UV Effect on Human Anterior Lens Capsule Macro-Molecular Composition Studied by Synchrotron-Based FTIR Micro-Spectroscopy
Abstract
1. Introduction
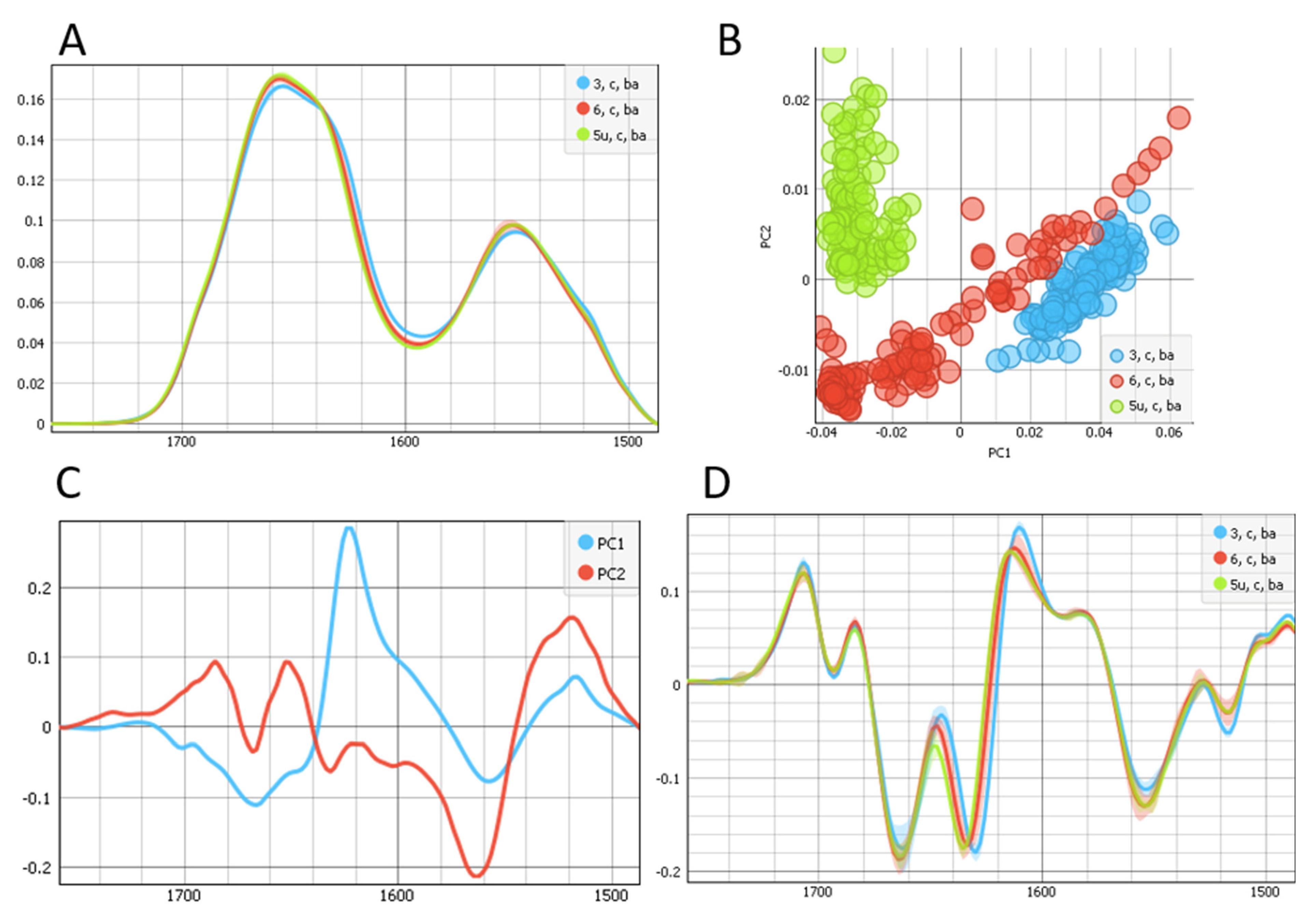
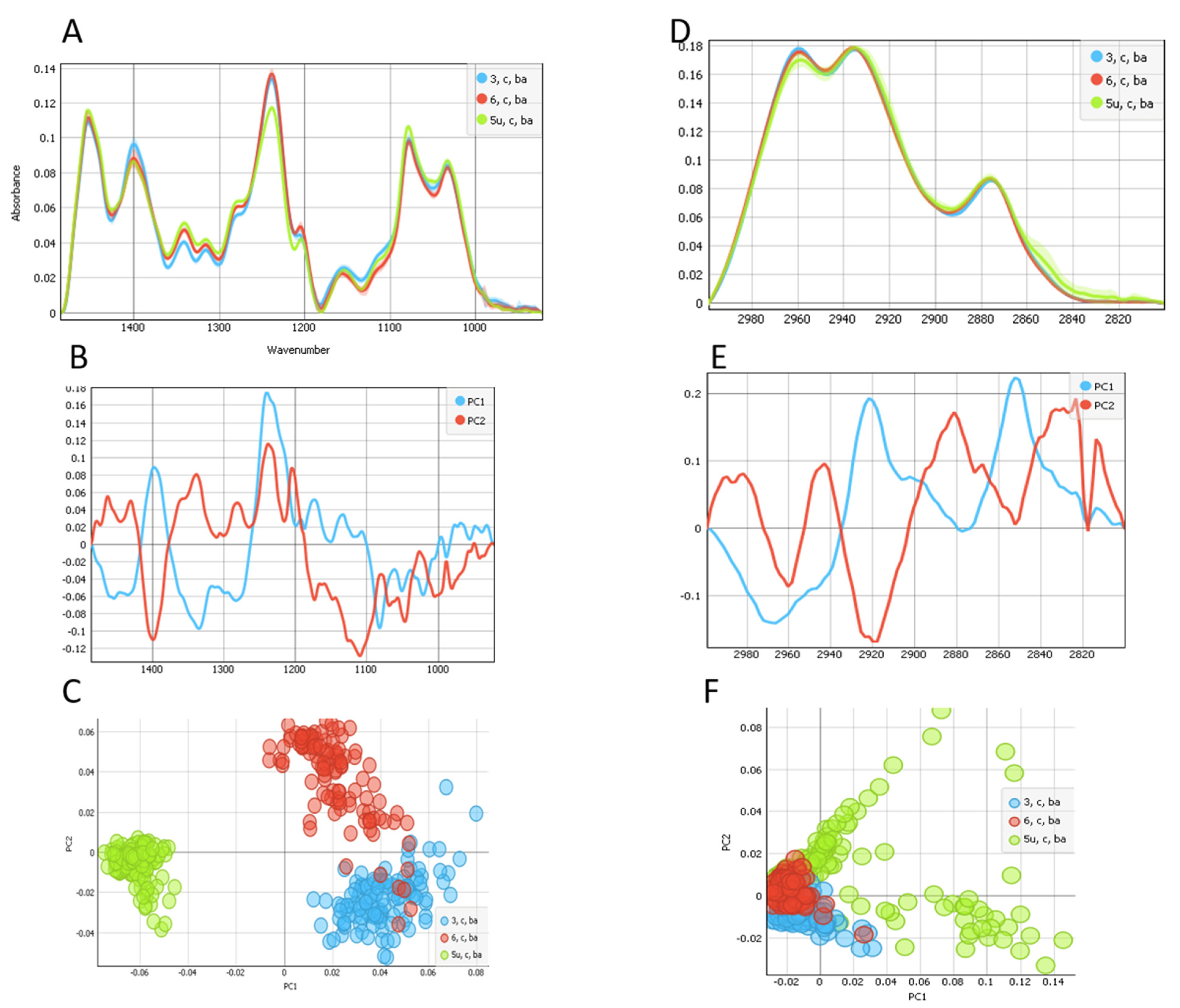
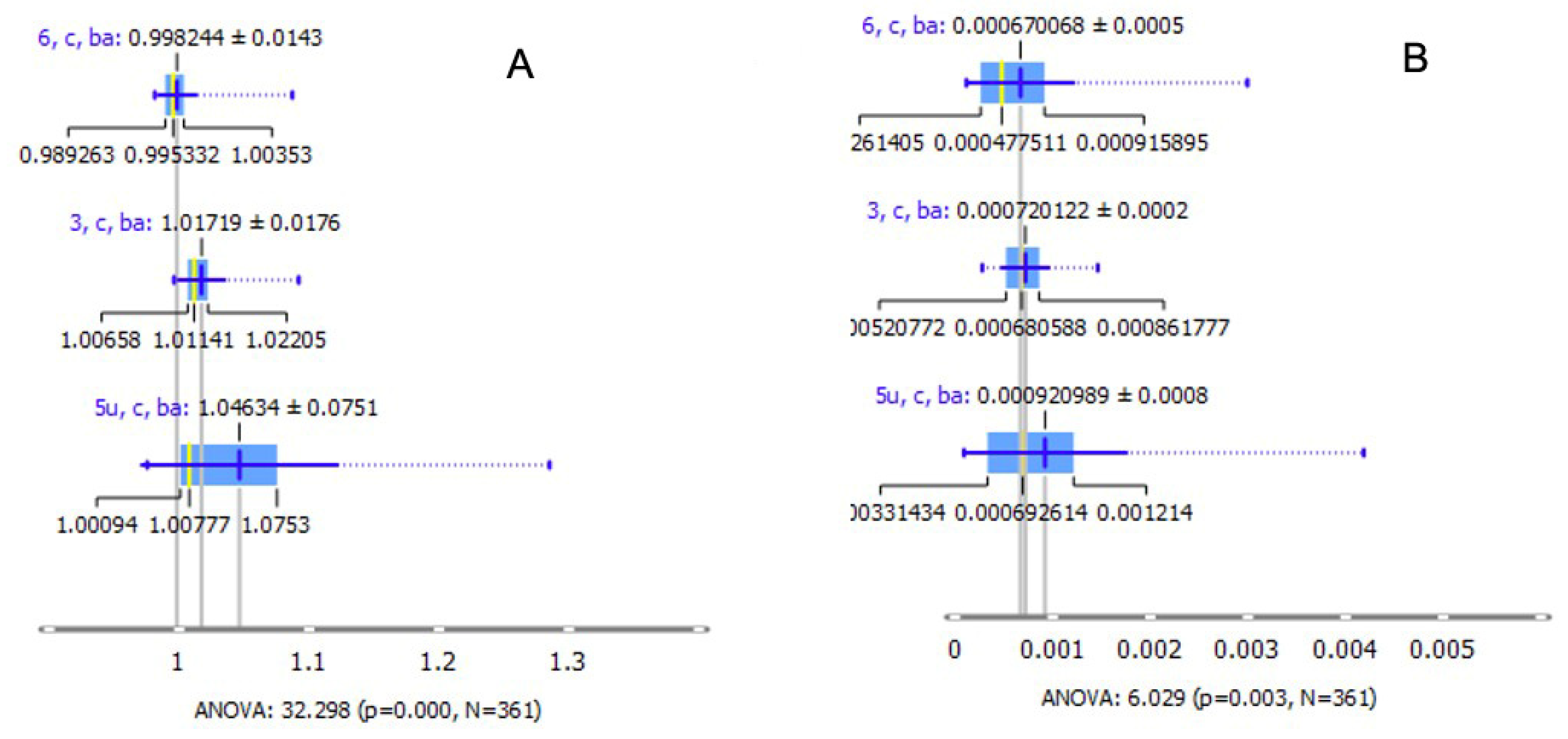
2. Results
3. Discussion
4. Materials and Methods
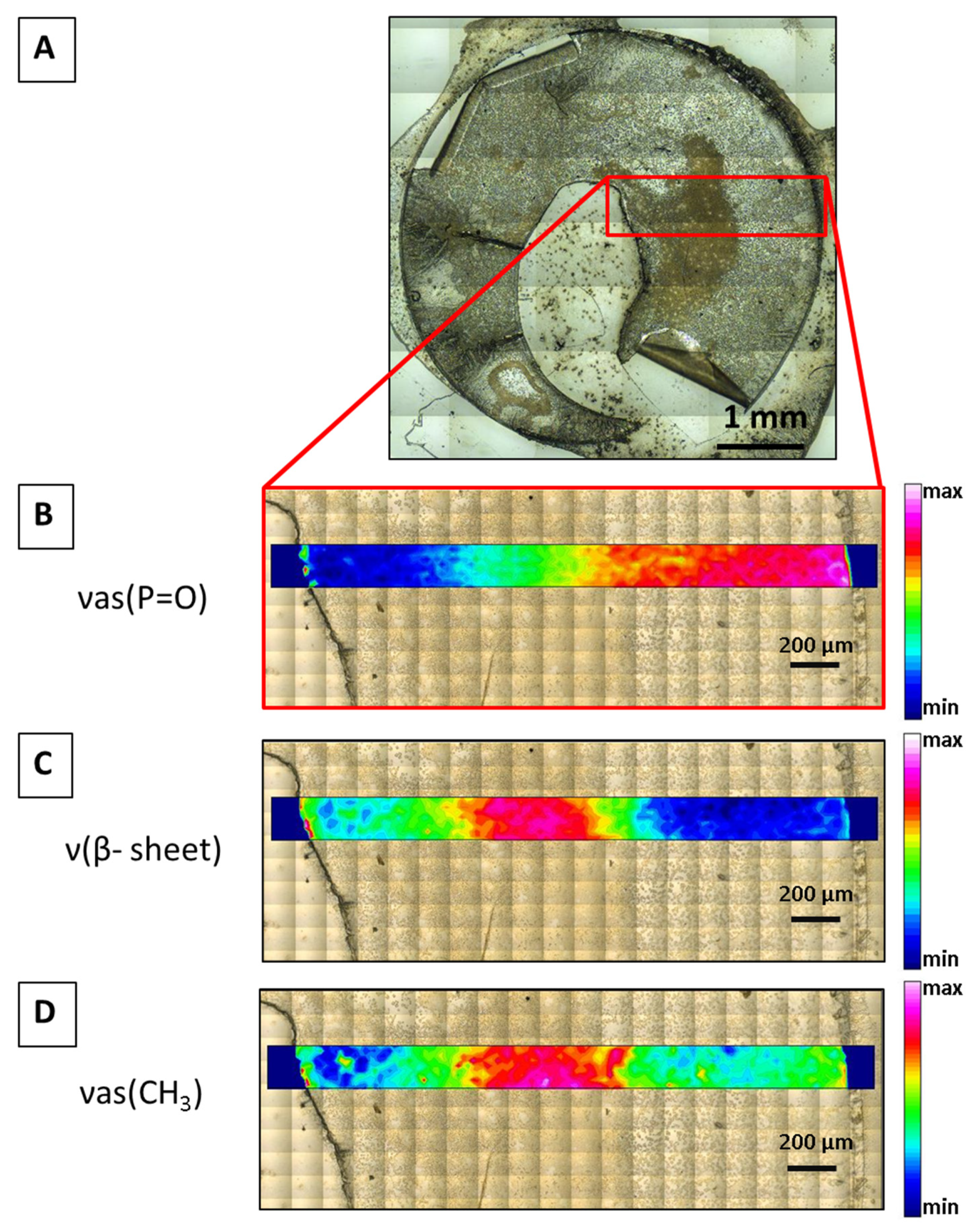
4.1. Synchrotron Radiation-Based FTIR Micro-Spectroscopy
4.2. FTIR Micro-Spectroscopy Imaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | ultraviolet |
| LC | lens capsules |
| LEC | lens epithelial cells |
| SR-FTIR | synchrotron radiation-based Fourier transform infrared |
| PCA | principal component analysis |
References
- Roberts, J.E. Ultraviolet Radiation as a Risk Factor for Cataract and Macular Degeneration. Eye Contact Lens 2011, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Kuszak, J.R.; Dunn, K.; Wang, R.R.; Ma, W.; Wang, G.M.; Spector, A.; Leib, M.; Cotliar, A.M.; Weiss, M.; et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J. Cell Biol. 1995, 130, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Kamari, F.; Hallaj, S.; Dorosti, F.; Alinezhad, F.; Taleschian-Tabrizi, N.; Farhadi, F.; Aslani, H. Phototoxicity of environmental radiations in human lens: Revisiting the pathogenesis of UV-induced cataract. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2065–2077. [Google Scholar] [CrossRef]
- Wang, S.S.; Wen, W.S. Examining the influence of ultraviolet C irradiation on recombinant human γD-crystallin. Mol. Vis. 2010, 16, 2777–2790. [Google Scholar]
