The Tumor Microenvironment in Follicular Lymphoma: Its Pro-Malignancy Role with Therapeutic Potential
Abstract
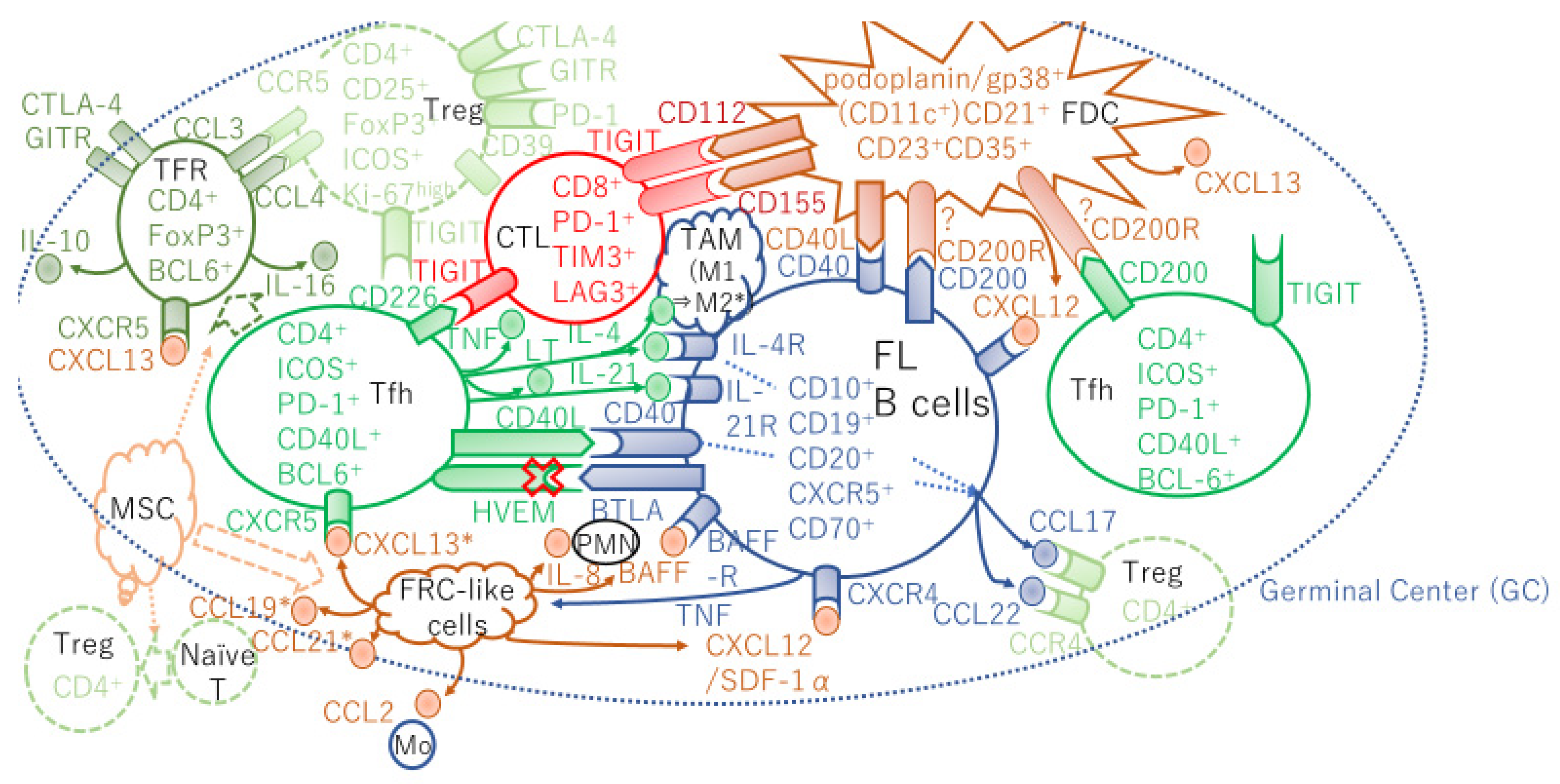
1. Introduction
2. Heterogeneity of Lymphoid Stromal Cells
3. Follicular Dendritic Cell Role in Healthy and Neoplastic Follicles
4. Mesenchymal Stem Cells Orchestrate the FL Cell Niche and Cancer-Associated Fibroblasts in the FL Microenvironment
5. Follicular Helper T Cells
6. The Herpes Virus Entry Mediator/B- and T-Lymphocyte Attenuator Axis
7. T-follicular Regulatory Cells
8. Other T Cell Dysfunctions in FL
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2AR | adenosine 2A receptor |
| ABC | adenosine triphosphate-binding cassette |
| ABCC1 | ABC subfamily C member 1 |
| ABCG2 | ABC sub-family G member 2 |
| APRIL | a proliferation-inducing ligand |
| BAFF | B-cell-activating factor belonging to the tumor necrosis factor family |
| BCL2 | B-cell lymphoma 2 |
| BCL6 | B-cell lymphoma 6 |
| BCL-XL | B-cell lymphoma-extra-large |
| BM | bone marrow |
| B-NHL | B-cell non-Hodgkin lymphoma |
| BTK | Bruton’s tyrosine kinase |
| BTLA | B- and T-lymphocyte attenuator |
| CAFs | cancer-associated fibroblasts |
| CAR | chimeric antigen receptor |
| CCL | C-C motif chemokine ligand |
| CCR7 | C-C chemokine receptor type 7 |
| CD | cluster of differentiation |
| CD40L | CD40 ligand |
| CRCs | CXCL12-expressing reticular cells |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| CXCL | C-X-C motif chemokine ligand |
| CXCR5 | C-X-C chemokine receptor type 5 |
| CTSS | cathepsin S |
| DCs | dendritic cells |
| FDCs | follicular dendritic cells |
| ERK | extracellular signal-regulated kinase |
| EZH2 | epigenetic regulator zeste hololog 2 |
| FL | follicular lymphoma |
| FoxP3 | forkhead box protein 3 |
| FRCs | fibroblastic reticular cells |
| GC | germinal center |
| GITR | glucocorticoid-induced tumor necrosis factor-related protein |
| HVEM | herpes virus entry mediator |
| ICAM-1 | intercellular adhesion molecule 1 |
| ICOS | inducible T-cell co-stimulator |
| ICOSL | ICOS ligand |
| IFN-γ | interferon-γ |
| IL | interleukin |
| ITIM | immunoreceptor tyrosine-based inhibition motif |
| KMT2D | lysin N-methyltransferase 2D |
| LAG-3 | lymphocyte-activation gene 3 |
| LFA-1 | lymphocyte function-associated antigen-1 |
| LN | lymph node |
| LT | lymphotoxin-α1β2 |
| MedRC | medullary FRCs |
| MSCs | mesenchymal stem cells |
| MIP-1 | macrophage inflammatory protein-1 |
| MRCs | marginal reticular cells |
| OS | overall survival |
| PD-1 | programmed cell death-1 |
| PDPN | podoplanin |
| PFS | progression-free survival |
| PGE2 | prostaglandin E2 |
| p-STAT6 | phospho-signal transducer and activator of transcription 6 |
| R/R | relapsed or refractory |
| SH2 | Src homology domain 2 |
| SHP-1 | SH2-containing protein tyrosine phosphatase-1 |
| Syk | spleen tyrosine kinase |
| TAM | tumor-associated macrophages |
| Tfh | follicular helper T cells |
| tFL | transformed FL |
| TFRs | T-follicular regulatory cells |
| TG | transglutaminase |
| TIGIT | T-cell immunoglobulin and ITIM domain |
| TIM-3 | T-cell immunoglobulin and mucin domain-containing protein 3 |
| TNF | tumor necrosis factor |
| TNFRSF14 | Tumor necrosis factor receptor superfamily 14 |
| TRCs | T-cell zone reticular cells |
| Treg | regulatory T cell |
| TTT | time to transformation |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Montoto, S.; Davies, A.J.; Matthews, J.; Calaminici, M.; Norton, A.J.; Amess, J.; Vinnicombe, S.; Waters, R.; Rohatiner, A.Z.; Lister, T.A. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J. Clin. Oncol. 2007, 25, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Al-Tourah, A.J.; Gill, K.K.; Chhanabhai, M.; Hoskins, P.J.; Klasa, R.J.; Savage, K.J.; Sehn, L.H.; Shenkier, T.N.; Gascoyne, R.D.; Connors, J.M. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J. Clin. Oncol. 2008, 26, 5165–5169. [Google Scholar] [CrossRef]
- Watanabe, T.; Tobinai, K.; Wakabayashi, M.; Morishima, Y.; Kobayashi, H.; Kinoshita, T.; Suzuki, T.; Yamaguchi, M.; Ando, K.; Ogura, M.; et al. Outcomes after R-CHOP in patients with newly diagnosed advanced follicular lymphoma: A 10-year follow-up analysis of the JCOG0203 trial. Lancet Haematol. 2018, 5, e520–e531. [Google Scholar] [CrossRef]
