SWATH Based Quantitative Proteomics Reveals Significant Lipid Metabolism in Early Myopic Guinea Pig Retina
Abstract
1. Introduction
2. Results
2.1. Ocular Parameters Changes
2.2. Generation of the Ion Library Using a Pooled Retinal Proteome
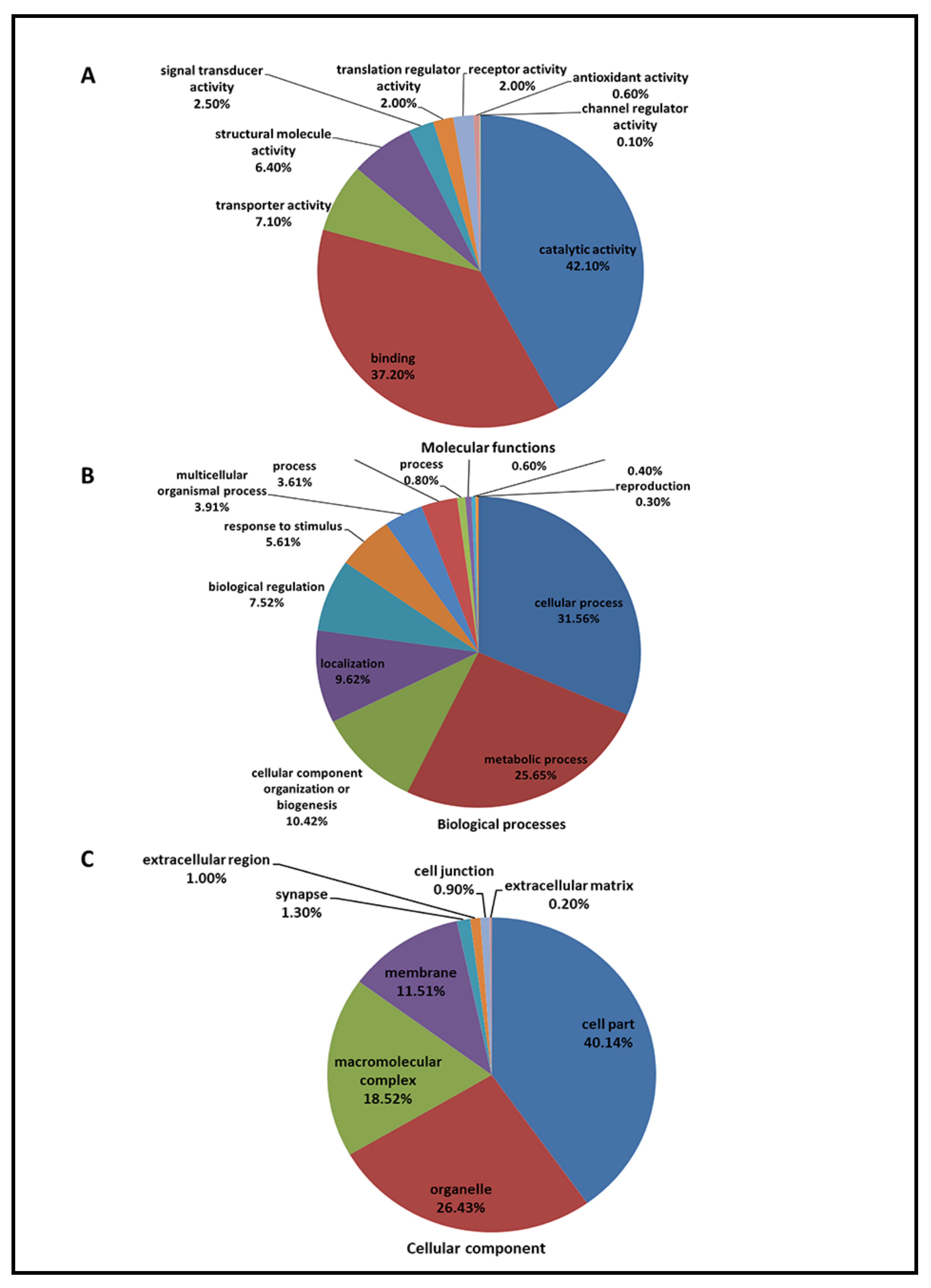
2.3. Gene Ontology Analysis of a Pooled Retinal Proteome
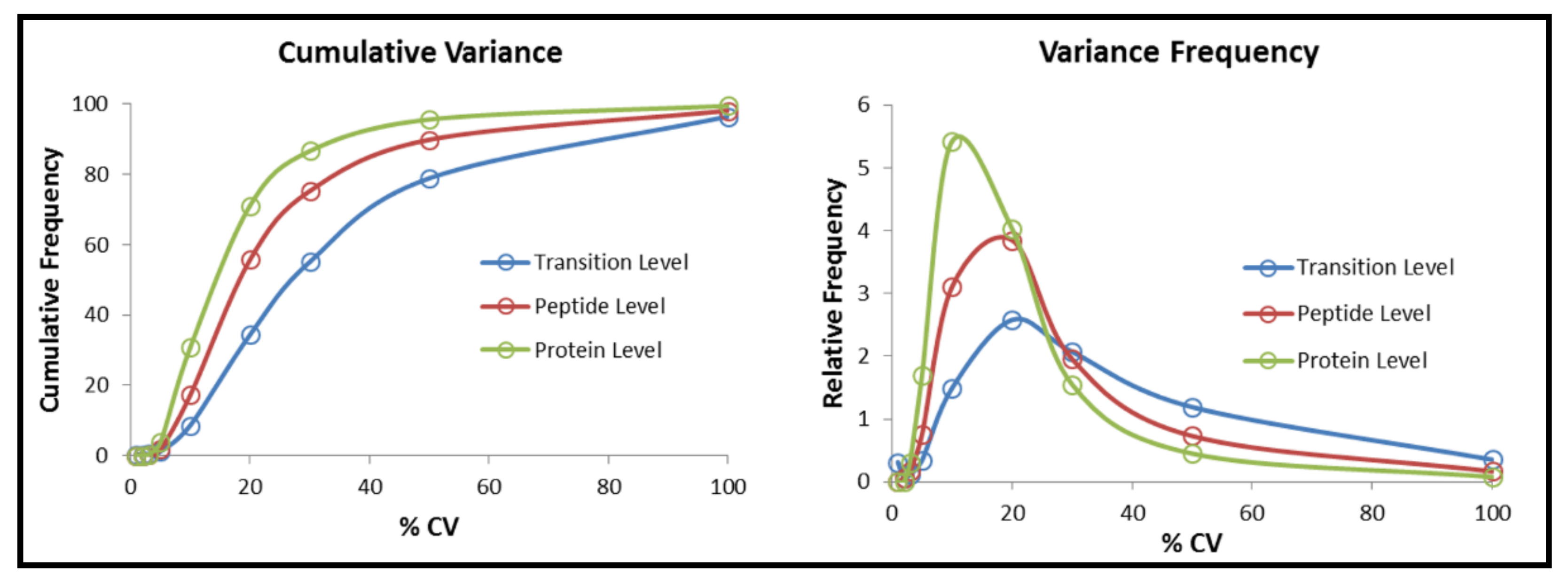
2.4. Data Quality Check of All SWATH Dataset
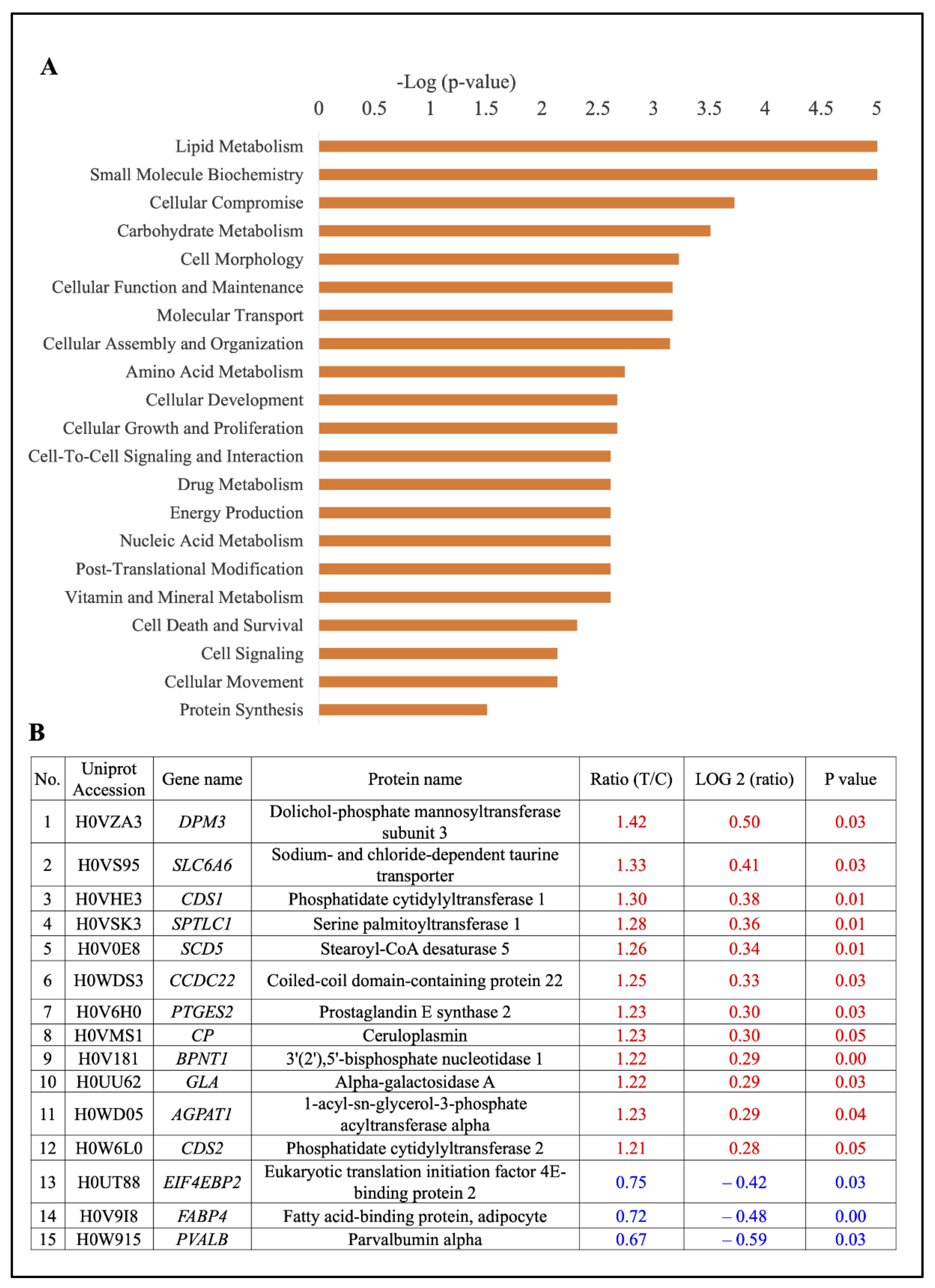
2.5. Pathways Analysis of All Significantly Expressed Proteins
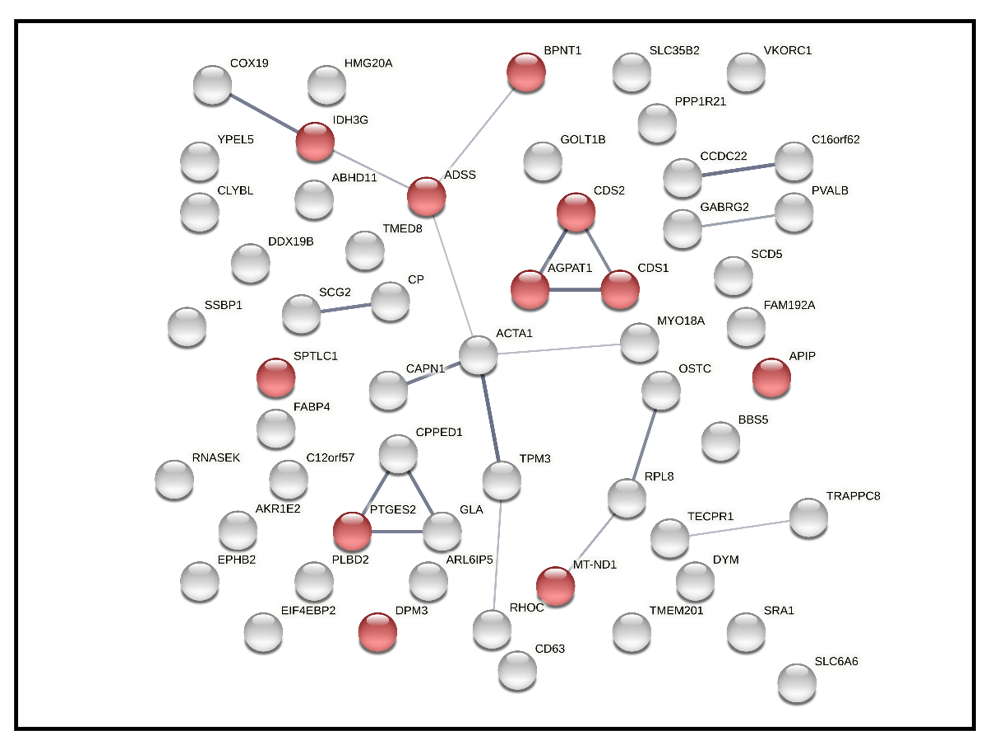
2.6. Quantitative Analysis of Differentially Expressed Proteins
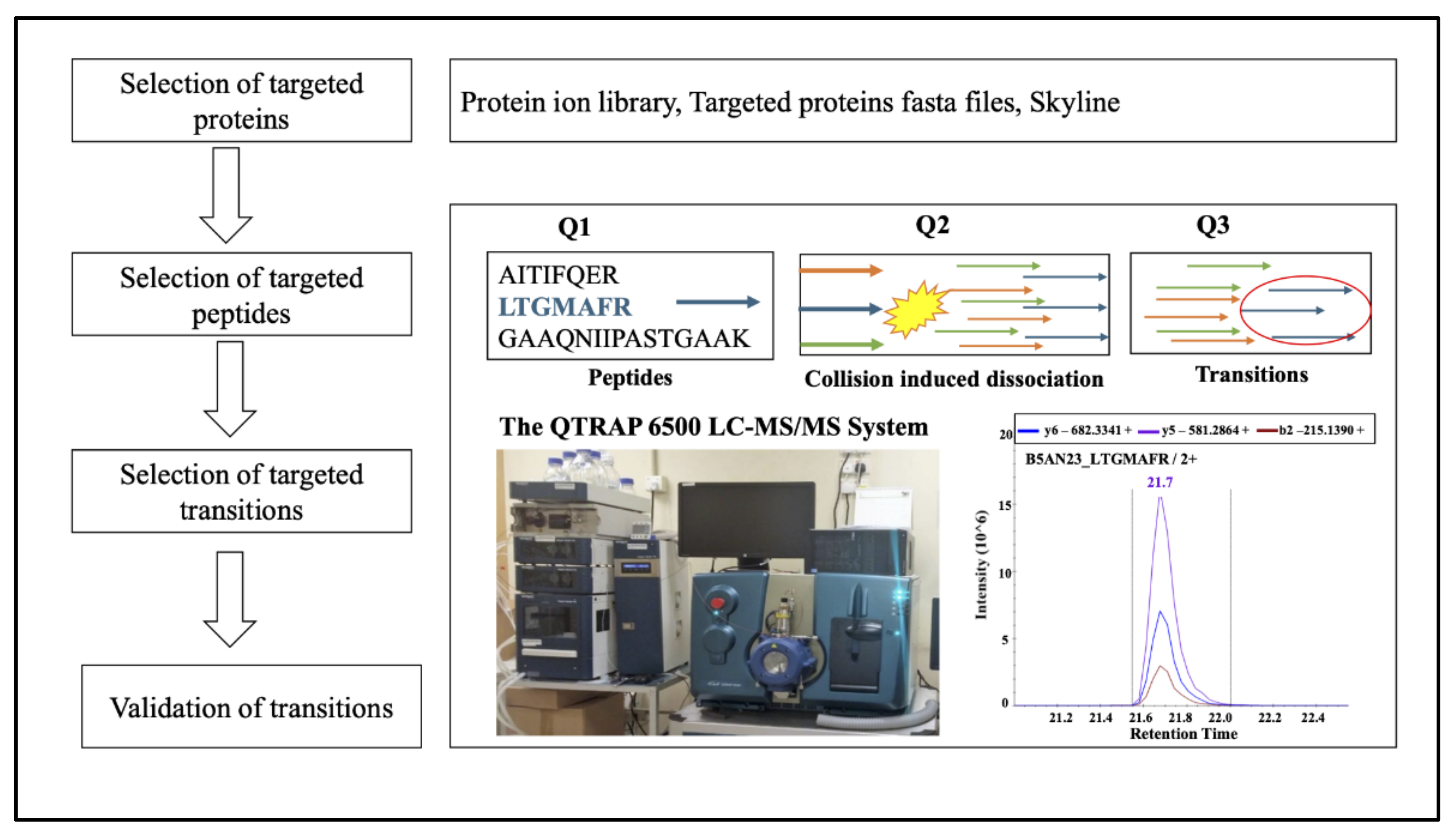
2.7. Validation of Index Proteins in Lipid Metabolism Using MRM Based Target Proteomic Approach
3. Discussion
3.1. Changes in Refractive Error and Ocular Dimension
3.2. Protein Ion Library
3.3. Significant Pathways
3.4. Index Proteins Invovled in Lipid Metabolism
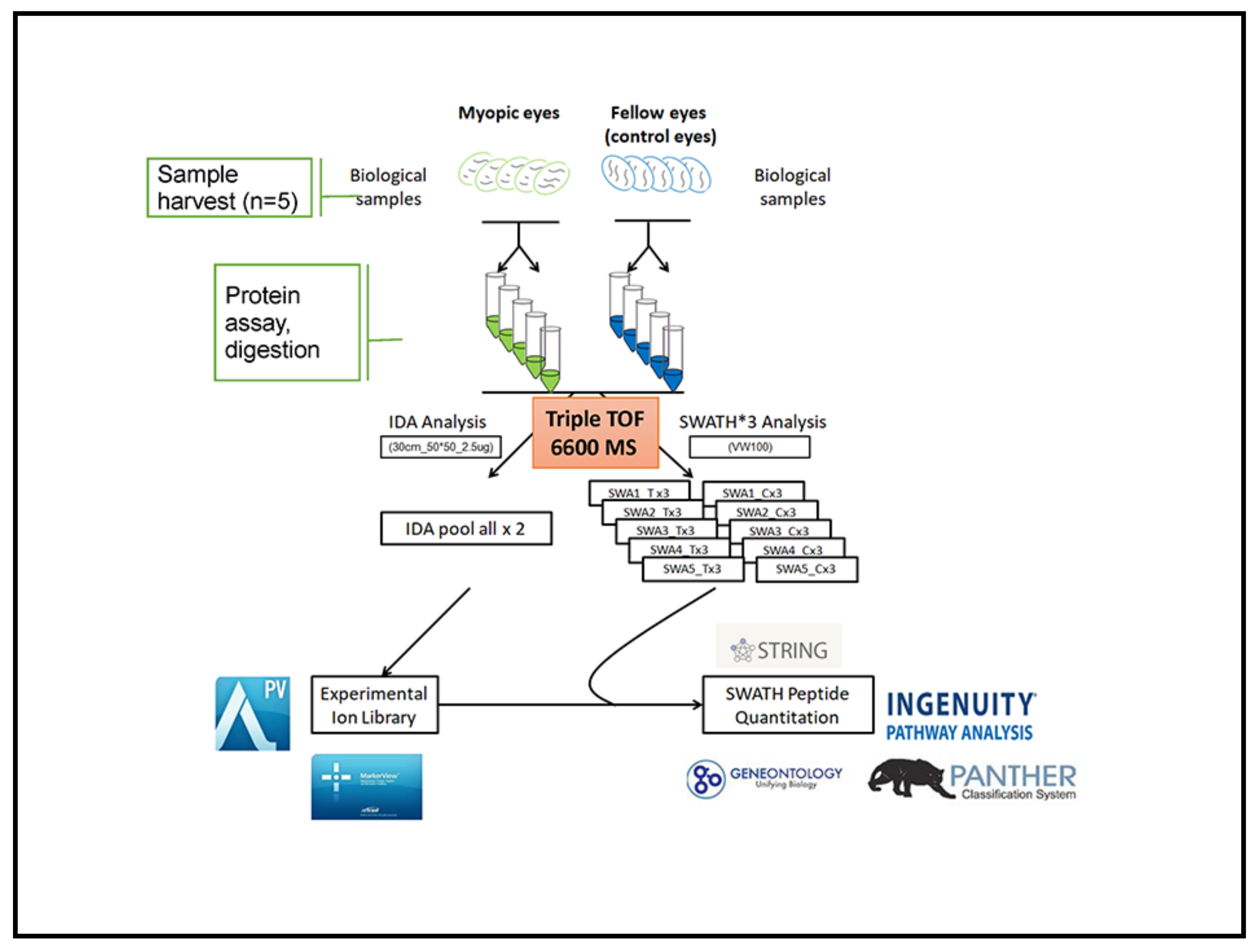
4. Materials and Methods
4.1. Animals
4.2. Refractive Error and Ocular Components Measurements
4.3. Retinal Harvest and Protein Extraction
4.4. Individual Retinal Samples Digestion for LC-MS/MS
4.5. Parameters Setting of IDA and SWATH-MS Experiments
4.6. Protein Ion Library Generation
4.7. SWATH Processing and Quantitative Analysis
4.8. Bioinformatics Analysis
4.9. Protein Validation Using MRM Based Proteomic Approach
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Dolgin, E. The myopia boom. Nature 2015, 519, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Curtin, B.J. The Myopias: Basic Science and Clinical Management; Harper and Row: Philadelphia, PA, USA, 1985. [Google Scholar]
- Zhou, Q.; Friedman, D.S.; Lu, H.; Duan, X.; Liang, Y.; Yang, X.; Wang, F.; Wang, N. The epidemiology of age-related eye diseases in Mainland China. Ophthalmic Epidemiol. 2007, 14, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Loon, S.C.; Saw, S.M. The epidemiology of age related eye diseases in Asia. Br. J. Ophthalmol. 2006, 90, 506–511. [Google Scholar] [CrossRef]
- Leske, M.C.; Chylack, L.T.; Wu, S.Y. The Lens Opacities Case-Control Study—Risk-Factors for Cataract. Arch. Ophthalmol. 1991, 109, 244–251. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, D.N.; Sun, J.; Zhou, J.B. Correlations Between MMPs and TIMPs Levels in Aqueous Humor from High Myopia and Cataract Patients. Curr. Eye Res. 2017, 42, 600–603. [Google Scholar] [CrossRef]
- Chen, S.J.; Lu, P.; Zhang, W.F.; Lu, J.H. High myopia as a risk factor in primary open angle glaucoma. Int. J. Ophthalmol. 2012, 5, 750–753. [Google Scholar]
- Chihara, E.; Liu, X.; Dong, J.; Takashima, Y.; Akimoto, M.; Hangai, M.; Kuriyama, S.; Tanihara, H.; Hosoda, M.; Tsukahara, S. Severe myopia as a risk factor for progressive visual field loss in primary open-angle glaucoma. Ophthalmologica 1997, 211, 66–71. [Google Scholar] [CrossRef]
- Mahfoudi, N.B.; Harbi, M.C.; Beddiar, F.B.; Chachoua, L. Bilateral retinal detachment and high myopia: Report of nine cases. J. Fr. D Ophtalmol. 2015, 38, 141–145. [Google Scholar]
- Jacobi, F.K.; Hessemer, V. Pseudophakic retinal detachment in high axial myopia. J. Cataract Refract. Surg. 1997, 23, 1095–1102. [Google Scholar] [CrossRef]
- Wallman, J.; Turkel, J.; Trachtman, J. Extreme myopia produced by modest change in early visual experience. Science 1978, 201, 1249–1251. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Tkatchenko, T.V.; Shen, Y.; Tkatchenko, A.V. Mouse experimental myopia has features of primate myopia. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1297–1303. [Google Scholar] [CrossRef]
- Barathi, V.A.; Boopathi, V.G.; Yap, E.P.; Beuerman, R.W. Two models of experimental myopia in the mouse. Vis. Res. 2008, 48, 904–916. [Google Scholar] [CrossRef]
- Frost, M.R.; Norton, T.T. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest. Ophthalmol. Vis. Sci. 2012, 53, 322–336. [Google Scholar] [CrossRef]
- Siegwart, J.T., Jr.; Norton, T.T. The susceptible period for deprivation-induced myopia in tree shrew. Vis. Res. 1998, 38, 3505–3515. [Google Scholar] [CrossRef]