- Kovécs, G.; Fekete, A.; Berces, A.; Ronto, G. The effect of the short wavelength ultraviolet radiation. An extension of biological dosimetry to the UV-C range. J. Photochem. Photobiol. B 2007, 88, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Tenkate, T.D. Occupational Exposure to Ultraviolet Radiation: A Health Risk Assessment. Rev. Environ. Health 1999, 14, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Uchida, H.; Saito, T. The damaging effect of UV-C irradiation on lens alpha-crystallin. Mol. Vis. 2004, 10, 814–820. [Google Scholar]
- Liao, J.H.; Wu, T.H.; Hsu, F.L.; Huang, Y.S.; Chiang, P.H.; Huang, Z.Y.; Huang, C.H.; Wu, S.H.; Lin, M.H. Anti-UVC Irradiation and Metal Chelation Properties of 6-Benzoyl-5,7-dihydroxy-4-phenyl-chromen-2-one: An Implications for Anti-Cataract Agent. Int. J. Mol. Sci. 2011, 12, 7059–7076. [Google Scholar] [CrossRef]
- Kim, B.; Kim, S.Y.; Chung, S.K. Changes in apoptosis factors in lens epithelial cells of cataract patients with diabetes mellitus. J. Cataract. Refract. Surg. 2012, 38, 1376–1381. [Google Scholar] [CrossRef]
- Hightower, K.R.; Reddan, J.R.; McCready, J.P.; Dziedzic, D.C. Lens Epithelium: A Primary Target of UVB Irradiation. Exp. Eye Res. 1994, 59, 557–564. [Google Scholar] [CrossRef]
- Hightower, K.R.; Mccready, J.P.; Borchman, D. Membrane damage in UV-irradiated lenses. Photochem. Photobiol. 1994. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, J.; Wu, D.; Zhang, J. Ultraviolet A exposure induces reversible disruption of gap junction intercellular communication in lens epithelial cells. Int. J. Mol. Med. 2011, 28, 239–245. [Google Scholar] [CrossRef][Green Version]
- Hua, H.; Yang, T.; Huang, L.; Chen, R.; Li, M.; Zou, Z.; Wang, N.; Yang, D.; Liu, Y. Protective Effects of Lanosterol Synthase Up-Regulation in UV-B-Induced Oxidative Stress. Front. Pharmacol. 2019, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Cai, L.; Zheng, T.; Ye, H.; Rong, X.; Rao, J.; Lu, Y. The mechanism of UVB irradiation induced-apoptosis in cataract. Mol. Cell. Biochem. 2015, 401, 87–95. [Google Scholar] [CrossRef]