- Kridel, R.; Xerri, L.; Gelas-Dore, B.; Tan, K.; Feugier, P.; Vawda, A.; Canioni, D.; Farinha, P.; Boussetta, S.; Moccia, A.A.; et al. The prognostic impact of CD163-positive macrophages in follicular lymphoma: A study from the BC cancer agency and the lymphoma study association. Clin. Cancer Res. 2015, 21, 3428–3435. [Google Scholar] [CrossRef]
- Farinha, P.; Al-Tourah, A.; Gill, K.; Klasa, R.; Connors, J.M.; Gascoyne, R.D. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood 2010, 115, 289–295. [Google Scholar] [CrossRef]
- Carreras, J.; Lopez-Guillermo, A.; Fox, B.C.; Colomo, L.; Martinez, A.; Roncador, G.; Montserrat, E.; Campo, E.; Banham, A.H. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 2006, 108, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Grote, D.M.; Ziesmer, S.C.; Xiu, B.; Novak, A.J.; Ansell, S.M. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015, 5. [Google Scholar] [CrossRef]
- Wang, H.W.; Joyce, J.A. Alternative activation of tumor-associated macrophages by IL-4 Priming for protumoral functions. Cell Cycle 2010, 9, 4824–4835. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Fletcher, A.L.; Astarita, J.; Lukacs-Kornek, V.; Tayalia, P.; Gonzalez, S.F.; Elpek, K.G.; Chang, S.K.; Knoblich, K.; Helmer, M.E.; et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 2012, 13, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Tanaka, S.; Chu, F.; I Nurieva, R.; Martinez, G.J.; Rawal, S.; Wang, Y.-H.; Lim, H.; Reynolds, J.M.; Zhou, X.-H.; et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011, 17, 983–988. [Google Scholar] [CrossRef]
- Linterman, M.A.; Pierson, W.; Lee, S.K.; Kallies, A.; Kawamoto, S.; Rayner, T.F.; Srivastava, M.; Divekar, D.P.; Beaton, L.; Hogan, J.J.; et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011, 17, 975–982. [Google Scholar] [CrossRef]
- Wollenberg, I.; Agua-Doce, A.; Hernández, A.; Almeida, C.; Oliveira, V.G.; Faro, J.; Graca, L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 2011, 187, 4553–4560. [Google Scholar] [CrossRef]
- Brady, M.T.; Hilchey, S.P.; Hyrien, O.; Spence, S.A.; Bernstein, S.H. Mesenchymal stromal cells support the viability and differentiation of follicular lymphoma-infiltrating follicular helper T-cells. PLoS ONE 2014, 9, e97597. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Vogt, T.K.; Favre, S.; Britschgi, M.R.; Acha-Orbea, H.; Hinz, B.; Cyster, J.G.; Luther, S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tech, L.; George, L.A.; Acs, A.; Durrett, R.E.; Hess, H.; Walker, L.S.K.; Tarlinton, D.M.; Fletcher, A.L.; Hauser, A.E.; et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J. Exp. Med. 2018, 215, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Astarita, J.L.; Cremasco, V.; Fu, J.; Darnell, M.C.; Peck, J.R.; Nieves-Bonilla, J.M.; Song, K.; Kondo, Y.; Woodruff, M.C.; Gogineni, A.; et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 2015, 16, 75–84. [Google Scholar] [CrossRef]
- Brown, F.D.; Turley, S.J. Fibroblastic reticular cells: Organization and regulation of the T lymphocyte life cycle. J. Immunol. 2015, 194, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Lukacs-Kornek, V.; Malhotra, D.; Fletcher, A.L.; E Acton, S.; Elpek, K.G.; Tayalia, P.; Collier, A.R.; Turley, S.J. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 2011, 12, 1096–1104. [Google Scholar] [CrossRef]
- Knoblich, K.; Migoni, S.C.; Siew, S.M.; Jinks, E.; Kaul, B.; Jeffery, H.C.; Baker, A.T.; Suliman, M.; Vrzalikova, K.; Mehenna, H.; et al. The human lymph node microenvironment unilaterally regulates T-cell activation and differentiation. PLoS Biol. 2018, 16, e2005046. [Google Scholar] [CrossRef]
- Routy, J.-P.; Routy, B.; Graziani, G.M.; Mehraj, V. The kynurenine pathway Is a double-edged sword in immune-privileged sites and in cancer: Implications for immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Fresno, M.; Cuesta, N. Prostaglandin E2 and T cells: Friends or foes? Immunol. Cell Biol. 2011, 90, 579–586. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.-C.; Heuzé-Vourc’H, N.; Zeng, G.; Reckamap, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, J.; Hatfield, S.; Jones, G.; Ohta, A.; Sitkovsky, M. A2A adenosine receptor gene deletion or synthetic A2A antagonist liberate tumor-reactive CD8(+) T cells from tumor-induced immunosuppression. J. Immunol. 2018, 201, 782–791. [Google Scholar] [CrossRef]