- Sherman, S.M.; Norton, T.T.; Casagrande, V.A. Myopia in the lid-sutured tree shrew (Tupaia glis). Brain Res. 1977, 124, 154–157. [Google Scholar] [CrossRef]
- Howlett, M.H.; McFadden, S.A. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vis. Res. 2007, 47, 1178–1190. [Google Scholar] [CrossRef]
- Howlett, M.H.; McFadden, S.A. Spectacle lens compensation in the pigmented guinea pig. Vis. Res. 2009, 49, 219–227. [Google Scholar] [CrossRef]
- Jiang, L.; Schaeffel, F.; Zhou, X.; Zhang, S.; Jin, X.; Pan, M.; Ye, L.; Wu, X.; Huang, Q.; Lu, F.; et al. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus). Invest. Ophthalmol. Vis. Sci. 2009, 50, 1013–1019. [Google Scholar] [CrossRef]
- Tkatchenko, A.V.; Walsh, P.A.; Tkatchenko, T.V.; Gustincich, S.; Raviola, E. Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proc. Natl. Acad. Sci. USA 2006, 103, 4681–4686. [Google Scholar] [CrossRef]
- Young, F.A. Primate myopia. Am. J. Optom. Physiol. Opt. 1981, 58, 560–566. [Google Scholar] [CrossRef]
- Hung, L.F.; Crawford, M.L.; Smith, E.L. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat. Med. 1995, 1, 761–765. [Google Scholar] [CrossRef]
- Smith, E.L., 3rd; Bradley, D.V.; Fernandes, A.; Boothe, R.G. Form deprivation myopia in adolescent monkeys. Optom. Vis. Sci. 1999, 76, 428–432. [Google Scholar] [CrossRef]
- McFadden, S.A.; Howlett, M.H.; Mertz, J.R. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vis. Res. 2004, 44, 643–653. [Google Scholar] [CrossRef]
- Howlett, M.H.C.; McFadden, S.A. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vis. Res. 2006, 46, 267–283. [Google Scholar] [CrossRef]
- Loeliger, M.; Rees, S. Immunocytochemical development of the guinea pig retina. Exp. Eye Res. 2005, 80, 9–21. [Google Scholar] [CrossRef]
- Troilo, D.; Smith, E.L.; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.S.; et al. IMI—Report on Experimental Models of Emmetropization and Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef]
- Wildsoet, C.; Wallman, J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis. Res. 1995, 35, 1175–1194. [Google Scholar] [CrossRef]
- Troilo, D.; Gottlieb, M.D.; Wallman, J. Visual deprivation causes myopia in chicks with optic nerve section. Curr. Eye Res. 1987, 6, 993–999. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Z.; He, L.; He, N.; Xi, Z.; Li, Z.; Deng, Y.; Zeng, X. Mass spectrometry-assisted gel-based proteomics in cancer biomarker discovery: Approaches and application. Theranostics 2017, 7, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Carberry, S.; Ohlendieck, K. Gel electrophoresis-based proteomics of senescent tissues. Methods Mol. Biol. 2013, 1048, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.C.; Yates, J.R., 3rd. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [PubMed]
- Wolters, D.A.; Washburn, M.P.; Yates, J.R., 3rd. An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 2001, 73, 5683–5690. [Google Scholar] [CrossRef]
- Yates, J.R., 3rd. Mass spectral analysis in proteomics. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 297–316. [Google Scholar] [CrossRef]
- Abdallah, C.; Dumas-Gaudot, E.; Renaut, J.; Sergeant, K. Gel-based and gel-free quantitative proteomics approaches at a glance. Int. J. Plant Genom. 2012, 2012, 494572. [Google Scholar] [CrossRef]
- Lam, T.C.; Li, K.K.; Lo, S.C.; Guggenheim, J.A.; To, C.H. A chick retinal proteome database and differential retinal protein expressions during early ocular development. J. Proteome Res. 2006, 5, 771–784. [Google Scholar] [CrossRef]
- Lam, T.C.; Li, K.K.; Lo, S.C.; Guggenheim, J.A.; To, C.H. Application of fluorescence difference gel electrophoresis technology in searching for protein biomarkers in chick myopia. J. Proteome Res. 2007, 6, 4135–4149. [Google Scholar] [CrossRef]
- Wu, Y.; Lam, C.S.; Tse, D.Y.; To, C.H.; Liu, Q.; McFadden, S.A.; Chun, R.K.; Li, K.K.; Bian, J.; Lam, C. Early quantitative profiling of differential retinal protein expression in lens-induced myopia in guinea pig using fluorescence difference two-dimensional gel electrophoresis. Mol. Med. Rep. 2018, 17, 5571–5580. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; To, C.H.; Li, K.K.; Chun, R.K.M.; Yu, J.F.J.; Lam, T.C. Differential Retinal Protein Expressions During form Deprivation Myopia in Albino Guinea Pigs. Curr. Proteom. 2014, 11, 37–47. [Google Scholar] [CrossRef]
