Glia and Orofacial Pain: Progress and Future Directions
Abstract
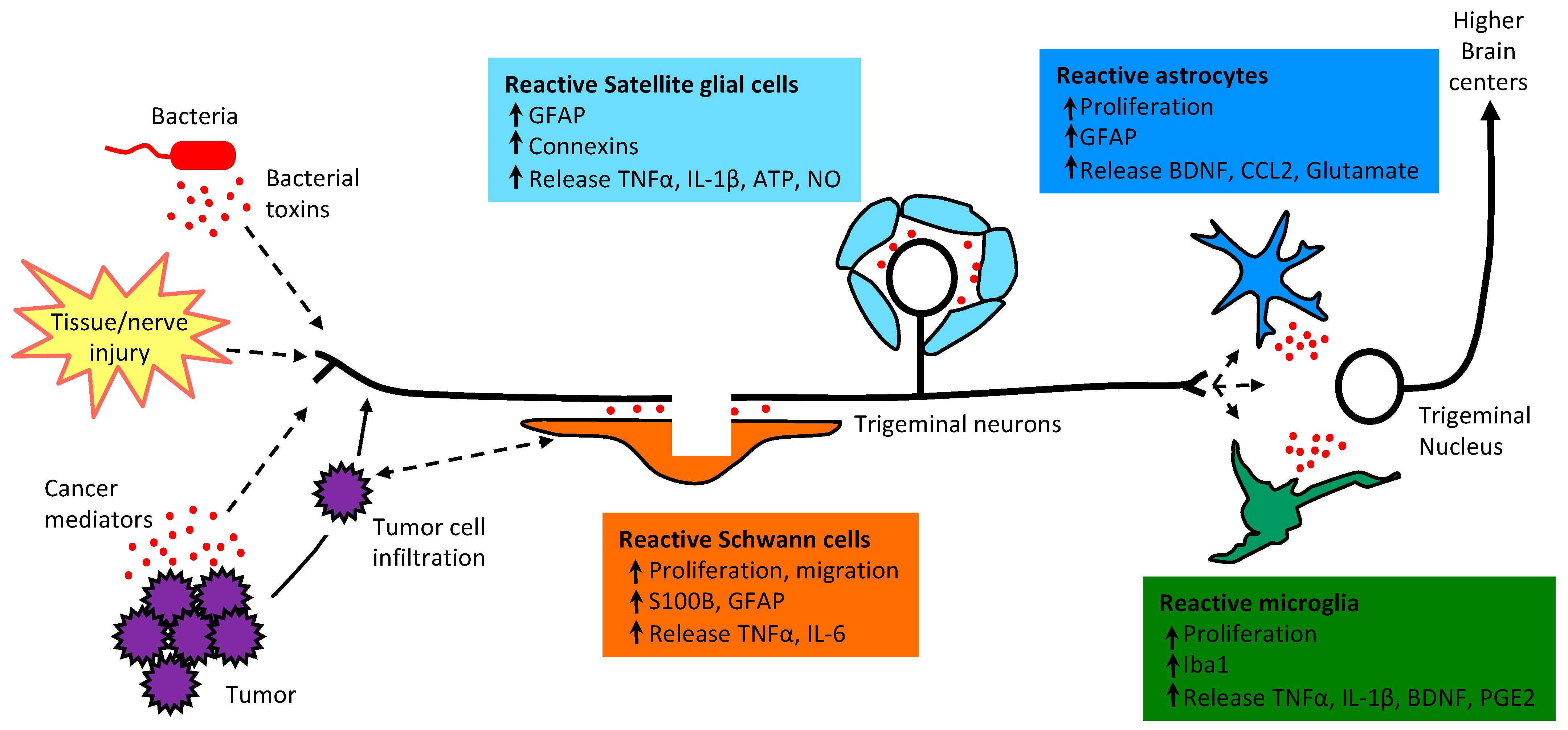
1. Introduction
2. Headache
3. TMD
3.1. Glial Cell Activation in the TG in Rodent Models of CFA-induced TMJ Inflammation
3.2. Microglia and Astrocyte Activation in the TNC in Rodent Models of TMJ Inflammation
3.3. Glial Cells Activation in Other Animal Models of TMD
4. Dental Pulp Injury
4.1. Glial Cells within the Pulp
4.2. The Effect of Pulp Injury on Glial Changes in the TG and TN
5. HNC
6. Remaining Questions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araujo-Filho, H.G.; Pereira, E.W.M.; Campos, A.R.; Quintans-Junior, L.J.; Quintans, J.S.S. Chronic orofacial pain animal models—Progress and challenges. Expert Opin. Drug Discov. 2018, 13, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M. Orofacial pain. Pain 2011, 152 (Suppl. 3), S25–S32. [Google Scholar] [CrossRef]
- De Rossi, S.S. Orofacial pain: A primer. Dent. Clin. N. Am. 2013, 57, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Dostrovsky, J.O.; Iwata, K.; Sessle, B.J. Role of glia in orofacial pain. Neuroscientist 2011, 17, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef]
- Berta, T.; Qadri, Y.J.; Chen, G.; Ji, R.R. Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence. J. Dent. Res 2016, 95, 1124–1131. [Google Scholar] [CrossRef]
- Hucho, T.; Levine, J.D. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron 2007, 55, 365–376. [Google Scholar] [CrossRef]
- Iwata, K.; Sessle, B.J. The Evolution of Neuroscience as a Research Field Relevant to Dentistry. J. Dent. Res. 2019, 98, 1407–1417. [Google Scholar] [CrossRef]
- Shinoda, M.; Kubo, A.; Hayashi, Y.; Iwata, K. Peripheral and Central Mechanisms of Persistent Orofacial Pain. Front. Neurosci. 2019, 13, 1227. [Google Scholar] [CrossRef]
- Lee, C.; Ramsey, A.; De Brito-Gariepy, H.; Michot, B.; Podborits, E.; Melnyk, J.; Gibbs, J.L.G. Molecular, cellular and behavioral changes associated with pathological pain signaling occur after dental pulp injury. Mol. Pain 2017, 13, 1744806917715173. [Google Scholar] [CrossRef]
- Salvo, E.; Campana, W.M.; Scheff, N.N.; Nguyen, T.H.; Jeong, S.H.; Wall, I.; Wu, A.K.; Zhang, S.; Kim, H.; Bhattacharya, A.; et al. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain 2020, 161, 2592–2602. [Google Scholar] [CrossRef]
- Kuchukulla, M.; Boison, D. Are glia targets for neuropathic orofacial pain therapy? J. Am. Dent. Assoc. 2020, 9. [Google Scholar] [CrossRef]
- Lukacs, M.; Haanes, K.A.; Majlath, Z.; Tajti, J.; Vecsei, L.; Warfvinge, K.; Edvinsson, L. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J. Headache Pain 2015, 16, 564. [Google Scholar] [CrossRef]
- Fried, N.T.; Maxwell, C.R.; Elliott, M.B.; Oshinsky, M.L. Region-specific disruption of the blood-brain barrier following repeated inflammatory dural stimulation in a rat model of chronic trigeminal allodynia. Cephalalgia Int. J. Headache 2018, 38, 674–689. [Google Scholar] [CrossRef]
- Fried, N.T.; Elliott, M.B.; Oshinsky, M.L. The Role of Adenosine Signaling in Headache: A Review. Brain Sci. 2017, 7, 30. [Google Scholar] [CrossRef]
- Su, M.; Ran, Y.; He, Z.; Zhang, M.; Hu, G.; Tang, W.; Zhao, D.; Yu, S. Inhibition of toll-like receptor 4 alleviates hyperalgesia induced by acute dural inflammation in experimental migraine. Mol. Pain 2018, 14, 1744806918754612. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Brennan, K. Cortical spreading depression-new insights and persistent questions. Cephalalgia Int. J. Headache 2009, 29, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Kocak, E.; Sen, Z.D.; Dalkara, T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013, 339, 1092–1095. [Google Scholar] [CrossRef]
- Aizawa, H.; Sun, W.; Sugiyama, K.; Itou, Y.; Aida, T.; Cui, W.; Toyoda, S.; Terai, H.; Yanagisawa, M.; Tanaka, K. Glial glutamate transporter GLT-1 determines susceptibility to spreading depression in the mouse cerebral cortex. Glia 2020, 68, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Gui, B.; Xie, J.; Chen, L.; Chen, L.; Cui, Z.; Zhou, J.; Tan, G. Tetrandrine Alleviates Nociception in a Rat Model of Migraine via Suppressing S100B and p-ERK Activation in Satellite Glial Cells of the Trigeminal Ganglia. J. Mol. Neurosci. 2018, 64, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; He, W.; Pan, Q.; Zhang, S.; Zhang, Y.; Liu, C.; Liu, Q.; Qin, G.; Chen, L.; Zhou, J. Microglia P2X4 receptor contributes to central sensitization following recurrent nitroglycerin stimulation. J. Neuroinflamm. 2018, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Z.; Zhang, P.; Hao, T.; Wang, L.M.; Guo, M.D.; Gan, Y.H. Connexin 43 contributes to temporomandibular joint inflammation induced-hypernociception via sodium channel 1.7 in trigeminal ganglion. Neurosci. Lett. 2019, 707, 134301. [Google Scholar] [CrossRef]
- Villa, G.; Ceruti, S.; Zanardelli, M.; Magni, G.; Jasmin, L.; Ohara, P.T.; Abbracchio, M.P. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol. Pain 2010, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Merli, D.; Verderio, C.; Abbracchio, M.P.; Ceruti, S. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: Role of satellite glial cells. Glia 2015, 63, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bi, R.Y.; Gan, Y.H. Glial interleukin-1beta upregulates neuronal sodium channel 1.7 in trigeminal ganglion contributing to temporomandibular joint inflammatory hypernociception in rats. J. Neuroinflamm. 2018, 15, 117. [Google Scholar] [CrossRef]