- Misiak, J.; Jean, R.; Rodriguez, S.; Deleurme, L.; Lamy, T.; Tarte, K.; Amé-Thomas, P. Human lymphoid stromal cells contribute to polarization of follicular T cells into IL-4 secreting cells. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Meli, A.P.; Fontés, G.; Avery, D.T.; Leddon, S.A.; Tam, M.; Elliot, M.; Ballesteros-Tato, A.; Miller, J.; Stevenson, M.M.; Fowell, D.J.; et al. The Integrin LFA-1 controls T follicular Helper CELL generation and maintenance. Immunity 2016, 45, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Mionnet, C.; Mondor, I.; Jorquera, A.; Loosveld, M.; Maurizio, J.; Arcangeli, M.-L.; Ruddle, N.H.; Nowak, J.; Aurrand-Lions, M.; Luche, H.; et al. Identification of a new stromal cell type involved in the regulation of inflamed B cell follicles. PLoS Biol. 2013, 11, e1001672. [Google Scholar] [CrossRef]
- Rodda, L.B.; Bannard, O.; Ludewig, B.; Nagasawa, T.; Cyster, J.G. Phenotypic and morphological properties of germinal center dark zone Cxcl12-expressing reticular cells. J. Immunol. 2015, 195, 4781–4791. [Google Scholar] [CrossRef]
- Rodda, L.B.; Lu, E.; Bennett, M.L.; Sokol, C.L.; Wang, X.; Luther, S.A.; Barres, B.A.; Luster, A.D.; Ye, C.J.; Cyster, J.G.; et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity 2018, 48, 1014–1028. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Rivas-Caicedo, A.; Renevey, F.; Cannelle, H.; Peranzoni, E.; Scarpellino, L.; Hardie, D.L.; Pommier, A.; Schaeuble, K.; Favre, S.; et al. Identification of a new subset of lymph node stromal cells involved in regulating plasma cell homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, E6826–E6835. [Google Scholar] [CrossRef]
- Bénézech, C.; Mader, E.; Desanti, G.; Khan, M.; Nakamura, K.; White, A.; Ware, C.F.; Anderson, G.; Caamaňo, J.H. Lymphotoxin-beta receptor signaling through NF-kappaB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity 2012, 37, 721–734. [Google Scholar] [CrossRef]
- Sitnik, K.M.; Wendland, K.; Weishaupt, H.; Uronen-Hansson, H.; White, A.J.; Anderson, G.; Kotarsky, K.; Agace, W.W. Context-dependent development of lymphoid stroma from adult CD34(+) adventitial progenitors. Cell Rep. 2016, 14, 2375–2388. [Google Scholar] [CrossRef] [PubMed]
- Golub, R.; Tan, J.; Watanabe, T.; Brendolan, A. Origin and immunological functions of spleen stromal cells. Trends Immunol. 2018, 39, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Mourcin, F.; Uhel, F.; Pangault, C.; Ruminy, P.; Dupré, L.; Guirriec, M.; Marchand, T.; Fest, T.; Lamy, T.; et al. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood 2015, 126, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, R.; Stevenson, F.K. Critical influences on the pathogenesis of follicular lymphoma. Blood 2018, 131, 2297–2306. [Google Scholar] [CrossRef]
- Chevalier, N.; Mueller, M.; Mougiakakos, D.; Ihorst, G.; Marks, R.; Schmitt-Graeff, A.; Veelken, H. Analysis of dendritic cell subpopulations in follicular lymphoma with respect to the tumor immune microenvironment. Leuk. Lymphoma 2015, 57, 2150–2160. [Google Scholar] [CrossRef]
- Pepe, G.; Di Napoli, A.; Cippitelli, C.; Scarpino, S.; Pilozzi, E.; Ruco, L. Reduced lymphotoxin-beta production by tumour cells is associated with loss of follicular dendritic cell phenotype and diffuse growth in follicular lymphoma. J. Pathol. Clin. Res. 2018, 4, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Blaker, Y.N.; Spetalen, S.; Brodtkorb, M.; Lingjaerde, O.C.; Beiske, K.; Østenstad, B.; Sander, B.; Wahlin, B.E.; Melen, C.M.; Myklebust, H.; et al. The tumour microenvironment influences survival and time to transformation in follicular lymphoma in the rituximab era. Br. J. Haematol. 2016, 175, 102–114. [Google Scholar] [CrossRef]
- Ohe, R.; Aung, N.Y.; Meng, H.; Kabasawa, T.; Suto, A.; Tamazawa, N.; Yang, S.; Kato, T.; Yamakawa, M. Localization of collagen modifying enzymes on fibroblastic reticular cells and follicular dendritic cells in non-neoplastic and neoplastic lymphoid tissues. Leuk. Lymphoma 2015, 57, 1687–1696. [Google Scholar] [CrossRef]