- Kim, S.T.; Koh, J.W. Mechanisms of Apoptosis on Human Lens Epithelium after Ultraviolet Light Exposure. Korean J. Ophthalmol. 2011, 25, 196–201. [Google Scholar] [CrossRef]
- Long, A.C.; Colitz, C.M.; Bomser, J.A. Apoptotic and Necrotic Mechanisms of Stress-Induced Human Lens Epithelial Cell Death. Exp. Biol. Med. 2004, 229, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Johar, S.R.K.; Rawal, U.M.; Jain, N.K.; Vasavada, A.R. Sequential Effects of Ultraviolet Radiation on the Histomorphology, Cell Density and Antioxidative Status of the Lens Epithelium—An In Vivo Study. Photochem. Photobiol. 2003, 78, 306. [Google Scholar] [CrossRef]
- Taillandier, E.; Liquier, J. Infrared spectroscopy of DNA. Methods Enzymol. 1992, 211, 307–335. [Google Scholar] [PubMed]
- Surewicz, W.K.; Mantsch, H.H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim. Biophys. Acta Protein Struct. Mol. 1988, 952, 115–130. [Google Scholar] [CrossRef]
- Mantsch, H.H.; McElhaney, R.N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef]
- Kreuzer, M.; Dučić, T.; Hawlina, M.; Andjelic, S. Synchrotron based FTIR microspectroscopy of protein aggregation and lipids peroxidation changes in human cataractous lens epithelial cells. Sci. Rep. 2020, 10, 15489. [Google Scholar] [CrossRef]
- Malek, K.; Wood, B.R.; Bambery, K.R. FTIR Imaging of Tissues: Techniques and Methods of Analysis. In Optical Spectroscopy and Computational Methods in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2014; pp. 419–473. [Google Scholar]
- Hejtmancik, J.F.; Riazuddin, S.A.; McGreal, R.; Liu, W.; Cvekl, A.; Shiels, A. Lens Biology and Biochemistry. Prog. Mol. Biol. Transl. Sci. 2015, 134, 169–201. [Google Scholar] [CrossRef] [PubMed]
