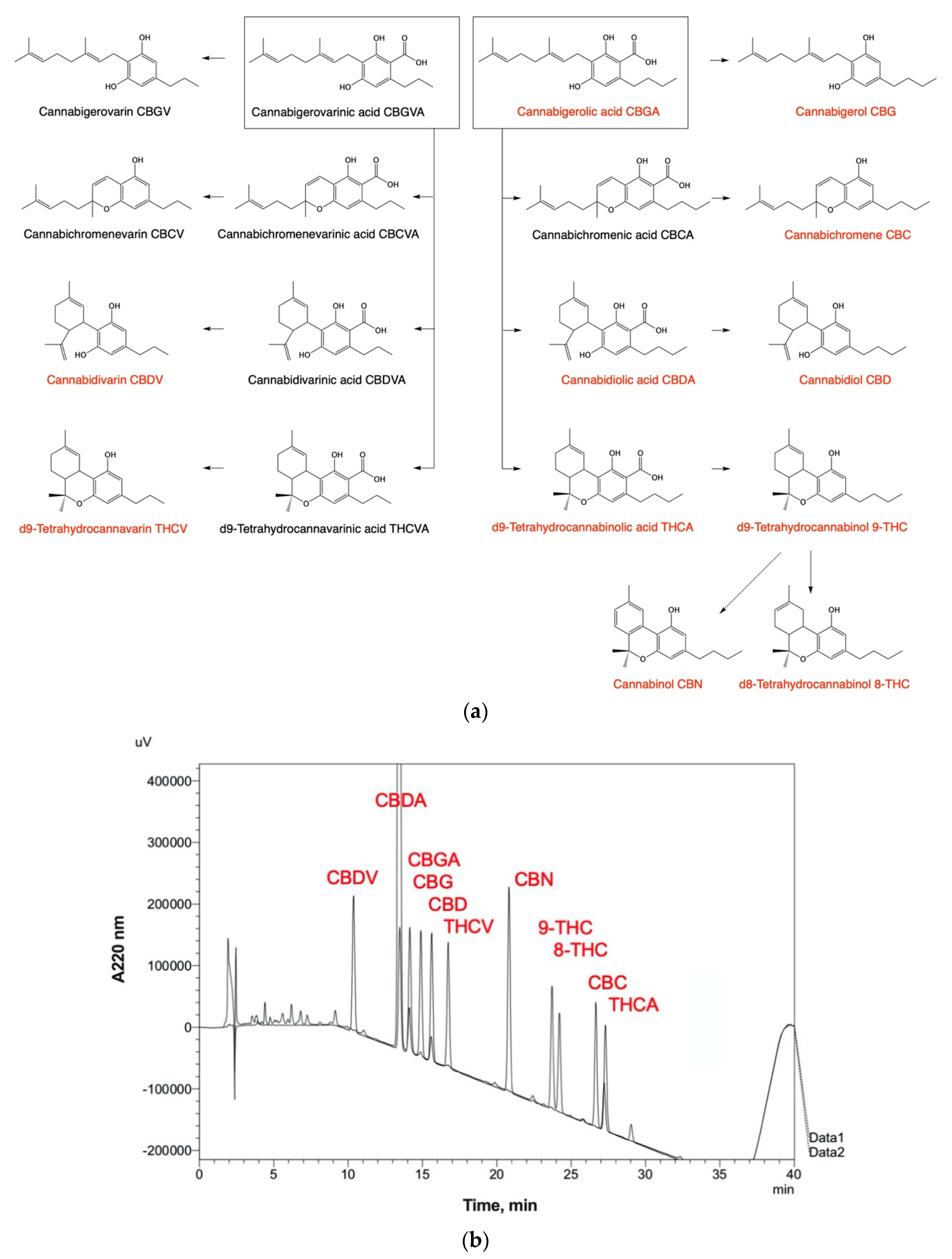
| Step | Detailed Description | Comments |
|---|---|---|
| 1a | Hemp tissues and powdered samples: Weigh 1 g of air-dried samples from sealed bags in triplicate Add 20 mL of methanol/chloroform (9:1, v:v) Agitate in an orbital shaker for 30 min at 200 rpm and 37 °C Sonicate in an ultrasonic bath for 30 min at 37 °C Centrifuge for 5 min at 3000 rpm and RT, and collect supernatant Repeat twice and combine supernatants into a single sample Evaporate to dryness and dissolve in 1 mL methanol Collect supernatant and filter through a 0.45 µm PTFE syringe filter | Whole plant complexity, minor cannabinoids of importance, and a variable terpenoid profile may all contribute to beneficial entourage effect of hemp |
| 1b | Hemp oils and liquid formulations: Add 400 µL of isopropanol to a 2 mL Eppendorf tube Add 10 µL of liquid sample and completely dissolve Vortex the sample for 30 sec at RT Add 400 µL of methanol to the mixture Vortex the sample for 30 sec at RT Filter through a 0.45 µm PTFE syringe filter | Hemp oil density is 0.92 (used as a conversion factor to calculate volume to weight ratio) |
| 1c | Biological fluids (urine or plasma): Add 1 mL urine (3 mL plasma) and 20 µL of DDT to a 20 mL glass vial Add 3 mL of 100 mM sodium acetate buffer (pH 4.8) and mix briefly Add 375 μL of β-glucuronidase in acetate buffer Vortex briefly and incubate at 37 °C for 16 h Add 15 mL of ice cold 1% formic acid in acetonitrile Vortex briefly and sonicate in an ultrasonic bath for 3 min at RT Centrifuge for 5 min at 10,000 rpm and RT, and collect supernatant Add 15 mL methanol to the pellet and vortex briefly Repeat once and combine supernatants into a single sample Evaporate to dryness and dissolve in 200 µL methanol | 4,4- Dichlorodiphenyltrichloro ethane (DDT, 50 µg/mL) is used as an internal analytical standard; β-glucuronidase (Abalone) |