- Zhang, P.; Gan, Y.H. Prostaglandin E2 Upregulated Trigeminal Ganglionic Sodium Channel 1.7 Involving Temporomandibular Joint Inflammatory Pain in Rats. Inflammation 2017, 40, 1102–1109. [Google Scholar] [CrossRef]
- Bi, R.Y.; Kou, X.X.; Meng, Z.; Wang, X.D.; Ding, Y.; Gan, Y.H. Involvement of trigeminal ganglionic Nav 1.7 in hyperalgesia of inflamed temporomandibular joint is dependent on ERK1/2 phosphorylation of glial cells in rats. Eur. J. Pain 2013, 17, 983–994. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Watanabe, M.; Shimizu, K.; Zou, S.; LaGraize, S.C.; Wei, F.; Dubner, R.; Ren, K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007, 27, 6006–6018. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, Y.S.; Kim, T.H.; Jin, M.U.; Ahn, D.K.; Noguchi, K.; Bae, Y.C. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J. Chem. Neuroanat. 2012, 45, 45–49. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Tan, Y.; Ma, Y.; Tian, Y.; Jin, X.; Lim, G.; Zhang, S.; Chen, L.; Mao, J. A functional relationship between trigeminal astroglial activation and NR1 expression in a rat model of temporomandibular joint inflammation. Pain Med. 2012, 13, 1590–1600. [Google Scholar] [CrossRef]
- Nascimento, G.C.; De Paula, B.B.; Gerlach, R.F.; Leite-Panissi, C.R.A. Temporomandibular inflammation regulates the matrix metalloproteinases MMP-2 and MMP-9 in limbic structures. J. Cell Physiol. 2021. [Google Scholar] [CrossRef]
- Cady, R.J.; Denson, J.E.; Sullivan, L.Q.; Durham, P.L. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience 2014, 269, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Koop, L.K.; Hawkins, J.L.; Cornelison, L.E.; Durham, P.L. Central Role of Protein Kinase A in Promoting Trigeminal Nociception in an In Vivo Model of Temporomandibular Disorders. J. Oral Facial Pain Headache 2017, 31, 264–274. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, S.; Shu, H.; Tang, Y.; George, S.; Dong, T.; Schmidt, B.L.; Tao, F. TNFalpha in the Trigeminal Nociceptive System Is Critical for Temporomandibular Joint Pain. Mol. Neurobiol. 2019, 56, 278–291. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, J.C.B.; Gondim, D.V.; Cavalcante, A.L.C.; Lisboa, M.R.P.; de Castro Brito, G.A.; Vale, M.L. Inflammatory pain assessment in the arthritis of the temporomandibular joint in rats: A comparison between two phlogistic agents. J. Pharmacol. Toxicol. Methods 2017, 88 Pt 1, 100–108. [Google Scholar] [CrossRef]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007, 47, 1008–1023. [Google Scholar] [CrossRef]
- Garrett, F.G.; Durham, P.L. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2008, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Won, K.A.; Kang, Y.M.; Lee, M.K.; Park, M.K.; Ju, J.S.; Bae, Y.C.; Ahn, D.K. Participation of microglial p38 MAPK in formalin-induced temporomandibular joint nociception in rats. J. Orofac. Pain 2012, 26, 132–141. [Google Scholar] [PubMed]
- Guo, W.; Wang, H.; Zou, S.; Wei, F.; Dubner, R.; Ren, K. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: A model of myogenic orofacial pain. Mol. Pain 2010, 6, 40. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, J.J.; Sun, L.; Chen, L.W.; Rao, Z.R.; Duan, L.; Cao, R.; Wang, M.Q. Involvement of medullary dorsal horn glial cell activation in mediation of masseter mechanical allodynia induced by experimental tooth movement. Arch Oral. Biol. 2009, 54, 1143–1150. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Liu, Y.; Zhao, Y.H.; Li, Q.; Zhang, M.; Chen, Y.J. Activation of satellite glial cells in the trigeminal ganglion contributes to masseter mechanical allodynia induced by restraint stress in rats. Neurosci. Lett. 2015, 602, 150–155. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Liu, Y.; Li, Q.; Zhao, Y.H.; Wang, J.; Zhang, M.; Chen, Y.J. Involvement of trigeminal astrocyte activation in masseter hyperalgesia under stress. Physiol. Behav. 2015, 142, 57–65. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Wang, J.; Xie, Y.F.; Zhang, S.; Hu, J.W.; Dostrovsky, J.O.; Sessle, B.J. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J. Neurosci. 2007, 27, 9068–9076. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.F.; Zhang, S.; Chiang, C.Y.; Hu, J.W.; Dostrovsky, J.O.; Sessle, B.J. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn). Brain Behav. Immun. 2007, 21, 634–641. [Google Scholar] [CrossRef]
- Filippini, H.F.; Scalzilli, P.A.; Costa, K.M.; Freitas, R.D.S.; Campos, M.M. Activation of trigeminal ganglion satellite glial cells in CFA-induced tooth pulp pain in rats. PLoS ONE 2018, 13, e0207411. [Google Scholar] [CrossRef]
- Komiya, H.; Shimizu, K.; Ishii, K.; Kudo, H.; Okamura, T.; Kanno, K.; Shinoda, M.; Ogiso, B.; Iwata, K. Connexin 43 expression in satellite glial cells contributes to ectopic tooth-pulp pain. J. Oral Sci. 2018, 60, 493–499. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Iwata, K.; Dostrovsky, J.O.; Chiang, C.Y.; Sessle, B.J.; Hu, J.W. Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur. J. Neurosci. 2011, 34, 292–302. [Google Scholar] [CrossRef]
- Hironaka, K.; Ozaki, N.; Hattori, H.; Nagamine, K.; Nakashima, H.; Ueda, M.; Sugiura, Y. Involvement of glial activation in trigeminal ganglion in a rat model of lower gingival cancer pain. Nagoya J. Med. Sci. 2014, 76, 323–332. [Google Scholar]
