Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of TiO2 Particles
2.2. Long-Term Exposure to TiO2 Affects the Cell Cycle
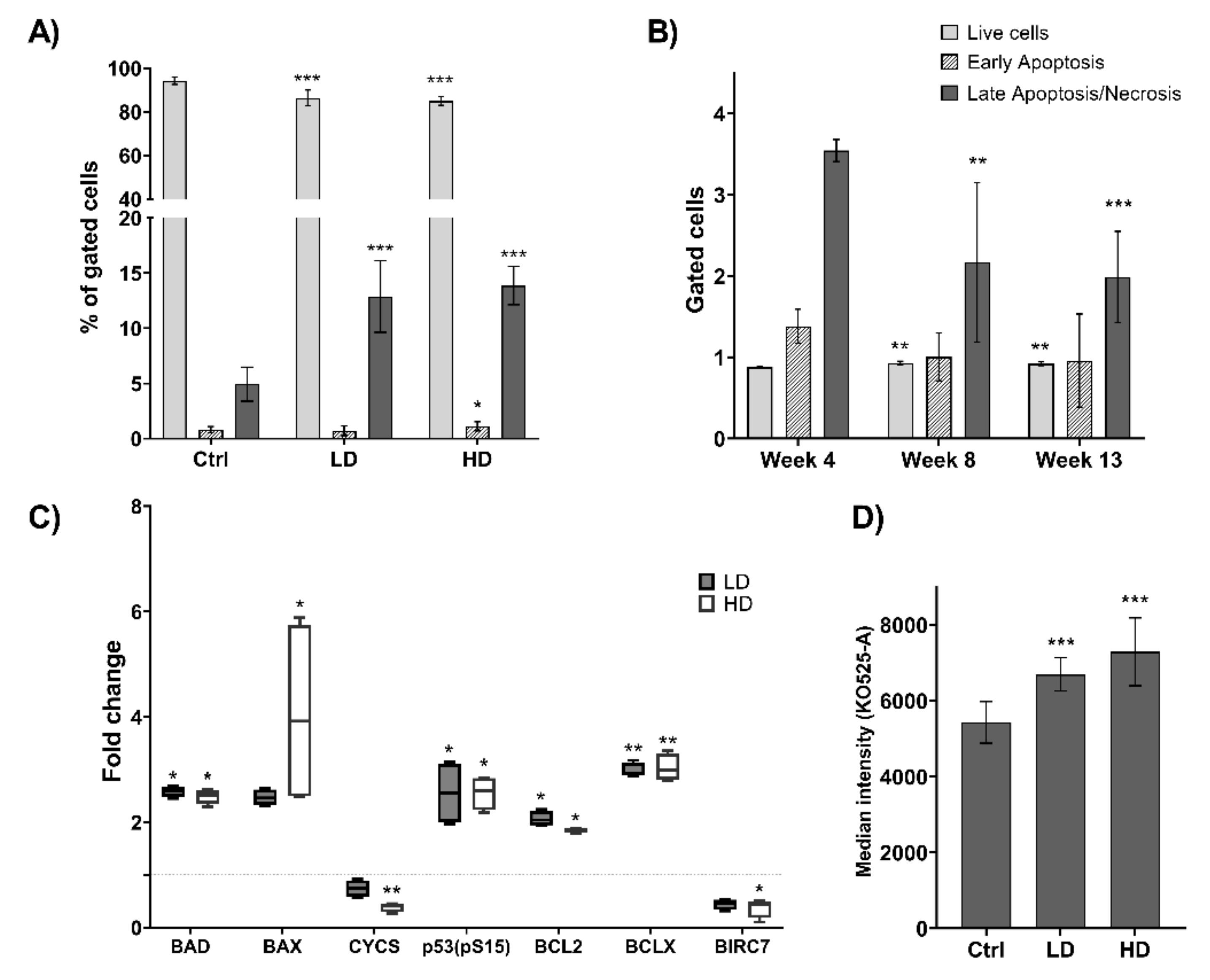
2.3. Long-Term TiO2 Exposure Induces Multiple Cell Death Pathways
2.4. Effect of Long-Term TiO2 Exposure on Telomere Length
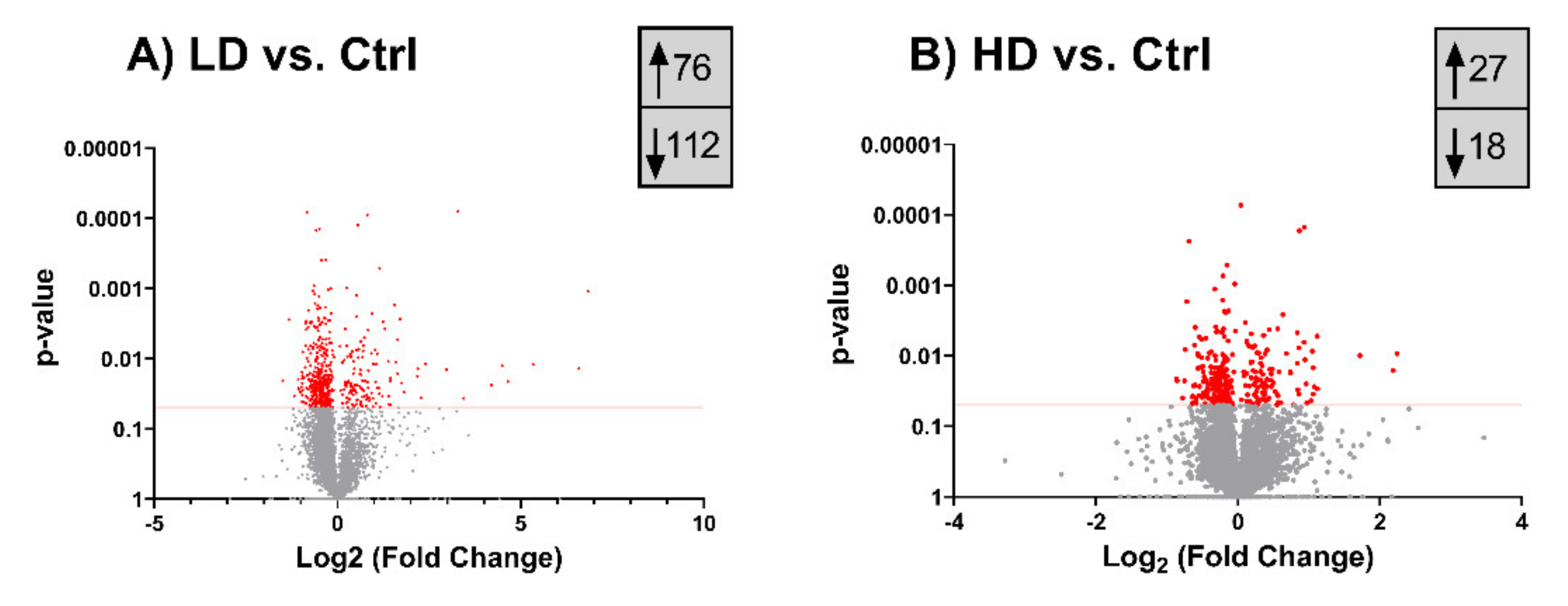
2.5. Long-Term TiO2 Exposure Affects the Expression of Genome Maintenance Proteins
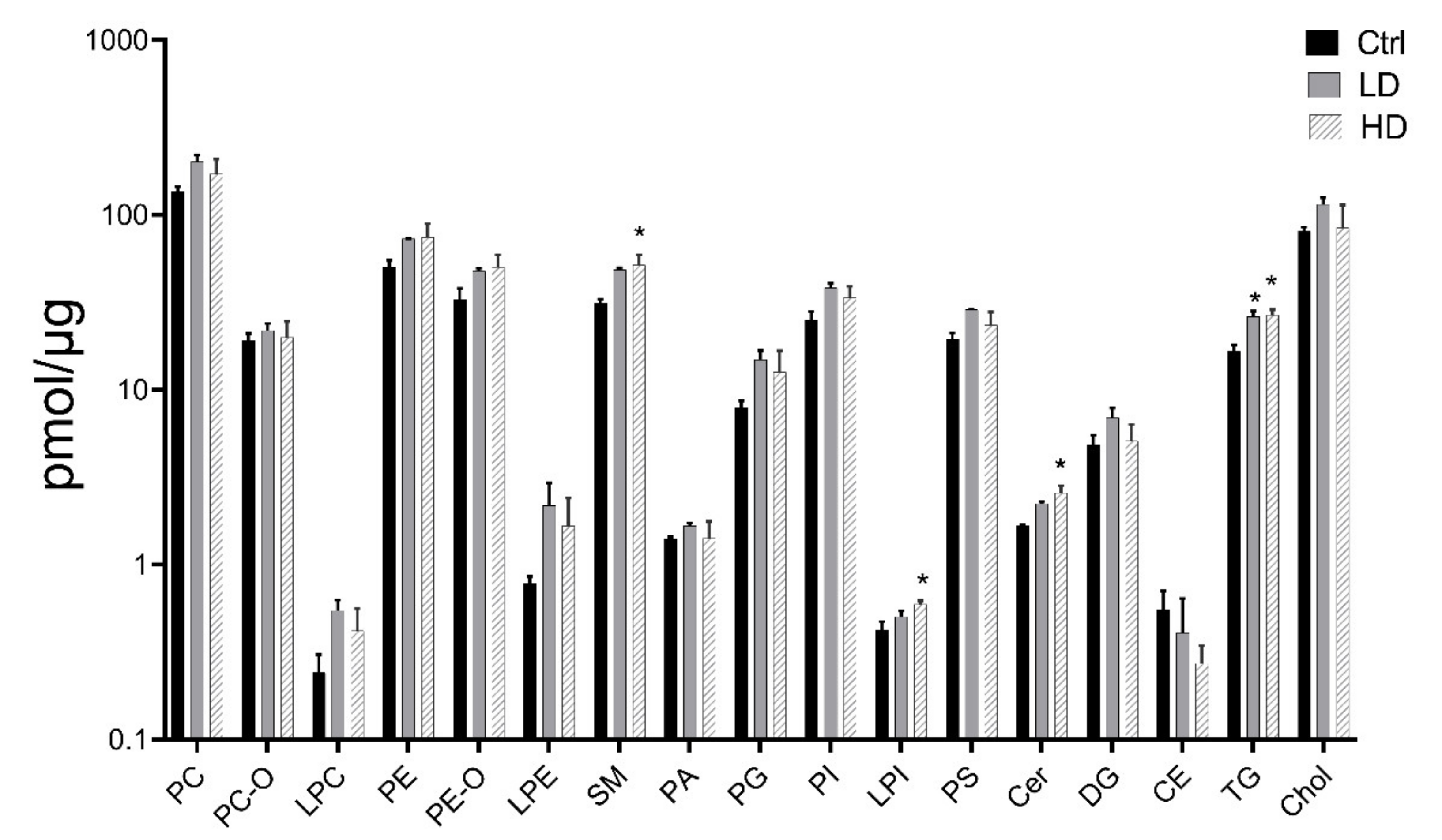
2.6. Analysis of Lipid Profiles after 13 Weeks of Exposure to TiO2
3. Discussion
4. Materials and Methods
4.1. Particle Dispersion and Characterization
4.2. Long-Term Cell Exposure
4.3. Flow Cytometry
4.4. Apoptosis Array
4.5. Telomere Length
4.6. Proteomics
4.7. Lipidomics
4.8. Statistical Anaysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.A.; Leso, V.; Niang, M.; Iavicoli, I. Current state of knowledge on the health effects of engineered nanomaterials in workers: A systematic review of human studies and epidemiological investigations. Scand. J. Work. Environ. Health 2019, 45, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Wu, W.T.; Liao, H.Y.; Chen, C.Y.; Tsai, C.Y.; Jung, W.T.; Lee, H.L. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017, 331, 329–335. [Google Scholar] [CrossRef]