| 2 | Standard curves over a linear dynamic range of 0.5–100 μg/mL (ppm) | Shimadzu #220-91239-21 |
| 3 | Instrument: Shimadzu Prominence LC-2030C UV Column: Restek Ultra C18 (250 mm × 4.6 mm, 5 μm dp) Guard column: Restek Ultra C18 Guard (10 mm × 2.1 mm, 5 μm dp) Mobile-phase A: 0.1% formic acid in water Mobile-phase B: 100% acetonitrile Flow rate: 1 mL/min; Column temperature: 30 °C Injection volume: 20 μL; Detection: 220 nm | Cannabinoid totals are calculated as the sum of the neutral form and the acid form multiplied by the conversion factors (0.877 for THCA and CBDA; 0.878 for CBGA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarnytsky, S.; Rathinasabapathy, T.; Wagner, C.; Metzger, B.; Carlisle, C.; Panda, C.; Le Brun-Blashka, S.; Troup, J.P.; Varadharaj, S. Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils. Int. J. Mol. Sci. 2021, 22, 5479. https://doi.org/10.3390/ijms22115479
Komarnytsky S, Rathinasabapathy T, Wagner C, Metzger B, Carlisle C, Panda C, Le Brun-Blashka S, Troup JP, Varadharaj S. Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils. International Journal of Molecular Sciences. 2021; 22(11):5479. https://doi.org/10.3390/ijms22115479
Chicago/Turabian StyleKomarnytsky, Slavko, Thirumurugan Rathinasabapathy, Charles Wagner, Brandon Metzger, Carolina Carlisle, Chinmayee Panda, Sara Le Brun-Blashka, John P. Troup, and Saradhadevi Varadharaj. 2021. "Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils" International Journal of Molecular Sciences 22, no. 11: 5479. https://doi.org/10.3390/ijms22115479
APA StyleKomarnytsky, S., Rathinasabapathy, T., Wagner, C., Metzger, B., Carlisle, C., Panda, C., Le Brun-Blashka, S., Troup, J. P., & Varadharaj, S. (2021). Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils. International Journal of Molecular Sciences, 22(11), 5479. https://doi.org/10.3390/ijms22115479