- Tamagawa, T.; Shinoda, M.; Honda, K.; Furukawa, A.; Kaji, K.; Nagashima, H.; Akasaka, R.; Chen, J.; Sessle, B.J.; Yonehara, Y.; et al. Involvement of Microglial P2Y12 Signaling in Tongue Cancer Pain. J. Dent. Res. 2016, 95, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, K.; Ono, K.; Harano, N.; Sago, T.; Nunomaki, M.; Shiiba, S.; Nakanishi, O.; Fukushima, H.; Inenaga, K. Central glial activation mediates cancer-induced pain in a rat facial cancer model. Neuroscience 2011, 180, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Sago, T.; Ono, K.; Harano, N.; Furuta-Hidaka, K.; Hitomi, S.; Nunomaki, M.; Yoshida, M.; Shiiba, S.; Nakanishi, O.; Matsuo, K.; et al. Distinct time courses of microglial and astrocytic hyperactivation and the glial contribution to pain hypersensitivity in a facial cancer model. Brain Res. 2012, 1457, 70–80. [Google Scholar] [CrossRef]
- Salvo, E.; Saraithong, P.; Curtin, J.G.; Janal, M.N.; Ye, Y. Reciprocal interactions between cancer and Schwann cells contribute to oral cancer progression and pain. Heliyon 2019, 5, e01223. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Ricci, J.A.; Chee, E.; Morganstein, D.; Lipton, R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003, 290, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Vila-Pueyo, M.; Hoffmann, J.; Romero-Reyes, M.; Akerman, S. Brain structure and function related to headache: Brainstem structure and function in headache. Cephalalgia Int. J. Headache 2019, 39, 1635–1660. [Google Scholar] [CrossRef] [PubMed]
- Kemper, R.H.; Meijler, W.J.; Korf, J.; Ter Horst, G.J. Migraine and function of the immune system: A meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia Int. J. Headache 2001, 21, 549–557. [Google Scholar] [CrossRef]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia Int. J. Headache 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Eising, E.; Datson, N.A.; van den Maagdenberg, A.M.; Ferrari, M.D. Epigenetic mechanisms in migraine: A promising avenue? BMC Med. 2013, 11, 26. [Google Scholar] [CrossRef]
- Bartley, J. Could glial activation be a factor in migraine? Med. Hypotheses 2009, 72, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 361–370. [Google Scholar] [CrossRef]
- Vermeiren, C.; Najimi, M.; Vanhoutte, N.; Tilleux, S.; de Hemptinne, I.; Maloteaux, J.M.; Hermans, E. Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J. Neurochem. 2005, 94, 405–416. [Google Scholar] [CrossRef]
- Johnson, J.L.; Kwok, Y.H.; Sumracki, N.M.; Swift, J.E.; Hutchinson, M.R.; Johnson, K.; Williams, D.B.; Tuke, J.; Rolan, P.E. Glial Attenuation With Ibudilast in the Treatment of Medication Overuse Headache: A Double-Blind, Randomized, Placebo-Controlled Pilot Trial of Efficacy and Safety. Headache 2015, 55, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Meng, I.D.; Cao, L. From migraine to chronic daily headache: The biological basis of headache transformation. Headache 2007, 47, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Maasumi, K.; Michael, R.L.; Rapoport, A.M. CGRP and Migraine: The Role of Blocking Calcitonin Gene-Related Peptide Ligand and Receptor in the Management of Migraine. Drugs 2018, 78, 913–928. [Google Scholar] [CrossRef]
- Edvinsson, L. The CGRP Pathway in Migraine as a Viable Target for Therapies. Headache 2018, 58 (Suppl. 1), 33–47. [Google Scholar] [CrossRef]
- Rodgers, K.M.; Deming, Y.K.; Bercum, F.M.; Chumachenko, S.Y.; Wieseler, J.L.; Johnson, K.W.; Watkins, L.R.; Barth, D.S. Reversal of established traumatic brain injury-induced, anxiety-like behavior in rats after delayed, post-injury neuroimmune suppression. J. Neurotrauma 2014, 31, 487–497. [Google Scholar] [CrossRef]
- Suzumura, A.; Ito, A.; Yoshikawa, M.; Sawada, M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999, 837, 203–212. [Google Scholar] [CrossRef]
- Kwok, Y.H.; Swift, J.E.; Gazerani, P.; Rolan, P. A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine. J. Pain Res. 2016, 9, 899–907. [Google Scholar] [CrossRef]
- Loggia, M.L.; Chonde, D.B.; Akeju, O.; Arabasz, G.; Catana, C.; Edwards, R.R.; Hill, E.; Hsu, S.; Izquierdo-Garcia, D.; Ji, R.R.; et al. Evidence for brain glial activation in chronic pain patients. Brain 2015, 138 Pt 3, 604–615. [Google Scholar] [CrossRef]
- Teepker, M.; Munk, K.; Mylius, V.; Haag, A.; Möller, J.C.; Oertel, W.H.; Schepelmann, K. Serum concentrations of s100b and NSE in migraine. Headache 2009, 49, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, O.; Soldatou, A.; Tsitsika, A.; Kariyannis, C.; Papandreou, T.; Zachariadi, A.; Papassotiriou, I.; Chrousos, G.P. Serum S100beta protein in children with acute recurrent headache: A potentially useful marker for migraine. Headache 2005, 45, 1313–1316. [Google Scholar] [CrossRef]
- Yilmaz, S.; Serum, N.O. S100B, NSE concentrations in migraine and their relationship. J. Clin. Neurosci. 2020, 82, 32–35. [Google Scholar] [CrossRef]
- Isaksen, T.J.; Lykke-Hartmann, K. Insights into the Pathology of the α2-Na(+)/K(+)-ATPase in Neurological Disorders; Lessons from Animal Models. Front. Physiol. 2016, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Leao, A.A. Further observations on the spreading depression of activity in the cerebral cortex. J. Neurophysiol. 1947, 10, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Larsen, B.; Lauritzen, M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981, 9, 344–352. [Google Scholar] [CrossRef]