- Pelclova, D.; Zdimal, V.; Fenclova, Z.; Vlckova, S.; Turci, F.; Corazzari, I.; Kacer, P.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Markers of oxidative damage of nucleic acids and proteins among workers exposed to TiO2 (nano) particles. Occup. Environ. Med. 2016, 73, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Zikova, N.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Makeš, O.; Syslova, K.; et al. Markers of lipid oxidative damage in the exhaled breath condensate of nano TiO(2) production workers. Nanotoxicology 2017, 11, 52–63. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Y.; Chen, Z.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Li, X.; Zhang, Y.; et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef]
- Bermudez, E.; Mangum, J.B.; Asgharian, B.; Wong, B.A.; Reverdy, E.E.; Janszen, D.B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol. Sci. 2002, 70, 86–97. [Google Scholar] [CrossRef]
- Li, B.; Ze, Y.; Sun, Q.; Zhang, T.; Sang, X.; Cui, Y.; Wang, X.; Gui, S.; Tan, D.; Zhu, M.; et al. Molecular mechanisms of nanosized titanium dioxide-induced pulmonary injury in mice. PLoS ONE 2013, 8, e55563. [Google Scholar] [CrossRef] [PubMed]
- Mohr, U.; Ernst, H.; Roller, M.; Pott, F. Pulmonary tumor types induced in Wistar rats of the so-called “19-dust study”. Exp. Toxicol. Pathol. 2006, 58, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takano, H.; Ohnuki, M.; Yanagisawa, R.; Sakurai, M.; Shimada, A.; Mizushima, K.; Yoshikawa, T. Size effects of nanomaterials on lung inflammation and coagulatory disturbance. Int. J. Immunopathol. Pharm. 2008, 21, 197–206. [Google Scholar] [CrossRef]
- Yoshiura, Y.; Izumi, H.; Oyabu, T.; Hashiba, M.; Kambara, T.; Mizuguchi, Y.; Lee, B.W.; Okada, T.; Tomonaga, T.; Myojo, T.; et al. Pulmonary toxicity of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J. Nanopart. Res. 2015, 17, 241. [Google Scholar] [CrossRef]
- Relier, C.; Dubreuil, M.; Lozano Garcìa, O.; Cordelli, E.; Mejia, J.; Eleuteri, P.; Robidel, F.; Loret, T.; Pacchierotti, F.; Lucas, S.; et al. Study of TiO2 P25 Nanoparticles Genotoxicity on Lung, Blood, and Liver Cells in Lung Overload and Non-Overload Conditions After Repeated Respiratory Exposure in Rats. Toxicol. Sci. 2017, 156, 