- Collins, B.C.; Hunter, C.L.; Liu, Y.; Schilling, B.; Rosenberger, G.; Bader, S.L.; Chan, D.W.; Gibson, B.W.; Gingras, A.C.; Held, J.M.; et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 2017, 8, 291. [Google Scholar] [CrossRef]
- Selevsek, N.; Chang, C.Y.; Gillet, L.C.; Navarro, P.; Bernhardt, O.M.; Reiter, L.; Cheng, L.Y.; Vitek, O.; Aebersold, R. Reproducible and consistent quantification of the Saccharomyces cerevisiae proteome by SWATH-mass spectrometry. Mol. Cell. Proteom. 2015, 14, 739–749. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Rost, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Shan, S.W.; Tse, D.Y.-y.; Zuo, B.; To, C.H.; Liu, Q.; McFadden, S.A.; Chun, R.K.-M.; Bian, J.; Li, K.K.; Lam, T.C. Integrated SWATH-based and targeted-based proteomics provide insights into the retinal emmetropization process in guinea pig. J. Proteom. 2018, 181, 1–15. [Google Scholar] [CrossRef]
- Nättinen, J.; Aapola, U.; Jylhä, A.; Vaajanen, A.; Uusitalo, H. Comparison of Capillary and Schirmer Strip Tear Fluid Sampling Methods Using SWATH-MS Proteomics Approach. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef]
- Shan, S.W.; Do, C.W.; Lam, T.C.; Kong, R.P.W.; Li, K.K.; Chun, K.M.; Stamer, W.D.; To, C.H. New Insight of Common Regulatory Pathways in Human Trabecular Meshwork Cells in Response to Dexamethasone and Prednisolone Using an Integrated Quantitative Proteomics: SWATH and MRM-HR Mass Spectrometry. J. Proteome Res. 2017, 16, 3753–3765. [Google Scholar] [CrossRef]
- Vahatupa, M.; Nattinen, J.; Jylha, A.; Aapola, U.; Kataja, M.; Koobi, P.; Jarvinen, T.A.H.; Uusitalo, H.; Uusitalo-Jarvinen, H. SWATH-MS Proteomic Analysis of Oxygen-Induced Retinopathy Reveals Novel Potential Therapeutic Targets. Invest. Ophthalmol. Vis. Sci. 2018, 59, 3294–3306. [Google Scholar] [CrossRef]
- Picotti, P.; Aebersold, R. Selected reaction monitoring-based proteomics: Workflows, potential, pitfalls and future directions. Nat. Methods 2012, 9, 555–566. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Yao, M.; Ma, L.; Humphreys, W.G.; Zhu, M.S. Rapid screening and characterization of drug metabolites using a multiple ion monitoring-dependent MS/MS acquisition method on a hybrid triple quadrupole-linear ion trap mass spectrometer. J. Mass Spectrom. 2008, 43, 1364–1375. [Google Scholar] [CrossRef]
- Kitteringham, N.R.; Jenkins, R.E.; Lane, C.S.; Elliott, V.L.; Park, B.K. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J. Chromatogr. 2009, 877, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Wright, B. A sensitive and cost-effective high-performance liquid chromatography/tandem mass spectrometry (multiple reaction monitoring) method for the clinical measurement of serum hepcidin. Rapid Commun. Mass Spectrom. 2020, 34 (Suppl. 1), e8644. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, H.W.; Wu, C.F.; Chu, L.J.; Chiang, W.F.; Wu, C.C.; Yu, J.S.; Tsai, C.H.; Liang, K.H.; Chang, Y.S.; et al. Development of a Multiplexed Liquid Chromatography Multiple-Reaction-Monitoring Mass Spectrometry (LC-MRM/MS) Method for Evaluation of Salivary Proteins as Oral Cancer Biomarkers. Mol. Cell. Proteom. 2017, 16, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Govaert, E.; Van Steendam, K.; Willems, S.; Vossaert, L.; Dhaenens, M.; Deforce, D. Comparison of fractionation proteomics for local SWATH library building. Proteomics 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Schubert, K.O.; Focking, M.; Wynne, K.; Cotter, D.R. Proteome and pathway effects of chronic haloperidol treatment in mouse hippocampus. Proteomics 2016, 16, 532–538. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Zhou, J.; Zhou, M.; Prieto, D.; Rotimi, C.N.; Adeyemo, A. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: A proteomics study. Obesity 2016, 24, 1257–1265. [Google Scholar] [CrossRef]