- Capuani, C.; Melone, M.; Tottene, A.; Bragina, L.; Crivellaro, G.; Santello, M.; Casari, G.; Conti, F.; Pietrobon, D. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. Embo Mol. Med. 2016, 8, 967–986. [Google Scholar] [CrossRef]
- Romanos, J.; Benke, D.; Pietrobon, D.; Zeilhofer, H.U.; Santello, M. Astrocyte dysfunction increases cortical dendritic excitability and promotes cranial pain in familial migraine. Sci. Adv. 2020, 6, eaaz1584. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.T.; Dessirier, J.M.; Jinks, S.L.; Carstens, E. An animal model to assess aversion to intra-oral capsaicin: Increased threshold in mice lacking substance p. Chem. Senses 2001, 26, 491–497. [Google Scholar] [CrossRef]
- Damodaram, S.; Thalakoti, S.; Freeman, S.E.; Garrett, F.G.; Durham, P.L. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache 2009, 49, 5–20. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Schoenen, J.; Göbel, H.; Diener, H.C.; Elkind, A.H.; Klapper, J.A.; Howard, R.A. Tonabersat, a gap-junction modulator: Efficacy and safety in two randomized, placebo-controlled, dose-ranging studies of acute migraine. Cephalalgia Int. J. Headache 2009, 29 (Suppl. 2), 17–27. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Ferrari, M.D.; Csanyi, A.; Olesen, J.; Mills, J.G.; Tonabersat, T.O.N.S.G. Randomized, double-blind, placebo-controlled, proof-of-concept study of the cortical spreading depression inhibiting agent tonabersat in migraine prophylaxis. Cephalalgia Int. J. Headache 2009, 29, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Hauge, A.W.; Asghar, M.S.; Schytz, H.W.; Christensen, K.; Olesen, J. Effects of tonabersat on migraine with aura: A randomised, double-blind, placebo-controlled crossover study. Lancet Neurol. 2009, 8, 718–723. [Google Scholar] [CrossRef]
- Suzuki, M.; Van Paesschen, W.; Stalmans, I.; Horita, S.; Yamada, H.; Bergmans, B.A.; Legius, E.; Riant, F.; De Jonghe, P.; Li, Y.; et al. Defective membrane expression of the Na(+)-HCO(3)(-) cotransporter NBCe1 is associated with familial migraine. Proc. Natl. Acad. Sci. USA 2010, 107, 15963–15968. [Google Scholar] [CrossRef]
- Weir, G.A.; Cader, M.Z. New directions in migraine. BMC Med. 2011, 9, 116. [Google Scholar] [CrossRef]
- Epstein, J.B.; Hong, C.; Logan, R.M.; Barasch, A.; Gordon, S.M.; Oberle-Edwards, L.; McGuire, D.; Napenas, J.J.; Elting, L.S.; Spijkervet, F.K.; et al. A systematic review of orofacial pain in patients receiving cancer therapy. Support Care Cancer 2010, 18, 1023–1031. [Google Scholar] [CrossRef]
- Gooz, M. ADAM-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169. [Google Scholar] [CrossRef]
- Eising, E.; de Leeuw, C.; Min, J.L.; Anttila, V.; Verheijen, M.H.; Terwindt, G.M.; Dichgans, M.; Freilinger, T.; Kubisch, C.; Ferrari, M.D.; et al. Involvement of astrocyte and oligodendrocyte gene sets in migraine. Cephalalgia Int. J. Headache 2016, 36, 640–647. [Google Scholar] [CrossRef]
- Wright, E.F.; North, S.L. Management and treatment of temporomandibular disorders: A clinical perspective. J. Man. Manip. 2009, 17, 247–254. [Google Scholar] [CrossRef]
- Chung, M.K.; Ro, J.Y. Peripheral glutamate receptor and transient receptor potential channel mechanisms of craniofacial muscle pain. Mol. Pain 2020, 16, 1744806920914204. [Google Scholar] [CrossRef]
- Damlar, I.; Esen, E.; Tatli, U. Effects of glucosamine-chondroitin combination on synovial fluid IL-1β, IL-6, TNF-α and PGE2 levels in internal derangements of temporomandibular joint. Med. Oralpatologia Oral Y Cir. Bucal 2015, 20, e278–e283. [Google Scholar] [CrossRef]
- Güven, O.; Tekin, U.; Salmanoğlu, B.; Kaymak, E. Tumor necrosis factor-alpha levels in the synovial fluid of patients with temporomandibular joint internal derangement. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2015, 43, 102–105. [Google Scholar] [CrossRef]
- Ibi, M.; Horie, S.; Kyakumoto, S.; Chosa, N.; Yoshida, M.; Kamo, M.; Ohtsuka, M.; Ishisaki, A. Cell-cell interactions between monocytes/macrophages and synoviocyte-like cells promote inflammatory cell infiltration mediated by augmentation of MCP-1 production in temporomandibular joint. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Okamoto, K.; Bereiter, D.F.; Thompson, R.; Tashiro, A.; Bereiter, D.A. Estradiol replacement modifies c-fos expression at the spinomedullary junction evoked by temporomandibular joint stimulation in ovariectomized female rats. Neuroscience 2008, 156, 729–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rozanski, G.M.; Li, Q.; Stanley, E.F. Transglial transmission at the dorsal root ganglion sandwich synapse: Glial cell to postsynaptic neuron communication. Eur. J. Neurosci. 2013, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, H.; Li, X.; Liu, J.; Cao, S.; Huang, B.; Huang, D.; Wu, L. Inhibition of Connexin 43 and Phosphorylated NR2B in Spinal Astrocytes Attenuates Bone Cancer Pain in Mice. Front. Cell Neurosci. 2018, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Roh, D.H.; Yoon, S.Y.; Choi, S.R.; Kwon, S.G.; Kang, S.Y.; Moon, J.Y.; Han, H.J.; Beitz, A.J.; Lee, J.H. Differential involvement of ipsilateral and contralateral spinal cord astrocyte D-serine in carrageenan-induced mirror-image pain: Role of σ1 receptors and astrocyte gap junctions. Br. J. Pharm. 2018, 175, 558–572. [Google Scholar] [CrossRef]