527–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, N.; Watanabe, S.; Verma, R.; Jablonski, R.P.; Chen, C.I.; Cheresh, P.; Markov, N.S.; Reyfman, P.A.; McQuattie-Pimentel, A.C.; Sichizya, L.; et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Guarda, G.; Riteau, N.; Drexler, S.K.; Tardivel, A.; Couillin, I.; Tschopp, J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proc. Natl. Acad. Sci. USA 2010, 107, 19449–19454. [Google Scholar] [CrossRef]
- Lee, K.P.; Trochimowicz, H.J.; Reinhardt, C.F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol. Appl. Pharm. 1985, 79, 179–192. [Google Scholar] [CrossRef]
- Heinrich, U.; Fuhst, R.; Rittinghausen, S.; Creutzenberg, O.; Bellmann, B.; Koch, W.; Levsen, K. Chronic Inhalation Exposure of Wistar Rats and two Different Strains of Mice to Diesel Engine Exhaust, Carbon Black, and Titanium Dioxide. Inhal. Toxicol. 1995, 7, 533–556. [Google Scholar] [CrossRef]
- IARC. International Agency for Research on Cancer (IARC): Carbon Black, Titanium Dioxide, and Talc. 2010. Available online: https://monographs.iarc.fr/ENG/Monographs/vol93/mono93-97.pdf (accessed on 15 March 2021).
- ECHA. Substance Infocard: Titanium Dioxide 2020. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.033.327 (accessed on 15 March 2021).