- Ohe, R.; Meng, H.-X.; Aung, N.Y.; Yamada, A.; Kabasawa, T.; Utsunomiya, A.; Tamazawa, N.; Tamura, Y.; Kitaoka, T.; Hashimoto, T.; et al. Differential expression of estrogen receptor-alpha on follicular dendritic cells from patients with grade 1-2 and grade 3 follicular lymphoma. Hematol. Oncol. 2019, 37, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kurshumliu, F.; Sadiku-Zehri, F.; Qerimi, A.; Vela, Z.; Jashari, F.; Bytyci, S.; Rashiti, V.; Sadiku, S. Divergent immunohistochemical expression of CD21 and CD23 by follicular dendritic cells with increasing grade of follicular lymphoma. World J. Surg. Oncol. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Ohe, R.; Meng, H.; Yamada, A.; Aung, N.Y.; Kabasawa, T.; Tamura, Y.; Utsunomiya, A.; Tamazawa, N.; Kawamura, I.; Kitaoka, T.; et al. Good prognosis for follicular lymphoma with estrogen receptor alpha-positive follicular dendritic cells. Hematol. Oncol. 2020, 38, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.K.; Kahl, B.S.; de Vos, S.; Wagner-Johnston, N.D.; Schuster, S.J.; Jurczak, W.J.; Flinn, I.W.; Flowers, C.R.; Martin, P.; Viardot, A.; et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 2014, 370, 1008–1018. [Google Scholar] [CrossRef]
- Serrat, N.; Guerrero-Hernandez, M.; Matas-Cespedes, A.; Yahiaoui, A.; Valero, J.G.; Nadeu, F.; Clot, G.; Di Re, M.; Corbera-Bellalta, M.; Magnano, L.; et al. PI3Kdelta inhibition reshapes follicular lymphoma-immune microenvironment cross talk and unleashes the activity of venetoclax. Blood Adv. 2020, 4, 4217–4231. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Schnotalle, P.; Koch, K.; Au-Yeung, R.K.H.; Reinke, S.; Winter, K.; Loeffler, M.; Braumann, U.-D.; Klapper, W. T-cell clustering in neoplastic follicles of follicular lymphoma. Cancer Microenviron. 2018, 11, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Das, B.; Lin, T.L.; Grimes, C.; Zhang, X.; Lavezzi, T.; Huang, L.; Cole, J.; Yau, L.; Li, L. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br. J. Haematol. 2012, 158, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kagami, Y.; Jung, J.; Choi, Y.S.; Osumi, K.; Nakamura, S.; Morishima, Y.; Seto, M. Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell-like cell line HK. Leukemia 2001, 15, 148–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matas-Cespedes, A.; Rodriguez, V.; Kalko, S.G.; Vidal-Crespo, A.; Rosich, L.; Casserras, T.; Balsas, P.; Villamor, N.; Giné, E.; Campo, E.; et al. Disruption of Follicular Dendritic Cells-Follicular Lymphoma Cross-talk by the Pan-PI3K Inhibitor BKM120 (Buparlisib). Clin. Cancer Res. 2014, 20, 3458–3471. [Google Scholar] [CrossRef]
- Amé-Thomas, P.; Hajjami, H.M.-E.; Monvoisin, C.; Jean, R.; Monnier, D.; Caulet-Maugendre, S.; Guillaudeux, T.; Lamy, T.; Fest, T.; Tarte, K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: Role of stromal cells in follicular lymphoma pathogenesis. Blood 2007, 109, 693–702. [Google Scholar] [CrossRef]
- Lwin, T.; Crespo, L.A.; Wu, A.; Dessureault, S.; Shu, H.B.; Moscinski, L.C.; Sotomayor, E.; Dalton, W.S.; Tao, J. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non-Hodgkin’s B lymphoma cells from apoptosis. Leukemia 2009, 23, 170–177. [Google Scholar] [CrossRef]
- Dubey, L.K.; Lebon, L.; Mosconi, I.; Yang, C.-Y.; Scandella, E.; Ludewig, B.; Luther, S.A.; Harris, N.L. Lymphotoxin-dependent B cell-Frc crosstalk promotes de novo follicle formation and antibody production following intestinal helminth infection. Cell Rep. 2016, 15, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.L.; Walter, A.; Alexandre, Y.O.; Hor, J.L.; Liu, R.; Ma, J.Z.; Devi, S.; Tokuda, N.; Owada, Y.; Mackay, L.K.; et al. Infection programs sustained lymphoid stromal cell responses and shapes lymph node remodeling upon secondary challenge. Cell Rep. 2017, 18, 406–418. [Google Scholar] [CrossRef]
- Di Ianni, M.; Del Papa, B.; De Ioanni, M.; Moretti, L.; Bonifacio, E.; Cecchini, D.; Sportoletti, P.; Falzetti, F.; Tabilio, A. Mesenchymal cells recruit and regulate T regulatory cells. Exp. Hematol. 2008, 36, 309–318. [Google Scholar] [CrossRef]