- Zhou, Q.; Imbe, H.; Dubner, R.; Ren, K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: Implications for persistent orofacial pain. J. Comp. Neurol. 1999, 412, 276–291. [Google Scholar] [CrossRef]
- Bartolucci, M.L.; Marini, I.; Bortolotti, F.; Impellizzeri, D.; Di Paola, R.; Bruschetta, G.; Crupi, R.; Portelli, M.; Militi, A.; Oteri, G.; et al. Micronized palmitoylethanolamide reduces joint pain and glial cell activation. Inflamm. Res. 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Manuel Munoz-Lora, V.R.; Abdalla, H.B.; Del Bel Cury, A.A.; Clemente-Napimoga, J.T. Modulatory effect of botulinum toxin type A on the microglial P2X7/CatS/FKN activated-pathway in antigen-induced arthritis of the temporomandibular joint of rats. Toxicon 2020, 187, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kiyomoto, M.; Shinoda, M.; Honda, K.; Nakaya, Y.; Dezawa, K.; Katagiri, A.; Kamakura, S.; Inoue, T.; Iwata, K. p38 phosphorylation in medullary microglia mediates ectopic orofacial inflammatory pain in rats. Mol. Pain 2015, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.F.; Shi, X.Q.; Wu, W.; Gueorguieva, M.; Yang, M.; Zhang, J. Sustained and repeated mouth opening leads to development of painful temporomandibular disorders involving macrophage/microglia activation in mice. Pain 2018, 159, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Michot, B.; Casey, S.M.; Gibbs, J.L. Effects of Calcitonin Gene-related Peptide on Dental Pulp Stem Cell Viability, Proliferation, and Differentiation. J. Endod. 2020, 46, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Austah, O.N.; Ruparel, N.B.; Henry, M.A.; Fajardo, R.J.; Schmitz, J.E.; Diogenes, A. Capsaicin-sensitive Innervation Modulates the Development of Apical Periodontitis. J. Endod. 2016, 42, 1496–1502. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Taylor, P.E.; Byers, M.R.; Redd, P.E. Sprouting of CGRP nerve fibers in response to dentin injury in rat molars. Brain Res. 1988, 461, 371–376. [Google Scholar] [CrossRef]
- Suzuki, K.; Lovera, M.; Schmachtenberg, O.; Couve, E. Axonal Degeneration in Dental Pulp Precedes Human Primary Teeth Exfoliation. J. Dent. Res. 2015, 94, 1446–1453. [Google Scholar] [CrossRef]
- Yoshiba, N.; Edanami, N.; Ohkura, N.; Maekawa, T.; Takahashi, N.; Tohma, A.; Izumi, K.; Maeda, T.; Hosoya, A.; Nakamura, H.; et al. M2 Phenotype Macrophages Colocalize with Schwann Cells in Human Dental Pulp. J. Dent. Res. 2020, 99, 329–338. [Google Scholar] [CrossRef]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef]
- Nixdorf, D.R.; Law, A.S.; Lindquist, K.; Reams, G.J.; Cole, E.; Kanter, K.; Nguyen, R.H.N.; Harris, D.R.; National Dental, P.C.G. Frequency, impact, and predictors of persistent pain after root canal treatment: A national dental PBRN study. Pain 2016, 157, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Katagiri, A.; Shinoda, M. Neuron-glia interaction is a key mechanism underlying persistent orofacial pain. J. Oral Sci. 2017, 59, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Komiya, H.; Shimizu, K.; Noma, N.; Tsuboi, Y.; Honda, K.; Kanno, K.; Ohara, K.; Shinoda, M.; Ogiso, B.; Iwata, K. Role of Neuron-Glial Interaction Mediated by IL-1β in Ectopic Tooth Pain. J. Dent. Res. 2018, 97, 467–475. [Google Scholar] [CrossRef]
- Naftel, J.P.; Bernanke, J.M.; Qian, X.B. Quantitative study of the apical nerve fibers of adult and juvenile rat molars. Anat. Rec. 1994, 238, 507–516. [Google Scholar] [CrossRef]
- Sugimoto, T.; Takemura, M. Tooth pulp primary neurons: Cell size analysis, central connection, and carbonic anhydrase activity. Brain Res. Bull 1993, 30, 221–226. [Google Scholar] [CrossRef]
- Gibbs, J.L.; Melnyk, J.L.; Basbaum, A.I. Differential TRPV1 and TRPV2 channel expression in dental pulp. J. Dent. Res. 2011, 90, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.K.; Lee, D.S.; Kim, J.Y.; Bae, J.Y.; Cho, Y.S.; Ahn, D.K.; Yoshida, A.; Bae, Y.C. Quantitative ultrastructural analysis of the neurofilament 200-positive axons in the rat dental pulp. J. Endod. 2010, 36, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.A.; Luo, S.; Levinson, S.R. Unmyelinated nerve fibers in the human dental pulp express markers for myelinated fibers and show sodium channel accumulations. BMC Neurosci. 2012, 13, 29. [Google Scholar] [CrossRef]
- Fried, K.; Sessle, B.J.; Devor, M. The paradox of pain from tooth pulp: Low-threshold “algoneurons”? Pain 2011, 152, 2685–2689. [Google Scholar] [CrossRef]
- Couve, E.; Schmachtenberg, O. Schwann Cell Responses and Plasticity in Different Dental Pulp Scenarios. Front. Cell Neurosci. 2018, 12, 299. [Google Scholar] [CrossRef]
- Houshmandi, M.; Ye, P.; Hunter, N. Glial network responses to polymicrobial invasion of dentin. Caries Res. 2014, 48, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Couve, E.; Lovera, M.; Suzuki, K.; Schmachtenberg, O. Schwann Cell Phenotype Changes in Aging Human Dental Pulp. J. Dent. Res. 2018, 97, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.W.; Brosius Lutz, A.; Cheng, Y.C.; Latremoliere, A.; Duong, K.; Miller, C.M.; Posada, S.; Cobos, E.J.; Zhang, A.X.; Wagers, A.J.; et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014, 83, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Ozawa, S.; Nukada, H.; Tsukamoto, M.; Sango, K.; Himeno, T.; Kamiya, H.; et al. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. 2017, 8, 279. [Google Scholar] [CrossRef]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Stephenson, J.L.; Byers, M.R. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp. Neurol. 1995, 131, 11–22. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, L.; Gu, W.; Liu, Q.; Gao, Z.; Zhu, X.; Wu, Z.; He, H.; Huang, F.; Fan, W. Activation of satellite glial cells in trigeminal ganglion following dental injury and inflammation. J. Mol. Histol. 2018, 49, 257–263. [Google Scholar] [CrossRef]