- Jugan, M.L.; Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 2012, 6, 501–513. [Google Scholar] [CrossRef]
- Park, E.-J.; Yi, J.; Chung, K.-H.; Ryu, D.-Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, F.; He, J.; Yadav, S.; Wang, H. Titanium dioxide nanoparticles cause apoptosis in BEAS-2B cells through the caspase 8/t-Bid-independent mitochondrial pathway. Toxicol. Lett. 2010, 196, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sund, J.; Palomäki, J.; Ahonen, N.; Savolainen, K.; Alenius, H.; Puustinen, A. Phagocytosis of nano-sized titanium dioxide triggers changes in protein acetylation. J. Proteom. 2014, 108, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Tilton, S.C.; Karin, N.J.; Tolic, A.; Xie, Y.; Lai, X.; Hamilton, R.F., Jr.; Waters, K.M.; Holian, A.; Witzmann, F.A.; Orr, G. Three human cell types respond to multi-walled carbon nanotubes and titanium dioxide nanobelts with cell-specific transcriptomic and proteomic expression patterns. Nanotoxicology 2014, 8, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Majaron, H.; Kokot, B.; Sebastijanović, A.; Voss, C.; Podlipec, R.; Zawilska, P.; Berthing, T.; López, C.B.; Danielsen, P.H.; Contini, C.; et al. From the Roundabout of Molecular Events to Nanomaterial-Induced Chronic Inflammation Prediction. bioRxiv 2020. [Google Scholar] [CrossRef]
- Armand, L.; Biola-Clier, M.; Bobyk, L.; Collin-Faure, V.; Diemer, H.; Strub, J.M.; Cianferani, S.; Van Dorsselaer, A.; Herlin-Boime, N.; Rabilloud, T.; et al. Molecular responses of alveolar epithelial A549 cells to chronic exposure to titanium dioxide nanoparticles: A proteomic view. J. Proteom. 2016, 134, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chueh, P.J.; Lin, Y.W.; Shih, T.S.; Chuang, S.M. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol. Appl. Pharm. 2009, 241, 182–194. [Google Scholar] [CrossRef]
- Phuyal, S.; Kasem, M.; Rubio, L.; Karlsson, H.L.; Marcos, R.; Skaug, V.; Zienolddiny, S. Effects on human bronchial epithelial cells following low-dose chronic exposure to nanomaterials: A 6-month transformation study. Toxicol. In Vitro 2017, 44, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Armand, L.; Tarantini, A.; Beal, D.; Biola-Clier, M.; Bobyk, L.; Sorieul, S.; Pernet-Gallay, K.; Marie-Desvergne, C.; Lynch, I.; Herlin-Boime, N.; et al. Long-term exposure of A549 cells to titanium dioxide nanoparticles induces DNA damage and sensitizes cells towards genotoxic agents. Nanotoxicology 2016, 10, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Wallin, H.; Kyjovska, Z.O.; Poulsen, S.S.; Jacobsen, N.R.; Saber, A.T.; Bengtson, S.; Jackson, P.; Vogel, U. Surface modification does not influence the genotoxic and inflammatory effects of TiO2 nanoparticles after pulmonary exposure by instillation in mice. Mutagenesis 2017, 32, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Esmailzadeh, S.; Mansoori, B.; Mohammadi, A.; Shanehbandi, D.; Baradaran, B. siRNA-Mediated Silencing of HMGA2 Induces Apoptosis and Cell Cycle Arrest in Human Colorectal Carcinoma. J. Gastrointest. Cancer 2017, 48, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Dai, M.; Li, Q.; Wang, Z.; Lu, Y.; Song, Z. HMGA2 regulates lung cancer proliferation and metastasis. Thorac. Cancer 2017, 8, 501–510. [Google Scholar] [CrossRef]
- Sriraman, A.; Dickmanns, A.; Najafova, Z.; Johnsen, S.A.; Dobbelstein, M. CDK4 inhibition diminishes p53 activation by MDM2 antagonists. Cell Death Dis. 2018, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, C.I.; Cimpan, M.R.; Høl, P.J.; Sørnes, S.; Lie, S.A.; Gjerdet, N.R. Induction of cell death by TiO2 nanoparticles: Studies on a human monoblastoid cell line. Toxicol. In Vitro 2008, 22, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.; Cheung, C.; Kaul, Z.; Shah, N.; Sakaushi, S.; Sugimoto, K.; Oka, S.; Kaul, S.C.; Wadhwa, R. CARF Is a vital dual regulator of cellular senescence and apoptosis. J. Biol. Chem. 2009, 284, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Gama, V.; Swahari, V.; Schafer, J.; Kole, A.J.; Evans, A.; Huang, Y.; Cliffe, A.; Golitz, B.; Sciaky, N.; Pei, X.H.; et al. The E3 ligase PARC mediates the degradation of cytosolic cytochrome c to promote survival in neurons and cancer cells. Sci. Signal 2014, 7, ra67. [Google Scholar] [CrossRef]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Crighton, D.; Wilkinson, S.; O’Prey, J.; Syed, N.; Smith, P.; Harrison, P.R.; Gasco, M.; Garrone, O.; Crook, T.; Ryan, K.M. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 2006, 126, 121–134. [Google Scholar] [CrossRef]