- Tkatchenko, T.V.; Shah, R.L.; Nagasaki, T.; Tkatchenko, A.V. Analysis of genetic networks regulating refractive eye development in collaborative cross progenitor strain mice reveals new genes and pathways underlying human myopia. BMC Med Genom. 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Tkatchenko, T.V.; Troilo, D.; Benavente-Perez, A.; Tkatchenko, A.V. Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanism of visually guided eye growth. PLoS Biol. 2018, 16, e2006021. [Google Scholar] [CrossRef]
- Giummarra, L.; Crewther, S.G.; Riddell, N.; Murphy, M.J.; Crewther, D.P. Pathway analysis identifies altered mitochondrial metabolism, neurotransmission, structural pathways and complement cascade in retina/RPE/choroid in chick model of form-deprivation myopia. Peerj 2018, 6. [Google Scholar] [CrossRef]
- Riddell, N.; Crewther, S.G. Novel evidence for complement system activation in chick myopia and hyperopia models: A meta-analysis of transcriptome datasets. Sci. Rep. 2017, 7, 9719. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, J.; Nimri, N.; Wildsoet, C.F. Microarray Analysis of RPE Gene Expression in Chicks During Long-term Imposed Myopic Defocus. Investig. Ophthalmol. Vis. Sci. 2010, 51. [Google Scholar]
- Rada, J.A.; Wiechmann, A.F. Melatonin receptors in chick ocular tissues: Implications for a role of melatonin in ocular growth regulation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 25–33. [Google Scholar] [CrossRef]
- Srinivasalu, N.; McFadden, S.A.; Medcalf, C.; Fuchs, L.; Chung, J.; Philip, G.; Richardson, A.; Riaz, M.; Baird, P.N. Gene Expression and Pathways Underlying Form Deprivation Myopia in the Guinea Pig Sclera. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1425–1434. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W.; Zhao, F.; Zhou, Q.Y.; Reinach, P.S.; Deng, L.L.; Ma, L.; Luo, S.M.; Srinivasalu, N.; Pan, M.Z.; et al. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci. USA 2018, 115, E7091–E7100. [Google Scholar] [CrossRef]
- Zelinka, C.P.; Volkov, L.; Goodman, Z.A.; Todd, L.; Palazzo, I.; Bishop, W.A.; Fischer, A.J. mTor signaling is required for the formation of proliferating Muller glia-derived progenitor cells in the chick retina. Development 2016, 143, 1859–1873. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Gu, J.; Guo, M.; Xia, H.; Liu, Y. UPR Activation and the Down-Regulation of α-Crystallin in Human High Myopia-Related Cataract Lens Epithelium. PLoS ONE 2015, 10, e0137582. [Google Scholar] [CrossRef]
- Huang, J.; Qu, X.M.; Chu, R.Y. Expressions of cellular retinoic acid binding proteins I and retinoic acid receptor-beta in the guinea pig eyes with experimental myopia. Int. J. Ophthalmol. 2011, 4, 131–136. [Google Scholar] [CrossRef]
- Huo, L.J.; Cui, D.M.; Yang, X.; Gao, Z.Y.; Trier, K.; Zeng, J.W. All-trans retinoic acid modulates mitogen-activated protein kinase pathway activation in human scleral fibroblasts through retinoic acid receptor beta. Mol. Vis. 2013, 19, 1795–1803. [Google Scholar]
- Li, H.; Cui, D.; Zhao, F.; Huo, L.; Hu, J.; Zeng, J. BMP-2 Is Involved in Scleral Remodeling in Myopia Development. PLoS ONE 2015, 10, e0125219. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Cui, D.; Zeng, J. Retinal and choroidal expression of BMP-2 in lens-induced myopia and recovery from myopia in guinea pigs. Mol. Med. Rep. 2016, 13, 2671–2676. [Google Scholar] [CrossRef]
- Feldkaemper, M.; Schaeffel, F. An updated view on the role of dopamine in myopia. Exp. Eye Res. 2013, 114, 106–119. [Google Scholar] [CrossRef]
- Chen, S.; Zhi, Z.N.; Ruan, Q.Q.; Liu, Q.X.; Li, F.; Wan, F.; Reinach, P.S.; Chen, J.F.; Qu, J.; Zhou, X.T. Bright Light Suppresses Form-Deprivation Myopia Development With Activation of Dopamine D1 Receptor Signaling in the ON Pathway in Retina. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2306–2316. [Google Scholar] [CrossRef]
- Zhou, X.T.; Pardue, M.T.; Iuvone, P.M.; Qu, J. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- He, L.; Frost, M.R.; Siegwart, J.T., Jr.; Norton, T.T. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp. Eye Res. 2014, 123, 56–71. [Google Scholar] [CrossRef][Green Version]