- Guilloton, F.; Caron, G.; Ménard, C.; Pangault, C.; Amé-Thomas, P.; Dulong, J.; Amé-Thomas, P.; Flecher, E.; Fest, T.; Tarte, K. Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood 2012, 119, 2556–2567. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, M.; Guilloton, F.; Pangault, C.; Mourcin, F.; Sok, P.; Latour, M.; Amé-Thomas, P.; Flecher, E.; Fest, T.; Tarte, K. Neutrophils trigger a NF-kappaB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget 2015, 6, 16471–16487. [Google Scholar] [CrossRef]
- Pandey, S.; Mourcin, F.; Marchand, T.; Nayar, S.; Guirriec, M.; Pangault, C.; Monvoisin, C.; Amé-Thomas, P.; Guilloton, F.; Dulong, J.; et al. IL-4/CXCL12 loop is a key regulator of lymphoid stroma function in follicular lymphoma. Blood 2017, 129, 2507–2518. [Google Scholar] [CrossRef]
- Staiger, A.M.; Duppel, J.; Dengler, M.A.; Van Der Kuip, H.; Vöhringer, M.C.; Aulitzky, W.E.; Rosenwald, A.; Ott, G.; Horn, H. An analysis of the role of follicular lymphoma-associated fibroblasts to promote tumor cell viability following drug-induced apoptosis. Leuk. Lymphoma 2017, 58, 1922–1930. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kunou, S.; Shimada, K.; Tsunoda, M.; Aoki, T.; Iriyama, C.; Tomita, A.; Nakamura, S.; Hayakawa, F.; Kiyoi, H. Pyruvate secreted from patient-derived cancer-associated fibroblasts supports survival of primary lymphoma cells. Cancer Sci. 2019, 110, 269–278. [Google Scholar] [CrossRef]
- Fazilleau, N.; Mark, L.; McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Follicular helper T cells: Lineage and location. Immunity 2009, 30, 324–335. [Google Scholar] [CrossRef]
- Amé-Thomas, P.; Le Priol, J.; Yssel, H.; Caron, G.; Pangault, C.; Jean, R.; Martin, N.; Marafioti, T.; Gaulard, P.; Lamy, T.; et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: Role in the survival of malignant B cells. Leukemia 2012, 26, 1053–1063. [Google Scholar] [CrossRef]
- Rawal, S.; Chu, F.; Zhang, M.; Park, H.J.; Nattamai, D.; Kannan, S.; Sharma, R.; Delgado, D.; Chou, T.; Lin, H.Y.; et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J. Immunol. 2013, 190, 6681–6693. [Google Scholar] [CrossRef]
- Pangault, C.; Ame-Thomas, P.; Ruminy, P.; Rossille, D.; Caron, G.; Baia, M.; De Vos, J.; Roussel, M.; Monvoisin, C.; Lamy, T.; et al. Follicular lymphoma cell niche: Identification of a preeminent IL-4-dependent T(FH)-B cell axis. Leukemia 2010, 24, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Townsend, W.; Pasikowska, M.; Yallop, D.; Phillips, E.H.; Patten, P.; Salisbury, J.R.; Marcus, R.; Pepper, A.; Devereux, S. The architecture of neoplastic follicles in follicular lymphoma; analysis of the relationship between the tumor and follicular helper T cells. Haematologica 2020, 105, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Lüthje, K.; Kallies, A.; Shimohakamada, Y.; Belz, G.T.; Light, A.; Tarlinton, D.M.; Nutt, S.L. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 2012, 13, 491–498. [Google Scholar] [CrossRef]
- Amé-Thomas, P.; Hoeller, S.; Artchounin, C.; Misiak, J.; Braza, M.S.; Jean, R.; Priol, J.L.; Monvoisin, C.; Martin, N.; Gaulard, P.; et al. CD10 delineates a subset of human IL-4 producing follicular helper T cells involved in the survival of follicular lymphoma B cells. Blood 2015, 125, 2381–2385. [Google Scholar] [CrossRef]
- Dheilly, E.; Battistello, E.; Katanayeva, N.; Sungalee, S.; Michaux, J.; Duns, G.; Wehrle, S.; Sordet-Dessimoz, J.; Mina, M.; Racle, J.; et al. Cathepsin S regulates antigen processing and T cell activity in non-Hodgkin lymphoma. Cancer Cell 2020, 37, 674–689. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Chong, E.A.; Ruella, M.; Schuster, S.J. Lymphoma Program Investigators at the University of Pennsylvania. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N. Engl. J. Med. 2021, 384, 673–674. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Bunse, M.; Pfeilschifter, J.; Bluhm, J.; Zschummel, M.; Joedicke, J.J.; Wirges, A.; Stark, H.; Kretschmer, V.; Chmielewski, M.; Uckert, W.; et al. CXCR5 CAR-T cells simultaneously target B cell non-Hodgkin’s lymphoma and tumor-supportive follicular T helper cells. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Li, H.S.; Liu, X.; Cao, J.; Ma, W.; Ma, Y.; Weng, J.; Zhu, Z.; Cheng, X.; Wang, Z.; et al. CXCR5(+)CD8(+) T cells are a distinct functional subset with an antitumor activity. Leukemia 2019, 33, 2640–2653. [Google Scholar] [CrossRef] [PubMed]