- Wei, Y.; Sinha, S.; Levine, B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy 2008, 4, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.; Chang, S.H.; Park, S.J.; Lim, J.; Lee, J.; Yoon, T.J.; Kim, J.S.; Cho, M.H. Titanium Dioxide Nanoparticles Induce Endoplasmic Reticulum Stress-Mediated Autophagic Cell Death via Mitochondria-Associated Endoplasmic Reticulum Membrane Disruption in Normal Lung Cells. PLoS ONE 2015, 10, e0131208. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Yao, M.; Dong, T.; Mao, Z.; Hang, B.; Han, X.; Lin, Z.; Bian, Q.; Li, M.; et al. Titanium dioxide nanoparticles induce proteostasis disruption and autophagy in human trophoblast cells. Chem. Biol. Interact. 2018, 296, 124–133. [Google Scholar] [CrossRef]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef]
- Krajewska, M.; Xu, L.; Xu, W.; Krajewski, S.; Kress, C.L.; Cui, J.; Yang, L.; Irie, F.; Yamaguchi, Y.; Lipton, S.A.; et al. Endoplasmic reticulum protein BI-1 modulates unfolded protein response signaling and protects against stroke and traumatic brain injury. Brain Res. 2011, 1370, 227–237. [Google Scholar] [CrossRef][Green Version]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef]
- Zhou, C.; Liang, Y.; Zhou, L.; Yan, Y.; Liu, N.; Zhang, R.; Huang, Y.; Wang, M.; Tang, Y.; Ali, D.W.; et al. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy 2020, 1–21. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.K.; Park, H.; Lee, Y.J.; Park, S.H.; Lee, K.J.; Lee, D.G.; Kang, H.; Kim, J.E. Contribution of Autophagy-Notch1-Mediated NLRP3 Inflammasome Activation to Chronic Inflammation and Fibrosis in Keloid Fibroblasts. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; MacCormack, T.J.; Clark, R.J.; Ede, J.D.; Ortega, V.A.; Felix, L.C.; Dang, M.K.; Ma, G.; Fenniri, H.; Veinot, J.G.; et al. Widespread nanoparticle-assay interference: Implications for nanotoxicity testing. PLoS ONE 2014, 9, e90650. [Google Scholar] [CrossRef]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012, 86, 1123–1136. [Google Scholar] [CrossRef]
- Bohmer, N.; Rippl, A.; May, S.; Walter, A.; Heo, M.B.; Kwak, M.; Roesslein, M.; Song, N.W.; Wick, P.; Hirsch, C. Interference of engineered nanomaterials in flow cytometry: A case study. Colloids Surf. B Biointerfaces 2018, 172, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Franz, P.; Bürkle, A.; Wick, P.; Hirsch, C. Exploring Flow Cytometry-Based Micronucleus Scoring for Reliable Nanomaterial Genotoxicity Assessment. Chem. Res. Toxicol. 2020, 33, 2538–2549. [Google Scholar] [CrossRef]
- Thipperudrappa, J.; Raghavendra, U.P.; Basanagouda, M. Effect of TiO(2) nanoparticles on some photophysical characteristics of ketocyanine dyes. Luminescence 2017, 32, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Doak, S.H.; Yan, J.; Chen, D.H.; Zhou, M.; Mittelstaedt, R.A.; Chen, Y.; Li, C.; Chen, T. Factors affecting the in vitro micronucleus assay for evaluation of nanomaterials. Mutagenesis 2017, 32, 151–159. [Google Scholar] [CrossRef]
- Sharma, A.; Hayat, A.; Mishra, R.K.; Catanante, G.; Bhand, S.; Marty, J.L. Titanium Dioxide Nanoparticles (TiO2) Quenching Based Aptasensing Platform: Application to Ochratoxin a Detection. Toxins 2015, 7, 3771–3784. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Hoare, M.; Das, T.; Alexander, G. Ageing, telomeres, senescence, and liver injury. J. Hepatol. 2010, 53, 950–961. [Google Scholar] [CrossRef]