- Gobel, S.; Binck, J.M. Degenerative changes in primary trigeminal axons and in neurons in nucleus caudalis following tooth pulp extirpations in the cat. Brain Res. 1977, 132, 347–354. [Google Scholar] [CrossRef]
- Vena, D.A.; Collie, D.; Wu, H.; Gibbs, J.L.; Broder, H.L.; Curro, F.A.; Thompson, V.P.; Craig, R.G.; Group, P.N. Prevalence of persistent pain 3 to 5 years post primary root canal therapy and its impact on oral health-related quality of life: PEARL Network findings. J. Endod. 2014, 40, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Luckett, T.; Davidson, P.M.; Boyle, F.; Liauw, W.; Agar, M.; Green, A.; Lovell, M.; ImPaCct; PoCoG. Australian survey of current practice and guideline use in adult cancer pain assessment and management: Perspectives of oncologists. Asia Pac. J. Clin. Oncol. 2014, 10, e99–e107. [Google Scholar] [CrossRef]
- Macfarlane, T.V.; Wirth, T.; Ranasinghe, S.; Ah-See, K.W.; Renny, N.; Hurman, D. Head and neck cancer pain: Systematic review of prevalence and associated factors. J. Oral Maxillofac. Res. 2012, 3, e1. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Teruel, A.; Ye, Y. Cancer and referred facial pain. Curr. Pain Headache Rep. 2015, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L. The neurobiology of cancer pain. Neuroscientist 2014, 20, 546–562. [Google Scholar] [CrossRef]
- Scheff, N.N.; Ye, Y.; Bhattacharya, A.; MacRae, J.; Hickman, D.N.; Sharma, A.K.; Dolan, J.C.; Schmidt, B.L. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain 2017, 158, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ono, K.; Bernabe, D.G.; Viet, C.T.; Pickering, V.; Dolan, J.C.; Hardt, M.; Ford, A.P.; Schmidt, B.L. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta Neuropathol. Commun. 2014, 2, 62. [Google Scholar] [CrossRef]
- Kolokythas, A.; Cox, D.P.; Dekker, N.; Schmidt, B.L. Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: Is there an association with perineural invasion? J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2010, 68, 1290–1295. [Google Scholar] [CrossRef]
- Demir, I.E.; Kujundzic, K.; Pfitzinger, P.L.; Saricaoglu, O.C.; Teller, S.; Kehl, T.; Reyes, C.M.; Ertl, L.S.; Miao, Z.; Schall, T.J.; et al. Early pancreatic cancer lesions suppress pain through CXCL12-mediated chemoattraction of Schwann cells. Proc. Natl. Acad. Sci. USA 2017, 114, e85–e94. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Saricaoglu, O.C.; Pfitzinger, P.L.; Teller, S.; Wang, K.; Waldbaur, C.; Kurkowski, M.U.; Wormann, S.M.; et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut 2016, 65, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D.; et al. Melanoma-induced reprogramming of Schwann cell signaling aids tumor growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef]
- Zhou, Y.; Shurin, G.V.; Zhong, H.; Bunimovich, Y.L.; Han, B.; Shurin, M.R. Schwann Cells Augment Cell Spreading and Metastasis of Lung Cancer. Cancer Res. 2018, 78, 5927–5939. [Google Scholar] [CrossRef] [PubMed]
- Bunimovich, Y.L.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Schwann cells: A new player in the tumor microenvironment. Cancer Immunol. Immunother. 2017, 66, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Deborde, S.; Wong, R.J. How Schwann cells facilitate cancer progression in nerves. Cell. Mol. Life Sci. CMLS 2017, 74, 4405–4420. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.-Y.; Barajas, F.; Chen, C.-H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Tu, N.H.; Scheff, N.N.; Dubeykovskaya, Z.A.; Chavan, S.A.; Aouizerat, B.E.; Ye, Y. TNFalpha promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021, 11, 1840. [Google Scholar] [CrossRef]
- Ein, L.; Mei, C.; Bracho, O.; Bas, E.; Monje, P.; Weed, D.; Sargi, Z.; Thomas, G.; Dinh, C. Modulation of BDNF-TRKB Interactions on Schwann Cell-induced Oral Squamous Cell Carcinoma Dispersion In Vitro. Anticancer Res. 2019, 39, 5933–5942. [Google Scholar] [CrossRef]
- Huang, T.; Fan, Q.; Wang, Y.; Cui, Y.; Wang, Z.; Yang, L.; Sun, X.; Wang, Y. Schwann Cell-Derived CCL2 Promotes the Perineural Invasion of Cervical Cancer. Front. Oncol. 2020, 10, 19. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann Cells at Neoplastic Cell Sites Before the Onset of Cancer Invasion. JNCI J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef]
- Gosselin, R.D.; Suter, M.R.; Ji, R.R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. 1), S10–S28. [Google Scholar] [CrossRef] [PubMed]
- Campana, W.M. Schwann cells: Activated peripheral glia and their role in neuropathic pain. Brain Behav. Immun. 2007, 21, 522–527. [Google Scholar] [CrossRef]
- Armati, P.J.; Mathey, E.K. An update on Schwann cell biology—Immunomodulation, neural regulation and other surprises. J. Neurol. Sci. 2013, 333, 68–72. [Google Scholar] [CrossRef]