- Penha, A.M.; Schaeffel, F.; Feldkaemper, M. Insulin, insulin-like growth factor-1, insulin receptor, and insulin-like growth factor-1 receptor expression in the chick eye and their regulation with imposed myopic or hyperopic defocus. Mol. Vis. 2011, 17, 1436–1448. [Google Scholar]
- Ritchey, E.R.; Zelinka, C.P.; Tang, J.H.; Liu, J.; Fischer, A.J. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp. Eye. Res. 2012, 99, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.D.; Cheng, Y.X.; Liu, G.B.; Guo, S.F.; Fan, C.L.; Zhan, L.H.; Xu, Y.C. Expressions of type I collagen, alpha 2 integrin and beta 1 integrin in sclera of guinea pig with defocus myopia and inhibitory effects of bFGF on the formation of myopia. Int. J. Ophthalmol. 2013, 6, 54–58. [Google Scholar] [CrossRef]
- Tao, Y.J.; Pan, M.Z.; Liu, S.F.; Fang, F.; Lu, R.X.; Lu, C.Y.; Zheng, M.; An, J.H.; Xu, H.J.; Zhao, F.X.; et al. cAMP Level Modulates Scleral Collagen Remodeling, a Critical Step in the Development of Myopia. PLoS ONE 2013, 8, e71441. [Google Scholar] [CrossRef]
- Chun, R.K.; Shan, S.W.; Lam, T.C.; Wong, C.L.; Li, K.K.; Do, C.W.; To, C.H. Cyclic Adenosine Monophosphate Activates Retinal Apolipoprotein A1 Expression and Inhibits Myopic Eye Growth. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8151–8157. [Google Scholar] [CrossRef] [PubMed]
- McFadden, S.A.; Tse, D.Y.; Bowrey, H.E.; Leotta, A.J.; Lam, C.S.; Wildsoet, C.F.; To, C.H. Integration of defocus by dual power Fresnel lenses inhibits myopia in the mammalian eye. Invest. Ophthalmol. Vis. Sci. 2014, 55, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.M.; Huynh, S.C.; Kifley, A.; Rose, K.A.; Morgan, I.G.; Varma, R.; Mitchell, P. Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4846–4853. [Google Scholar] [CrossRef] [PubMed]
- Badmus, S.A.; Ajaiyeoba, A.I.; Adegbehingbe, B.O.; Onakpoya, O.H.; Adeoye, A.O. Axial length/corneal radius of curvature ratio and refractive status in an adult Nigerian population. Niger. J. Clin. Pract. 2017, 20, 1328–1334. [Google Scholar] [CrossRef]
- Shan, S.W.; Tse, D.Y.-Y.; Zuo, B.; To, C.h.; Liu, Q.; McFadden, S.A.; Chun, R.K.-M.; Bian, J.; Li, K.K.; Lam, T.C. Data on differentially expressed proteins in retinal emmetropization process in guinea pig using integrated SWATH-based and targeted-based proteomics. Data Brief 2018, 21, 1750–1755. [Google Scholar] [CrossRef]
- Velez, G.; Machlab, D.A.; Tang, P.H.; Sun, Y.; Tsang, S.H.; Bassuk, A.G.; Mahajan, V.B. Proteomic analysis of the human retina reveals region-specific susceptibilities to metabolic- and oxidative stress-related diseases. PLoS ONE 2018, 13, e0193250. [Google Scholar] [CrossRef]
- Yu, F. Proteome Profiling and Relative Quantification of Protein Expressions in the Chick Vitreous and Retina During Compensated Ocular Growth; The Hong Kong Polytechnic University: Hong Kong, China, 2016. [Google Scholar]
- Yu, F.J.; Lam, T.C.; Liu, L.Q.; Chun, R.K.; Cheung, J.K.; Li, K.K.; To, C.H. Isotope-coded protein label based quantitative proteomic analysis reveals significant up-regulation of apolipoprotein A1 and ovotransferrin in the myopic chick vitreous. Sci. Rep. 2017, 7, 12649. [Google Scholar] [CrossRef]
- Ebrey, T.; Koutalos, Y. Vertebrate photoreceptors. Prog. Retin. Eye Res. 2001, 20, 49–94. [Google Scholar] [CrossRef]
- Park, H.; Tan, C.C.; Faulkner, A.; Jabbar, S.B.; Schmid, G.; Abey, J.; Iuvone, P.M.; Pardue, M.T. Retinal degeneration increases susceptibility to myopia in mice. Mol. Vis. 2013, 19, 2068–2079. [Google Scholar]
- Mylvaganam, G.H.; McGee, T.L.; Berson, E.L.; Dryja, T.P. A screen for mutations in the transducin gene GNB1 in patients with autosomal dominant retinitis pigmentosa. Mol. Vis. 2006, 12, 1496–1498. [Google Scholar]
- Stone, R.A.; Lin, T.; Laties, A.M.; Iuvone, P.M. Retinal dopamine and form-deprivation myopia. Proc. Natl. Acad. Sci. USA 1989, 86, 704–706. [Google Scholar] [CrossRef]
- Schmid, K.L.; Wildsoet, C.F. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom. Vis. Sci. 2004, 81, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhi, Z.N.; Pan, M.Z.; Xie, R.Z.; Qin, X.Y.; Lu, R.X.; Mao, X.J.; Chen, J.F.; Willcox, M.D.P.; Qu, J.; et al. Inhibition of experimental myopia by a dopamine agonist: Different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol. Vis. 2011, 17, 2824–2834. [Google Scholar]