- Dahm, R.; van Marle, J.; Quinlan, R.A.; Prescott, A.R.; Vrensen, G.F. Homeostasis in the vertebrate lens: Mechanisms of solute exchange. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1265–1277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paluszkiewicz, C.; Piergies, N.; Sozańska, A.; Chaniecki, P.; Rękas, M.; Miszczyk, J.; Gajda, M.; Kwiatek, W. Vibrational microspectroscopy analysis of human lenses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Andjelic, S.; Drašlar, K.; Hvala, A.; Lopic, N.; Strancar, J.; Hawlina, M. Anterior lens epithelial cells attachment to the basal lamina. Acta Ophthalmol. 2016, 94, e183–e188. [Google Scholar] [CrossRef]
- Durchschlag, H.; Fochler, C.; Feser, B.; Hausmann, S.; Seroneit, T.; Swientek, M.; Swoboda, E.; Winklmair, A.; Wlček, C.; Zipper, P. Effects of X- and UV-irradiation on proteins. Radiat. Phys. Chem. 1996, 47, 501–505. [Google Scholar] [CrossRef]
- Zandomeneghi, G.; Krebs, M.R.; McCammon, M.G.; Fändrich, M. FTIR reveals structural differences between native beta-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.O.; Alperstein, A.M.; Zanni, M.T. Amyloid β-Sheet Secondary Structure Identified in UV-Induced Cataracts of Porcine Lenses using 2D IR Spectroscopy. J. Mol. Biol. 2017, 429, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Alperstein, A.M.; Ostrander, J.S.; Zhang, T.O.; Zanni, M.T. Amyloid found in human cataracts with two-dimensional infrared spectroscopy. Proc. Natl. Acad. Sci. USA 2019, 116, 6602–6607. [Google Scholar] [CrossRef]
- Andley, U.P. Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 2007, 26, 78–98. [Google Scholar] [CrossRef]