- Rolf, J.; Bell, S.E.; Kovesdi, D.; Janas, M.L.; Soond, D.R.; Webb, L.M.; Santinelli, S.; Saunders, T.; Hebeis, B.; Killeen, N.; et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J. Immunol. 2010, 185, 4042–4052. [Google Scholar] [CrossRef]
- Hilchey, S.P.; De, A.; Rimsza, L.M.; Bankert, R.B.; Bernstein, S.H. Follicular lymphoma intratumoral CD4 + CD25 + GITR + regulatory T cells potently suppress CD3/CD28-costimulated autologous and allogeneic CD8 + CD25- and CD4 + CD25- T cells. J. Immunol. 2007, 178, 4051–4061. [Google Scholar] [CrossRef] [PubMed]
- Hilchey, S.P.; Rosenberg, A.F.; Hyrien, O.; Secor-Socha, S.; Cochran, M.R.; Brady, M.T.; Wang, J.-C.; Sanz, I.; Burack, W.R.; Quataert, S.A.; et al. Follicular lymphoma tumor-infiltrating T-helper (T(H)) cells have the same polyfunctional potential as normal nodal T(H) cells despite skewed differentiation. Blood 2011, 118, 3591–3602. [Google Scholar] [CrossRef]
- Nedelkovska, H.; Rosenberg, A.F.; Hilchey, S.P.; Hyrien, O.; Burack, W.R.; Quataert, S.A.; Baker, C.M.; Azadniv, M.; Welle, S.L.; Ansell, S.M.; et al. Follicular lymphoma tregs have a distinct transcription profile impacting their migration and retention in the malignant lymph node. PLoS ONE 2016, 11, e0155347. [Google Scholar] [CrossRef]
- Le, K.-S.; Thibult, M.-L.; Just-Landi, S.; Pastor, S.; Gondois-Rey, F.; Granjeaud, S.; Broussais, F.; Bouabdallah, R.; Colisson, R.; Caux, C.; et al. Follicular B lymphomas generate regulatory T cells via the ICOS/ICOSL pathway and are susceptible to treatment by anti-ICOS/ICOSL therapy. Cancer Res. 2016, 76, 4648–4660. [Google Scholar] [CrossRef]
- Boice, M.; Salloum, D.; Mourcin, F.; Sanghvi, V.; Amin, R.; Oricchio, E.; Jiang, M.; Mottok, A.; Denis-Lagache, N.; Ciriello, G.; et al. Loss of the HVEM tumor suppressor in lymphoma and restoration by modified CAR-T cells. Cell 2016, 167, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X.; et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef]
- Mintz, M.A.; Felce, J.H.; Chou, M.Y.; Mayya, V.; Xu, Y.; Shui, J.-W.; An, J.; Li, Z.; Marson, A.; Okada, T.; et al. The HVEM-BTLA axis restrains T cell help to germinal center B cells and functions as a cell-extrinsic suppressor in lymphomagenesis. Immunity 2019, 51, 310–323. [Google Scholar] [CrossRef]
- Cheung, K.-J.J.; Johnson, N.A.; Affleck, J.G.; Severson, T.; Steidl, C.; Ben-Neriah, S.; Schein, J.; Morin, R.D.; Moore, R.; Shah, S.P.; et al. Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res. 2010, 70, 9166–9174. [Google Scholar] [CrossRef]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef]
- Kotsiou, E.; Okosun, J.; Besley, C.; Iqbal, S.; Matthews, J.; Fitzgibbon, J.; Gribben, J.G.; Davies, J.K. TNFRSF14 aberrations in follicular lymphoma increase clinically significant allogeneic T-cell responses. Blood 2016, 128, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Lackraj, T.; Goswami, R.; Kridel, R. Pathogenesis of follicular lymphoma. Best Pract. Res. Clin. Haematol. 2018, 31, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Carreras, J.; Lopez-Guillermo, A.; Kikuti, Y.Y.; Itoh, J.; Masashi, M.; Ikoma, H.; Tomita, S.; Hiraiwa, S.; Hamoudi, R.; Rosenwald, A.; et al. High TNFRSF14 and low BTLA are associated with poor prognosis in Follicular Lymphoma and in Diffuse Large B-cell Lymphoma transformation. J. Clin. Exp. Hematop. 2019, 59, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Lugtenburg, P.; Mous, R.; Clausen, M.R.; Chamuleau, M.; Linton, K.; Rule, S.; Lopez, J.S.; Oliveri, R.S.; DeMarco, D.; et al. Epcoritamab (GEN3013; DuoBody-CD3xCD20) to induce complete response in patients with relapsed/refractory B-cell non-Hodgkin lymphoma (B-NHL): Complete dose escalation data and efficacy results from a phase I/II trial. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Van der Horst, H.J.; de Jonge, A.V.; Hiemstra, I.H.; Gelderloos, A.T.; Berry, D.R.A.I.; Hijmering, N.J.; van Essen, H.F.; de Jong, D.; Chamuleau, M.E.D.; Zweegman, S.; et al. Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J. 2021, 11. [Google Scholar] [CrossRef]