- Ydens, E.; Lornet, G.; Smits, V.; Goethals, S.; Timmerman, V.; Janssens, S. The neuroinflammatory role of Schwann cells in disease. Neurobiol. Dis. 2013, 55, 95–103. [Google Scholar] [CrossRef]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.D.; Usoskin, D.; El Manira, A.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized cutaneous Schwann cells initiate pain sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- De Logu, F.; Li Puma, S.; Landini, L.; Portelli, F.; Innocenti, A.; de Araujo, D.S.M.; Janal, M.N.; Patacchini, R.; Bunnett, N.W.; Geppetti, P.; et al. Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J. Clin. Investig. 2019, 129, 5424–5441. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Goncalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Horst, O.V.; Cunha-Cruz, J.; Zhou, L.; Manning, W.; Mancl, L.; DeRouen, T.A. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. J. Am. Dent. Assoc. 2015, 146, 721–728.e3. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2017, 18, 113. [Google Scholar] [CrossRef]
- Messlinger, K.; Balcziak, L.K.; Russo, A.F. Cross-talk signaling in the trigeminal ganglion: Role of neuropeptides and other mediators. J. Neural Transm. (Vienna) 2020, 127, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Megat, S.; Ray, P.R.; Tavares-Ferreira, D.; Moy, J.K.; Sankaranarayanan, I.; Wanghzou, A.; Fang Lou, T.; Barragan-Iglesias, P.; Campbell, Z.T.; Dussor, G.; et al. Differences between Dorsal Root and Trigeminal Ganglion Nociceptors in Mice Revealed by Translational Profiling. J. Neurosci. 2019, 39, 6829–6847. [Google Scholar] [CrossRef]
- Lopes, D.M.; Denk, F.; McMahon, S.B. The Molecular Fingerprint of Dorsal Root and Trigeminal Ganglion Neurons. Front. Mol. Neurosci. 2017, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int. Rev. Neurobiol. 2011, 97, 207–225. [Google Scholar]
- Okada, S.; Katagiri, A.; Saito, H.; Lee, J.; Ohara, K.; Iinuma, T.; Bereiter, D.A.; Iwata, K. Differential activation of ascending noxious pathways associated with trigeminal nerve injury. Pain 2019, 160, 1342–1360. [Google Scholar] [CrossRef]
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia Int. J. Headache 2017, 37, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Hitomi, S.; Suzuki, I.; Masuda, Y.; Kitagawa, J.; Tsuboi, Y.; Kondo, M.; Sessle, B.J.; Iwata, K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J. Neurophysiol. 2008, 99, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, J.; Kaneko, T.; Kaneko, M.; Sunakawa, M.; Kaneko, R.; Chokechanachaisakul, U.; Okiji, T.; Suda, H. Neuron-immune interactions in the sensitized thalamus induced by mustard oil application to rat molar pulp. J. Dent. Res. 2010, 89, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Totsch, S.K.; Sorge, R.E. Immune System Involvement in Specific Pain Conditions. Mol. Pain 2017, 13, 1744806917724559. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Q.; Guo, Z.; Wang, T.; Wu, H.; Ma, C.; Wang, W.; Su, F.; Zhang, H.; Su, X. Gadolinium-conjugated CB86: A novel TSPO-targeting MRI contrast agent for imaging of rheumatoid arthritis. J. Drug Target 2020, 28, 398–407. [Google Scholar] [CrossRef]
- Forsberg, A.; Lampa, J.; Estelius, J.; Cervenka, S.; Farde, L.; Halldin, C.; Lekander, M.; Olgart Hoglund, C.; Kosek, E. Disease activity in rheumatoid arthritis is inversely related to cerebral TSPO binding assessed by [(11)C]PBR28 positron emission tomography. J. Neuroimmunol. 2019, 334, 577000. [Google Scholar] [CrossRef]
- de Groot, M.; Patel, N.; Manavaki, R.; Janiczek, R.L.; Bergstrom, M.; Ostor, A.; Gerlag, D.; Roberts, A.; Graves, M.J.; Karkera, Y.; et al. Quantifying disease activity in rheumatoid arthritis with the TSPO PET ligand (18)F-GE-180 and comparison with (18)F-FDG and DCE-MRI. EJNMMI Res. 2019, 9, 113. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandstrom, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Hoglund, C.O.; Catana, C.; et al. Brain glial activation in fibromyalgia—A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Gelesko, S.; Long, L.; Faulk, J.; Phillips, C.; Dicus, C.; White, R.P., Jr. Cryotherapy and topical minocycline as adjunctive measures to control pain after third molar surgery: An exploratory study. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2011, 69, e324–e332. [Google Scholar] [CrossRef]
- Martinez, V.; Szekely, B.; Lemarie, J.; Martin, F.; Gentili, M.; Ben Ammar, S.; Lepeintre, J.F.; Garreau de Loubresse, C.; Chauvin, M.; Bouhassira, D.; et al. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: A randomized, double-blind, controlled study. Pain 2013, 154, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Syngle, A.; Verma, I.; Krishan, P.; Garg, N.; Syngle, V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014, 35, 1067–1073. [Google Scholar] [CrossRef]
- Darcey, E.; Boyle, T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 70, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Vanelderen, P.; Van Zundert, J.; Kozicz, T.; Puylaert, M.; De Vooght, P.; Mestrum, R.; Heylen, R.; Roubos, E.; Vissers, K. Effect of minocycline on lumbar radicular neuropathic pain: A randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 2015, 122, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Sumracki, N.M.; Hutchinson, M.R.; Gentgall, M.; Briggs, N.; Williams, D.B.; Rolan, P. The effects of pregabalin and the glial attenuator minocycline on the response to intradermal capsaicin in patients with unilateral sciatica. PLoS ONE 2012, 7, e38525. [Google Scholar] [CrossRef] [PubMed]