- Kumar, P.A.; Reddy, G.B. Modulation of alpha-crystallin chaperone activity: A target to prevent or delay cataract? IUBMB Life 2009, 61, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-related nuclear cataract—Oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Taylor, A. Nutritional antioxidants and age-related cataract and maculopathy. Exp. Eye Res. 2007, 84, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: Disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract. Cell Biochem. Funct. 2011, 29, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Bantseev, V.; Youn, H.Y. Mitochondrial “Movement” and Lens Optics following Oxidative Stress from UV-B Irradiation. Ann. N. Y. Acad. Sci. 2006, 1091, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.D.; Kovtun, S.; Hegde, K.R. Role of ultraviolet irradiation and oxidative stress in cataract formation-medical prevention by nutritional antioxidants and metabolic agonists. Eye Contact Lens 2011, 37, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the Aging Eye. Am. J. Ther. 2016, 23, 98–117. [Google Scholar] [CrossRef]
- Huang, L.; Estrada, R.; Yappert, M.C.; Borchman, D. Oxidation-induced changes in human lens epithelial cells. Free. Radic. Biol. Med. 2006, 41, 1425–1432. [Google Scholar] [CrossRef]
- Borchman, D.; Ozaki, Y.; Lamba, O.P.; Byrdwell, W.C.; Czarnecki, M.A.; Yappert, M.C. Structural characterization of clear human lens lipid membranes by near-infrared Fourier transform Raman spectroscopy. Curr. Eye Res. 1995, 14, 511. [Google Scholar] [CrossRef]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhang, G.; Kang, L.; Qin, B.; Guan, H. Altered DNA Methylation and Expression Profiles of 8-Oxoguanine DNA Glycosylase 1 in Lens Tissue from Age-related Cataract Patients. Curr. Eye Res. 2015, 40, 815–821. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Zhang, G.; Zhou, J.; Yang, L.; Guan, H. Expression and methylation of DNA repair genes in lens epithelium cells of age-related cataract. Mutat. Res. 2014, 766–767, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Zhang, G.; Kang, L.; Guan, H. Ultraviolet-B induces ERCC6 repression in lens epithelium cells of age-related nuclear cataract through coordinated DNA hypermethylation and histone deacetylation. Clin. Epigenetics 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.; Bassnett, S. UV-B-Induced DNA Damage and Repair in the Mouse Lens. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6789–6797. [Google Scholar] [CrossRef] [PubMed]
- Andley, U.P.; Becker, B.; Hebert, J.S.; Reddan, J.R.; Morrison, A.R.; Pentland, A.P. Enhanced prostaglandin synthesis after ultraviolet-B exposure modulates DNA synthesis of lens epithelial cells and lowers intraocular pressure in vivo. Investig. Ophthalmol. Vis. Sci. 1996, 37, 142–153. [Google Scholar]
- Babizhayev, M.A.; Vishnyakova, K.S.; Yegorov, Y.E. Telomere-dependent senescent phenotype of lens epithelial cells as a biological marker of aging and cataractogenesis: The role of oxidative stress intensity and specific mechanism of phospholipid hydroperoxide toxicity in lens and aqueous. Fundam. Clin. Pharmacol. 2011, 25, 139–162. [Google Scholar] [CrossRef]
- Williamson, L.; Saponaro, M.; Boeing, S.; East, P.; Mitter, R.; Kantidakis, T.; Kelly, G.P.; Lobley, A.; Walker, J.; Spencer-Dene, B.; et al. UV Irradiation Induces a Non-coding RNA that Functionally Opposes the Protein Encoded by the Same Gene. Cell 2017, 168, 843–855. [Google Scholar] [CrossRef]
- Zhang, H.; Rosdahl, I. Ultraviolet A and B differently induce intracellular protein expression in human skin melanocytes—A speculation of separate pathways in initiation of melanoma. Carcinogenesis 2003, 24, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Yousef, I.; Ribó, L.; Crisol, A.; Šics, I.; Ellis, G.; Ducic, T.; Kreuzer, M.; Benseny-Cases, N.; Quispe, M.; Dumas, P.; et al. MIRAS: The Infrared Synchrotron Radiation Beamline at ALBA. Synchrotron Radiat. News 2017, 30, 4–6. [Google Scholar] [CrossRef]