- Tuscano, J.M.; Maverakis, E.; Groshen, S.; Tsao-Wei, D.; Luxardi, G.; Merleev, A.A.; Beaven, A.; DiPersio, J.F.; Popplewell, L.; Chen, R.; et al. A phase I study of the combination of rituximab and ipilimumab in patients with relapsed/refractory B-cell lymphoma. Clin. Cancer Res. 2019, 25, 7004–7013. [Google Scholar] [CrossRef]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef]
- Arvey, A.; van der Veeken, J.; Samstein, R.M.; Feng, Y.; Stamatoyannopoulos, J.A.; Rudensky, A.Y. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014, 15, 580–587. [Google Scholar] [CrossRef]
- Goswami, S.; Apostolou, I.; Zhang, J.; Skepner, J.; Anandhan, S.; Zhang, X.; Xiong, L.; Trojer, P.; Aparicio, A.; Subundhi, S.K.; et al. Modulation of EZH2 expression in T cells improves efficacy of anti-CTLA-4 therapy. J. Clin. Investig. 2018, 128, 3813–3818. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Tilly, H.; Chaidos, A.; McKay, P.; Phillips, T.; Assouline, S.; Batlevi, C.L.; Cambell, P.; Ribrag, V.; Damaj, G.L.; et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: An open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 1433–1442. [Google Scholar] [CrossRef]
- Bödör, C.; O’Riain, C.; Wrench, D.; Matthews, J.; Iyengar, S.; Tayyib, H.; Calaminchi, M.; Clear, A.; Iqbal, S.; Quentmeier, H.; et al. EZH2 Y641 mutations in follicular lymphoma. Leukemia 2011, 25, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chan, J.K.; Iqbal, J.; McKeithan, T.; Fu, K.; Meng, B.; Pan, Y.; Cheuk, W.; Luo, D.; Wang, R.; et al. EZH2 mutations in follicular lymphoma from different ethnic groups and associated gene expression alterations. Clin. Cancer Res. 2014, 20, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Quiros, J.; Mahuron, K.; Pai, C.C.; Ranzani, V.; Young, A.; Silveria, S.; Harwin, T.; Abnousian, A.; Pagani, M.; et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep. 2018, 23, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.; Kushekhar, K.; Munthe, L.A.; Tjonnfjord, G.E.; Aandahl, E.M.; Okkenhaug, K.; Tasken, K. The PI3K p110delta Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function. J. Immunol. 2019, 202, 1397–1405. [Google Scholar] [CrossRef]
- Leonard, J.P.; Trneny, M.; Izutsu, K.; Fowler, N.H.; Hong, X.; Zhu, J.; Zhang, H.; Offner, F.; Scheliga, A.; Nowakowski, G.S.; et al. AUGMENT: A phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J. Clin. Oncol. 2019, 37, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.; Trisal, P.; Bjorklund, C.; Carrancio, S.; Toraño, E.G.; Guarinos, C.; Papazoglou, D.; Hagner, P.R.; Beldi-Ferchiou, A.; Tarte, K.; et al. Combination lenalidomide-rituximab immunotherapy activates anti-tumour immunity and induces tumour cell death by complementary mechanisms of action in follicular lymphoma. Br. J. Haematol. 2019, 185, 240–253. [Google Scholar] [CrossRef]
- Josefsson, S.E.; Beiske, K.; Blaker, Y.N.; Førsund, M.S.; Holte, H.; Østenstad, B.; Kimby, E.; Köksal, H.; Wälchli, S.; Bai, B.; et al. TIGIT and PD-1 mark intratumoral T cells with reduced effector function in B-cell non-Hodgkin lymphoma. Cancer Immunol. Res. 2019, 7, 355–362. [Google Scholar] [CrossRef]
- Josefsson, S.E.; Huse, K.; Kolstad, A.; Beiske, K.; Pende, D.; Steen, C.B.; Inderberg, E.M.; Lingjærde, O.C.; Østenstad, B.; Smeland, E.B.; et al. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T-cell receptor signaling. Clin. Cancer Res. 2018, 24, 870–881. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Kim, H.J.; Wu, H.; Jalali, S.; Tang, X.; Krull, J.E.; Ding, W.; Novak, A.J.; Ansell, S.M. TIGIT expression is associated with T-cell suppression and exhaustion and predicts clinical outcome and anti-PD-1 response in follicular lymphoma. Clin. Cancer Res. 2020, 26, 5217–5231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Kim, H.J.; Villasboas, J.C.; Chen, Y.-P.; Price-Troska, T.; Jalali, S.; Wilson, M.; Novak, A.J.; Ansell, S.M. Expression of LAG-3 defines exhaustion of intratumoral PD-1(+) T cells and correlates with poor outcome in follicular lymphoma. Oncotarget 2017, 8, 61425–61439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Kim, H.J.; Villasboas, J.C.; Price-Troska, T.; Jalali, S.; Wu, H.; Luchtel, R.A.; Polley, M.-Y.; Novak, A.J.; Ansell, S.M. Mass cytometry analysis reveals that specific intratumoral CD4(+) T cell subsets correlate with patient survival in follicular lymphoma. Cell Rep. 2019, 26, 2178–2193. [Google Scholar] [CrossRef] [PubMed]