- Iuvone, P.M.; Tigges, M.; Stone, R.A.; Lambert, S.; Laties, A.M. Effects of Apomorphine, a Dopamine Receptor Agonist, on Ocular Refraction and Axial Elongation in a Primate Model of Myopia. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1674–1677. [Google Scholar]
- Yan, T.T.; Xiong, W.W.; Huang, F.R.; Zheng, F.; Ying, H.F.; Chen, J.F.; Qu, J.; Zhou, X.T. Daily Injection But Not Continuous Infusion of Apomorphine Inhibits Form-Deprivation Myopia in Mice. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2475–2485. [Google Scholar] [CrossRef]
- Ashton-Beaucage, D.; Therrien, M. How Genetics Has Helped Piece Together the MAPK Signaling Pathway. In ERK Signaling: Methods and Protocols; Jimenez, G., Ed.; Springer: New York, NY, USA, 2017; pp. 1–21. [Google Scholar]
- Williams, K.M.; Hammond, C.J. GWAS in myopia: Insights into disease and implications for the clinic. Expert Rev. Ophthalmol. 2016, 11, 101–110. [Google Scholar] [CrossRef]
- Beuerman, R.W.; Goh, L.K.; Barathi, V.A. Gene Analysis in Experimental Animal Models of Myopia. In Myopia; World Scientific: Singapore, 2010; pp. 331–342. [Google Scholar]
- Huang, Q.; Yang, L.; Luo, J.; Guo, L.; Wang, Z.; Yang, X.; Jin, W.; Fang, Y.; Ye, J.; Shan, B.; et al. SWATH enables precise label-free quantification on proteome scale. Proteomics 2015, 15, 1215–1223. [Google Scholar] [CrossRef]
- Basak, T.; Bhat, A.; Malakar, D.; Pillai, M.; Sengupta, S. In-depth comparative proteomic analysis of yeast proteome using iTRAQ and SWATH based MS. Mol. Biosyst. 2015, 11, 2135–2143. [Google Scholar] [CrossRef]
- Simonelli, F.; Nesti, A.; Pensa, M.; Romano, L.; Savastano, S.; Rinaldi, E.; Auricchio, G. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp. Eye Res. 1989, 49, 181–187. [Google Scholar] [CrossRef]
- Francisco, B.M.; Salvador, M.; Amparo, N. Oxidative stress in myopia. Oxid. Med. Cell. Longev. 2015, 2015, 750637. [Google Scholar] [CrossRef]
- Yang, J.; Reinach, P.S.; Zhang, S.; Pan, M.; Sun, W.; Liu, B.; Li, F.; Li, X.; Zhao, A.; Chen, T.; et al. Changes in retinal metabolic profiles associated with form deprivation myopia development in guinea pigs. Sci. Rep. 2017, 7, 2777. [Google Scholar] [CrossRef] [PubMed]
- Holman, S.W.; Sims, P.F.; Eyers, C.E. The use of selected reaction monitoring in quantitative proteomics. Bioanalysis 2012, 4, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Kiyonami, R.; Domon, B. Selected reaction monitoring applied to quantitative proteomics. Methods Mol. Biol. 2010, 658, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Preising, M.N.; Görg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmüller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. 2019, 33, 11507–11527. [Google Scholar] [CrossRef]
- Gether, U.; Andersen, P.H.; Larsson, O.M.; Schousboe, A. Neurotransmitter transporters: Molecular function of important drug targets. Trends Pharmacol. Sci. 2006, 27, 375–383. [Google Scholar] [CrossRef]
- Tomi, M.; Tajima, A.; Tachikawa, M.; Hosoya, K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 2138–2142. [Google Scholar] [CrossRef]
- Sha, F.; Ye, X.; Zhao, W.; Xu, C.L.; Wang, L.; Ding, M.H.; Bi, A.L.; Wu, J.F.; Jiang, W.J.; Guo, D.D.; et al. Effects of Electroacupuncture on the Levels of Retinal Gamma-Aminobutyric Acid and Its Receptors in a Guinea Pig Model of Lens-Induced Myopia. Neuroscience 2015, 287, 164–174. [Google Scholar] [CrossRef]
- Zhao, W.; Bi, A.L.; Xu, C.L.; Ye, X.; Chen, M.Q.; Wang, X.T.; Zhang, X.Y.; Guo, J.G.; Jiang, W.J.; Zhang, J.; et al. GABA and GABA receptors alterations in the primary visual cortex of concave lens-induced myopic model. Brain Res. Bull. 2017, 130, 173–179. [Google Scholar] [CrossRef]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Xiang, Y.; Tian, Y.; Xia, F.; Zhou, Y.-S.; Peng, J.; Peng, Q.-H. Hub Genes of Astrocyte Involved in Glaucoma with Ocular Hypertension by Integrated Bioinformatics Analysis. Digit. Chin. Med. 2018, 1, 280–288. [Google Scholar] [CrossRef]