- Rhee, D.B.; Ghosh, A.; Lu, J.; Bohr, V.A.; Liu, Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair 2011, 10, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Løhr, M.; Sheykhzade, M.; Lykkesfeldt, J.; Wils, R.S.; Loft, S.; Møller, P. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis 2019, 34, 203–214. [Google Scholar] [CrossRef]
- Gonzalo, S.; Coll-Bonfill, N. Genomic instability and innate immune responses to self-DNA in progeria. GeroScience 2019, 41, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Pandita, R.K.; Chow, T.T.; Udayakumar, D.; Bain, A.L.; Cubeddu, L.; Hunt, C.R.; Shi, W.; Horikoshi, N.; Zhao, Y.; Wright, W.E.; et al. Single-strand DNA-binding protein SSB1 facilitates TERT recruitment to telomeres and maintains telomere G-overhangs. Cancer Res. 2015, 75, 858–869. [Google Scholar] [CrossRef] [PubMed]
- D’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Jacobs, J.J.; de Lange, T. p16INK4a as a second effector of the telomere damage pathway. Cell Cycle 2005, 4, 1364–1368. [Google Scholar] [CrossRef]
- Mahootchi, E.; Cannon Homaei, S.; Kleppe, R.; Winge, I.; Hegvik, T.A.; Megias-Perez, R.; Totland, C.; Mogavero, F.; Baumann, A.; Glennon, J.C.; et al. GADL1 is a multifunctional decarboxylase with tissue-specific roles in β-alanine and carnosine production. Sci. Adv. 2020, 6, eabb3713. [Google Scholar] [CrossRef]
- Bolderson, E.; Petermann, E.; Croft, L.; Suraweera, A.; Pandita, R.K.; Pandita, T.K.; Helleday, T.; Khanna, K.K.; Richard, D.J. Human single-stranded DNA binding protein 1 (hSSB1/NABP2) is required for the stability and repair of stalled replication forks. Nucleic Acids Res. 2014, 42, 6326–6336. [Google Scholar] [CrossRef]
- Salas-Armenteros, I.; Barroso, S.I.; Rondón, A.G.; Pérez, M.; Andújar, E.; Luna, R.; Aguilera, A. Depletion of the MFAP1/SPP381 Splicing Factor Causes R-Loop-Independent Genome Instability. Cell Rep. 2019, 28, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Parplys, A.C.; Zhao, W.; Sharma, N.; Groesser, T.; Liang, F.; Maranon, D.G.; Leung, S.G.; Grundt, K.; Dray, E.; Idate, R.; et al. NUCKS1 is a novel RAD51AP1 paralog important for homologous recombination and genome stability. Nucleic Acids Res. 2015, 43, 9817–9834. [Google Scholar] [CrossRef] [PubMed]
- Maranon, D.G.; Sharma, N.; Huang, Y.; Selemenakis, P.; Wang, M.; Altina, N.; Zhao, W.; Wiese, C. NUCKS1 promotes RAD54 activity in homologous recombination DNA repair. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Hannss, R.; Dubiel, W. COP9 signalosome function in the DDR. FEBS Lett. 2011, 585, 2845–2852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montaldo, N.P.; Bordin, D.L.; Brambilla, A.; Rösinger, M.; Fordyce Martin, S.L.; Bjørås, K.; Bradamante, S.; Aas, P.A.; Furrer, A.; Olsen, L.C.; et al. Alkyladenine DNA glycosylase associates with transcription elongation to coordinate DNA repair with gene expression. Nat. Commun. 2019, 10, 5460. [Google Scholar] [CrossRef]
- Oka, S.; Kato, J.; Moss, J. Identification and characterization of a mammalian 39-kDa poly(ADP-ribose) glycohydrolase. J. Biol. Chem. 2006, 281, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Zehethofer, N.; Bermbach, S.; Hagner, S.; Garn, H.; Müller, J.; Goldmann, T.; Lindner, B.; Schwudke, D.; König, P. Lipid Analysis of Airway Epithelial Cells for Studying Respiratory Diseases. Chromatographia 2015, 78, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, V.; Ulrich, M.; Endlich, N.; Riethmüller, J.; Wilker, B.; De Oliveira-Munding, C.C.; van Heeckeren, A.M.; Barr, M.L.; von Kürthy, G.; Schmid, K.W.; et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008, 14, 382–391. [Google Scholar] [CrossRef]
- Phuyal, S.; Kasem, M.; Knittelfelder, O.; Sharma, A.; Fonseca, D.M.; Vebraite, V.; Shaposhnikov, S.; Slupphaug, G.; Skaug, V.; Zienolddiny, S. Characterization of the proteome and lipidome profiles of human lung cells after low dose and chronic exposure to multiwalled carbon nanotubes. Nanotoxicology 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Haniu, H. Nanoparticle-mediated intracellular lipid accumulation during C2C12 cell differentiation. Biochem. Biophys. Res. Commun. 2011, 406, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Whitefield, P.D.; Ma, Y. Quantification of F(2)-isoprostane isomers in cultured human lung epithelial cells after silica oxide and metal oxide nanoparticle treatment by liquid chromatography/tandem mass spectrometry. Talanta 2010, 81, 1599–1606. [Google Scholar] [CrossRef]