- Pangault, C.; Amé-Thomas, P.; Rossille, D.; Dulong, J.; Caron, G.; Nonn, C.; Chatonnet, F.; Desmots, F.; Launay, V.; Lamy, T.; et al. Integrative analysis of cell crosstalk within follicular lymphoma cell niche: Towards a definition of the FL supportive synapse. Cancers 2020, 12, 2865. [Google Scholar] [CrossRef]
| Cells | Phenotype | Cytokines/Chemo-kine Production | Functions in FL | Reference |
|---|---|---|---|---|
| FRC (mice) | PDPN/gp38+ CD31− | IL-8 CXCL12,13 CCL2,7,19,21a (BAFF) | FL cell survival | [9] |
| FRC-like cells | - | IL-6,15, 33 CCL2, 5,11 CXCL10 (ICAM-1↑) | Tfh generation IL-4 production by Tfh (CXCR5+PD-1dimCD4+) | [24] |
| (cDCs) | CD11c+DCs | - | Assistance of Treg infiltration | [35] |
| FDCs | CCL21+CD23+↓ | - | Diffuse growth pattern of FL | [36] |
| CD21+ | - | Shorten TTT, PFS, OS | [37] | |
| TGII+FRC/CD35+ | (CMEslo) | Reduction in FRCs/FDCs | [38] | |
| CD23+CD23+ERα+ | - | Support of G1-2 FL microenvironment | [39] | |
| CD23+↓ | - | More haphazard distribution of S-phase FL cells (i.e., G3 FL) | [40] | |
| (HK cells) | (FDC-like cell derived from the human tonsil) | - | FL cell survival and proliferation | [51] |
| (HK cells) | - | CXCL12 | Cancer stem cell-like activities | [49] |
| (HK cells) | - | IL-6, 8, CXCL1, 2, 5,12, CCL2 | Angiogenesis, FL cell adhesion, migration, survival, and proliferation | [52] |
| Tfh | CD4+CXCR5hiCCR7lo ICOS+PD-1+BCL6+ | IL-2, 10 IFNγ | - | [59] |
| CD4+CXCR5hi ICOShiPD-1hi CD200hi CD127/IL7-Rαlo | TNF-α IFNγ IL-4 (CD40L expression) | FL cell viability support FL cell viability support FL cell viability support and rescue from apoptosis | [60] | |
| CD4+PD-1hi CXCR5hiBCL6hi | IL-4 IL-4 (CD40L expression) | pSTAT6 ↑ Treg-recruiting CCL17 and CCL22 production by FL cells | [61] | |
| CD4+CXCR5hi PD-1hi BCL6+ | IL-4 (CD40L expression) | CXCL12↑i FL cell migration, adhesion to SCs, Syk and ERK phosphorylation | [56] | |
| CD4+PD-1+ICOS+ | - | FL cell proliferation Increase in histological grade Immune synapse formation with Ki-67+ FL cells | [63] | |
| CD10+PAX5− CD3+CD4+CXCR5+ PD-1+ICOS+ CXCL13+ HLA-DR+Ki-67− | IL-4hiIFNγlo TNF-αhi IL-21hiIL-17lo (either CD10+ or CD10−) | B-cell activation and survival B-cell supportive lymphoid stromal network Treg-recruiting CCL17 and CCL22 production by FL cells | [65] | |
| TFRs | CD4+CD25+ CXCR5hiICOShi Blimp-1/PRDM1+ | - | - | [60] |
| CD4+CD25+ GITR+ | - | Inhibition of FL LN-infiltrating T-cell cytokine production | [74] | |
| Bcl-6+ CXCR5+Foxp3+ BTLA+PD1+ICOS+ CD44+CTLA4+ GITR+ | - | - | [10] | |
| CD4+Foxp3+Bcl-6+ Blimp-1/PRDM1+ CXCR5hiPD-1hi | - | - | [11] | |
| Foxp3+Ki-67+PD-1+ CXCR5+Bcl-6+ | - | - | [12] | |
| CD4+CD25+GITR+ PD-1dimCXCR5+ Foxp3+ | - | - | [75] | |
| CXCR5+ CD4+ PD-1+CD25+ BCL6+FoxP3+ CXCL13+ CTLA-4+IL-10+GITR+ | CCL4 IL-16 CXCL13 (S1PR1↓SELL↓ CCR7↓) | Treg migration to the GC in response to a CXCL13 (CXCR5 ligand) gradient Chemotactic for CCR5-expressing Tregs Treg recruitment Treg retention in the GC More suppressive than normal LN Tregs | [76] | |
| (TFR?) | CD25hiCD127lo/- FoxP3hiICOS+CXCR5+ CTLA-4+GITR+ CD39+PD-1+Ki-67hi (activated Treg) | - | - | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T. The Tumor Microenvironment in Follicular Lymphoma: Its Pro-Malignancy Role with Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 5352. https://doi.org/10.3390/ijms22105352
Watanabe T. The Tumor Microenvironment in Follicular Lymphoma: Its Pro-Malignancy Role with Therapeutic Potential. International Journal of Molecular Sciences. 2021; 22(10):5352. https://doi.org/10.3390/ijms22105352
Chicago/Turabian StyleWatanabe, Takashi. 2021. "The Tumor Microenvironment in Follicular Lymphoma: Its Pro-Malignancy Role with Therapeutic Potential" International Journal of Molecular Sciences 22, no. 10: 5352. https://doi.org/10.3390/ijms22105352
APA StyleWatanabe, T. (2021). The Tumor Microenvironment in Follicular Lymphoma: Its Pro-Malignancy Role with Therapeutic Potential. International Journal of Molecular Sciences, 22(10), 5352. https://doi.org/10.3390/ijms22105352