- El-Nimri, N.W.; Yao, M.; Huerta, A.; Hoang, M.; Wildsoet, C.F. Effect of chronic topical latanoprost on the sclera and lamina cribrosa of form-deprived myopic Guinea pigs. Exp. Eye Res. 2019, 186, 107740. [Google Scholar] [CrossRef]
- Harris, E.D. The transport of copper. Prog. Clin. Biol. Res. 1993, 380, 163–179. [Google Scholar]
- Klomp, L.W.; Farhangrazi, Z.S.; Dugan, L.L.; Gitlin, J.D. Ceruloplasmin gene expression in the murine central nervous system. J. Clin. Investig. 1996, 98, 207–215. [Google Scholar] [CrossRef]
- Stasi, K.; Nagel, D.; Yang, X.; Ren, L.; Mittag, T.; Danias, J. Ceruloplasmin upregulation in retina of murine and human glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 2007, 48, 727–732. [Google Scholar] [CrossRef][Green Version]
- Fedor, M.; Socha, K.; Urban, B.; Soroczynska, J.; Matyskiela, M.; Borawska, M.H.; Bakunowicz-Lazarczyk, A. Serum Concentration of Zinc, Copper, Selenium, Manganese, and Cu/Zn Ratio in Children and Adolescents with Myopia. Biol. Trace Elem. Res. 2017, 176, 1–9. [Google Scholar] [CrossRef]
- Pan, W.; Pham, V.N.; Stratman, A.N.; Castranova, D.; Kamei, M.; Kidd, K.R.; Lo, B.D.; Shaw, K.M.; Torres-Vazquez, J.; Mikelis, C.M.; et al. CDP-diacylglycerol synthetase-controlled phosphoinositide availability limits VEGFA signaling and vascular morphogenesis. Blood 2012, 120, 489–498. [Google Scholar] [CrossRef][Green Version]
- Cheng, C.L.; Molday, R.S. Changes in gene expression associated with retinal degeneration in the rd3 mouse. Mol. Vis. 2013, 19, 955–969. [Google Scholar]
- Mathis, U.; Ziemssen, F.; Schaeffel, F. Effects of a human VEGF antibody (Bevacizumab) on deprivation myopia and choroidal thickness in the chicken. Exp. Eye Res. 2014, 127, 161–169. [Google Scholar] [CrossRef]
- Couser, N.L.; Masood, M.M.; Aylsworth, A.S.; Stevenson, R.E. Ocular manifestations in the X-linked intellectual disability syndromes. Ophthalmic Genet. 2017, 38, 401–412. [Google Scholar] [CrossRef]
- Starokadomskyy, P.; Gluck, N.; Li, H.; Chen, B.; Wallis, M.; Maine, G.N.; Mao, X.; Zaidi, I.W.; Hein, M.Y.; McDonald, F.J.; et al. CCDC22 deficiency in humans blunts activation of proinflammatory NF-kappaB signaling. J. Clin. Investig. 2013, 123, 2244–2256. [Google Scholar] [CrossRef]
- Zhang, J.S.; Da Wang, J.; Zhu, G.Y.; Li, J.; Xiong, Y.; Yusufu, M.; He, H.L.; Sun, X.L.; Ju, T.; Tao, Y.; et al. The expression of cytokines in aqueous humor of high myopic patients with cataracts. Mol. Vis. 2020, 26, 150–157. [Google Scholar] [PubMed]
- Suh, B.C.; Hong, Y.B.; Nakhro, K.; Nam, S.H.; Chung, K.W.; Choi, B.O. Early-onset severe hereditary sensory and autonomic neuropathy type 1 with S331F SPTLC1 mutation. Mol. Med. Rep. 2014, 9, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Pitz, S.; Kalkum, G.; Arash, L.; Karabul, N.; Sodi, A.; Larroque, S.; Beck, M.; Gal, A. Ocular signs correlate well with disease severity and genotype in Fabry disease. PLoS ONE 2015, 10, e0120814. [Google Scholar] [CrossRef] [PubMed]
- Fledelius, H.C.; Sandfeld, L.; Rasmussen, A.K.; Madsen, C.V.; Feldt-Rasmussen, U. Ophthalmic experience over 10 years in an observational nationwide Danish cohort of Fabry patients with access to enzyme replacement. Acta Ophthalmol. 2015, 93, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Lindblad-Toh, K.; Garber, M.; Zuk, O.; Lin, M.F.; Parker, B.J.; Washietl, S.; Kheradpour, P.; Ernst, J.; Jordan, G.; Mauceli, E.; et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature 2011, 478, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Ashby, R.S.; Zeng, G.; Leotta, A.J.; Tse, D.Y.; McFadden, S.A. Egr-1 mRNA Expression Is a Marker for the Direction of Mammalian Ocular Growth. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5911–5921. [Google Scholar] [CrossRef]
- Zhang, Y.; Bilbao, A.; Bruderer, T.; Luban, J.; Strambio-De-Castillia, C.; Lisacek, F.; Hopfgartner, G.; Varesio, E. The Use of Variable Q1 Isolation Windows Improves Selectivity in LC-SWATH-MS Acquisition. J. Proteome Res. 2015, 14, 4359–4371. [Google Scholar] [CrossRef]