- Landry, R.P.; Jacobs, V.L.; Romero-Sandoval, E.A.; DeLeo, J.A. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: In vitro evidence for differential responses in human and rodent microglia and macrophages. Exp. Neurol. 2012, 234, 340–350. [Google Scholar] [CrossRef]
- Younger, J.; Noor, N.; McCue, R.; Mackey, S. Low-dose naltrexone for the treatment of fibromyalgia: Findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013, 65, 529–538. [Google Scholar] [CrossRef]
- Younger, J.; Mackey, S. Fibromyalgia symptoms are reduced by low-dose naltrexone: A pilot study. Pain Med. 2009, 10, 663–672. [Google Scholar] [CrossRef]
- Ostenfeld, T.; Krishen, A.; Lai, R.Y.; Bullman, J.; Baines, A.J.; Green, J.; Anand, P.; Kelly, M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: A double-blind, placebo-controlled study. Eur. J. Pain 2013, 17, 844–857. [Google Scholar] [CrossRef]
- Ostenfeld, T.; Krishen, A.; Lai, R.Y.; Bullman, J.; Green, J.; Anand, P.; Scholz, J.; Kelly, M. A randomized, placebo-controlled trial of the analgesic efficacy and safety of the p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain from lumbosacral radiculopathy. Clin. J. Pain 2015, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Shenoy, R.; Palmer, J.E.; Baines, A.J.; Lai, R.Y.; Robertson, J.; Bird, N.; Ostenfeld, T.; Chizh, B.A. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain 2011, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
| Pain Conditions | Animal Models | Glial Responses | |||
|---|---|---|---|---|---|
| Schwann Cells | SGCs | Astrocytes | Microglia | ||
| Headache | Acute/Chronic dural inflammatory soup | ND |
|
|
|
| Cortical Spreading Depression (CSD) | ND |
| |||
| Nitroglycerin | ND |
|
| ||
| TMD | CFA injection in the TMJ | ND |
|
| |
| Carrageenan injection in the TMJ | ND | ND | ND |
| |
| Zymosan injection in the TMJ | ND | ND | ND |
| |
| Capsaicin injection in the TMJ | ND |
| ND | ND | |
| Formalin injection into the TMJ | ND | ND | ND |
| |
| Masseter tendon ligation | ND | ND |
|
| |
| Tooth movement | ND | ND |
|
| |
| Chronic stress | ND |
| |||
| Dental pulp injury | Acute pulp exposure followed by mustard oil application | ND | ND |
|
|
| Pulp exposure followed by CFA application | ND |
|
| ND | |
| Pulp exposure alone | ND | ND |
|
| |
| HNC | Oral cancer cells inoculated into the rat gingiva | ND |
| Not activated [48] |
|
| Oral cancer cells inoculated into the rat tongue | ND | ND | ND |
| |
| Breast cancer cells inoculated into the rat vibrissa pad | ND |
|
|
| |
| Oral cancer cells inoculated into themouse sciatic nerve to mimic PNI |
| ND | ND | ND | |
| Schwann cell supernatant injection |
| ND | ND | ND | |
| Drug Name | Target | Indication | Efficacy | Reference |
|---|---|---|---|---|
| Minocycline | Microglia inhibitor | Third molar surgery | Yes | Gelesko et al., 2011 [181] |
| Lumbar discectomy | No | Martinez et al., 2013 [182] | ||
| Diabetic peripheral neuropathy | No | Syngle et al., 2014 [183] | ||
| Carpal tunnel and trigger finger release | No; longer pain in a patient subgroup | Curtin et al., 2017 [184] | ||
| Lumbar radiculopathy | Yes | Vanelderen et al., 2015 [185] | ||
| Unilateral sciatica | No | Sumracki et al., 2012 [186] | ||
| Propentofylline | Microglia and astrocytes modulator | Post-herpetic neuralgia | No | Landry et al., 2012 [187] |
| Ibudilast | cAMP phosphodiesterase inhibitor | Chronic migraine | No | Kwok et al., 2016 [70] |
| Medication overuse headache | No | Loggia et al., 2015 [71] | ||
| Tonabersat | Gap-junction modulator | Migraine prophylaxis | Yes, in migraine patients with aura | Hauge et al., 2009 [84] |
| Naltrexone | Toll-like receptor 4 antagonist | Fibromyalgia | Yes | Younger et al., 2009, 2013 [188,189] |
| Amitriptyline | P38 mitogen-activated protein kinase inhibitor | Lumbar radiculopathy | Yes | Vanelderen et al., 2015 [185] |
| Losmapimod | P38 mitogen-activated protein kinase inhibitor | Traumatic peripheral nerve injury | No | Ostenfeld et al., 2013 [190] |
| Lumbosacral radiculopathies | No | Ostenfeld et al., 2015 [191] | ||
| Dilmapimod | P38 mitogen-activated protein kinase inhibitor | Mixed neuropathic pain | Yes | Anand et al., 2011 [192] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Salvo, E.; Romero-Reyes, M.; Akerman, S.; Shimizu, E.; Kobayashi, Y.; Michot, B.; Gibbs, J. Glia and Orofacial Pain: Progress and Future Directions. Int. J. Mol. Sci. 2021, 22, 5345. https://doi.org/10.3390/ijms22105345
Ye Y, Salvo E, Romero-Reyes M, Akerman S, Shimizu E, Kobayashi Y, Michot B, Gibbs J. Glia and Orofacial Pain: Progress and Future Directions. International Journal of Molecular Sciences. 2021; 22(10):5345. https://doi.org/10.3390/ijms22105345
Chicago/Turabian StyleYe, Yi, Elizabeth Salvo, Marcela Romero-Reyes, Simon Akerman, Emi Shimizu, Yoshifumi Kobayashi, Benoit Michot, and Jennifer Gibbs. 2021. "Glia and Orofacial Pain: Progress and Future Directions" International Journal of Molecular Sciences 22, no. 10: 5345. https://doi.org/10.3390/ijms22105345
APA StyleYe, Y., Salvo, E., Romero-Reyes, M., Akerman, S., Shimizu, E., Kobayashi, Y., Michot, B., & Gibbs, J. (2021). Glia and Orofacial Pain: Progress and Future Directions. International Journal of Molecular Sciences, 22(10), 5345. https://doi.org/10.3390/ijms22105345