- Gómez-Muñoz, A. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim. Biophys. Acta 2006, 1758, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Merrill, A.H., Jr. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996, 10, 1388–1397. [Google Scholar] [CrossRef]
- Ding, T.; Li, Z.; Hailemariam, T.; Mukherjee, S.; Maxfield, F.R.; Wu, M.P.; Jiang, X.C. SMS overexpression and knockdown: Impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J. Lipid Res. 2008, 49, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Paland, N.; Aviram, M. Triglyceride accumulation in macrophages upregulates paraoxonase 2 (PON2) expression via ROS-mediated JNK/c-Jun signaling pathway activation. Biofactors 2012, 38, 458–469. [Google Scholar] [CrossRef]
- Arifin, S.A.; Falasca, M. Lysophosphatidylinositol Signalling and Metabolic Diseases. Metabolites 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- OECD. Testing Programme of Manufactured Nanomaterials. Available online: https://www.oecd.org/chemicalsafety/nanosafety/testing-programme-manufactured-nanomaterials.htm (accessed on 15 March 2021).
- Jensen, K.A. The NANOGENOTOX Dispersion Protocol for NANoREG; Version 1.0; National Research Centre for the Working Environment: Copenhagen, Denmark, 2014. [Google Scholar]
- Rasmussen, K.; Gilliland, D.; Pianella, F.; Ceccone, G.; Spampinato, V.; Cotogno, G.; Gibson, N.; Gaillard, C.; Mech, A.; Mast, J.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and PhysicoChemical Properties; Joint Research Centre: Ispra, Italy, 2014; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC86291 (accessed on 15 March 2021).
- Jensen, K.A. Towards a Method for Detecting the Potential Genotoxicity of Nanomaterials; Copenhagen, Denmark, 2011. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 15 March 2021).
- NIOSH. Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide; NIOSH Publication No. 2011–160; NIOSH: Cincinnati, OH, USA, 2011. [Google Scholar]
- De Pedro, N.; Díez, M.; García, I.; García, J.; Otero, L.; Fernández, L.; García, B.; González, R.; Rincón, S.; Pérez, D.; et al. Analytical Validation of Telomere Analysis Technology® for the High-Throughput Analysis of Multiple Telomere-Associated Variables. Biol. Proced. Online 2020, 22, 2. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Schuhmann, K.; Schwudke, D.; Sampaio, J.L.; Bornstein, S.R.; Schroeder, M.; Shevchenko, A. LipidXplorer: A software for consensual cross-platform lipidomics. PLoS ONE 2012, 7, e29851. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alswady-Hoff, M.; Erdem, J.S.; Phuyal, S.; Knittelfelder, O.; Sharma, A.; Fonseca, D.d.M.; Skare, Ø.; Slupphaug, G.; Zienolddiny, S. Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5349. https://doi.org/10.3390/ijms22105349
Alswady-Hoff M, Erdem JS, Phuyal S, Knittelfelder O, Sharma A, Fonseca DdM, Skare Ø, Slupphaug G, Zienolddiny S. Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells. International Journal of Molecular Sciences. 2021; 22(10):5349. https://doi.org/10.3390/ijms22105349
Chicago/Turabian StyleAlswady-Hoff, Mayes, Johanna Samulin Erdem, Santosh Phuyal, Oskar Knittelfelder, Animesh Sharma, Davi de Miranda Fonseca, Øivind Skare, Geir Slupphaug, and Shanbeh Zienolddiny. 2021. "Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells" International Journal of Molecular Sciences 22, no. 10: 5349. https://doi.org/10.3390/ijms22105349
APA StyleAlswady-Hoff, M., Erdem, J. S., Phuyal, S., Knittelfelder, O., Sharma, A., Fonseca, D. d. M., Skare, Ø., Slupphaug, G., & Zienolddiny, S. (2021). Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells. International Journal of Molecular Sciences, 22(10), 5349. https://doi.org/10.3390/ijms22105349