- Miller, L.M.; Dumas, P. Chemical imaging of biological tissue with synchrotron infrared light. Biochim. Biophys. Acta Biomembr. 2006, 1758, 846–857. [Google Scholar] [CrossRef]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Toplak, M.; Birarda, G.; Read, S.; Sandt, C.; Rosendahl, S.M.; Vaccari, L.; Demšar, J.; Borondics, F. Infrared Orange: Connecting Hyperspectral Data with Machine Learning. Synchrotron Radiat. News 2017, 30, 40–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumi, X.; Dučić, T.; Kreuzer, M.; Hawlina, M.; Andjelic, S. UV Effect on Human Anterior Lens Capsule Macro-Molecular Composition Studied by Synchrotron-Based FTIR Micro-Spectroscopy. Int. J. Mol. Sci. 2021, 22, 5249. https://doi.org/10.3390/ijms22105249
Lumi X, Dučić T, Kreuzer M, Hawlina M, Andjelic S. UV Effect on Human Anterior Lens Capsule Macro-Molecular Composition Studied by Synchrotron-Based FTIR Micro-Spectroscopy. International Journal of Molecular Sciences. 2021; 22(10):5249. https://doi.org/10.3390/ijms22105249
Chicago/Turabian StyleLumi, Xhevat, Tanja Dučić, Martin Kreuzer, Marko Hawlina, and Sofija Andjelic. 2021. "UV Effect on Human Anterior Lens Capsule Macro-Molecular Composition Studied by Synchrotron-Based FTIR Micro-Spectroscopy" International Journal of Molecular Sciences 22, no. 10: 5249. https://doi.org/10.3390/ijms22105249
APA StyleLumi, X., Dučić, T., Kreuzer, M., Hawlina, M., & Andjelic, S. (2021). UV Effect on Human Anterior Lens Capsule Macro-Molecular Composition Studied by Synchrotron-Based FTIR Micro-Spectroscopy. International Journal of Molecular Sciences, 22(10), 5249. https://doi.org/10.3390/ijms22105249