- Magrane, M.; UniProt, C. UniProt Knowledgebase: A hub of integrated protein data. Database 2011, 2011, bar009. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Guerra, C. Ingenuity Pathways Analysis: Software for discovering and modelling pathways and networks in your systems data. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2008, 150, S50. [Google Scholar] [CrossRef]
- Bisson, N.; James, D.A.; Ivosev, G.; Tate, S.A.; Bonner, R.; Taylor, L.; Pawson, T. Selected reaction monitoring mass spectrometry reveals the dynamics of signaling through the GRB2 adaptor. Nat. Biotechnol. 2011, 29, 653–658. [Google Scholar] [CrossRef]

| Ocular Parameters | Myopic Eyes | Control Eyes | p Value |
|---|---|---|---|
| Refractive errors (D) | +5.25 ± 2.33 | +6.94 ± 1.81 | 0.004 ** |
| ACD (mm) | 1.089 ± 0.035 | 1.106 ± 0.052 | 0.367 |
| Lens thickness (mm) | 3.033 ± 0.040 | 3.024 ± 0.059 | 0.499 |
| VCD (mm) | 2.979 ± 0.035 | 2.955 ± 0.024 | 0.253 |
| Retinal (mm) | 0.083 ± 0.002 | 0.083 ± 0.003 | 1.000 |
| Choroidal (mm) | 0.156 ± 0.013 | 0.174 ± 0.019 | 0.016 * |
| Axial length (mm) | 7.416 ± 0.084 | 7.397 ± 0.104 | 0.361 |
| Ocular length (mm) | 7.756 ± 0.095 | 7.757 ± 0.122 | 0.948 |
| No | Pathway Name | p Value | Z Score | Molecules | Evidence from Gene Expression | Evidence from Protein Expression |
|---|---|---|---|---|---|---|
| 1 | Phototransduction Pathway | 0.000 | NaN | PRKACB, GNB1, RGS9BP, GRK1, PDE6B, PRKAR1A, RCVRN | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | [45] (Shan et al., 2018) |
| 2 | Oxidative Phosphorylation | 0.000 | NaN | SDHA, ATP5B, COX5B, COX7A2, ATPAF2, CYB5A, NDUFAB1, NDUFB10 | [61] (Tkatchenko et al., 2018) [62] (Giummarra et al., 2018) | N/A |
| 3 | Mitochondrial Dysfunction | 0.000 | NaN | SDHA, ATP5B, COX5B, COX7A2, MAPK9, ATPAF2, CYB5A, NDUFAB1, NDUFB10 | [61] (Tkatchenko et al., 2018) [63] (Riddell and Crewther, 2017) | N/A |
| 4 | IL-1 Signaling | 0.000 | NaN | PRKACB, GNB1, MAPK9, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) | N/A |
| 5 | Huntington’s Disease Signaling | 0.000 | NaN | GNB1, SDHA, ATP5B, MAPK9, GNG13, DLG4, GNG3, AP2A2, SNAP25 | [60] (Tkatchenko et al., 2019) [62] (Giummarra et al., 2018) | N/A |
| 6 | Tight Junction Signaling | 0.001 | NaN | PRKACB, EPB41, TJP2, PPP2R2A, SNAP25, ACTA1, PRKAR1A | [60] (Tkatchenko et al., 2019) [64] (Zhang et al., 2010) | N/A |
| 7 | Tec Kinase Signaling | 0.001 | 1.000 | GNB1, RHOC, PAK2, MAPK9, GNG13, GNG3, ACTA1 | [60] (Tkatchenko et al., 2019) | N/A |
| 8 | Germ Cell-Sertoli Cell Junction Signaling | 0.001 | NaN | EPN1, RHOC, PAK2, ILK, MAPK9, ACTN4, ACTA1 | [60] (Tkatchenko et al., 2019) | N/A |
| 9 | Calcium Signaling | 0.001 | 1.000 | PRKACB, CAMK2A, CAMK2D, ATP2B3, TPM3, ACTA1, PRKAR1A | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | N/A |
| 10 | α-Adrenergic Signaling | 0.001 | NaN | PRKACB, GNB1, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | N/A |
| 11 | G Beta Gamma Signaling | 0.001 | 0.447 | PRKACB, GNB1, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) | N/A |
| 12 | TCA Cycle II (Eukaryotic) | 0.001 | NaN | SDHA, IDH3G, DLST | [62] (Giummarra et al., 2018) | N/A |
| 13 | CREB Signaling in Neurons | 0.001 | 1.000 | PRKACB, GNB1, CAMK2A, CAMK2D, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | N/A |
| 14 | CDP-diacylglycerol Biosynthesis I | 0.001 | NaN | CDS1, AGPAT1, LPGAT1 | [60] (Tkatchenko et al., 2019) | N/A |
| 15 | Phosphatidylglycerol Biosynthesis II (Non-plastidic) | 0.001 | NaN | CDS1, AGPAT1, LPGAT1 | [60] (Tkatchenko et al., 2019) | N/A |
| 16 | Relaxin Signaling | 0.002 | NaN | PRKACB, GNB1, GNG13, GNG3, PDE6B, PRKAR1A | [60] (Tkatchenko et al., 2019) | N/A |
| 17 | Gαs Signaling | 0.003 | NaN | PRKACB, GNB1, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | N/A |
| 18 | G Protein Signaling Mediated by Tubby | 0.003 | NaN | GNB1, GNG13, GNG3 | [60] (Tkatchenko et al., 2019) | N/A |
| 19 | Androgen Signaling | 0.003 | NaN | PRKACB, GNB1, GNG13, GNG3, PRKAR1A | [61] (Tkatchenko et al., 2018) | N/A |
| 20 | CXCR4 Signaling | 0.003 | 1.000 | GNB1, RHOC, PAK2, MAPK9, GNG13, GNG3 | [60] (Tkatchenko et al., 2019) | N/A |
| 21 | Melatonin Signaling | 0.003 | −1.000 | PRKACB, CAMK2A, CAMK2D, PRKAR1A | [60] (Tkatchenko et al., 2019) | [65] (Rada and Wiechmann, 2006) |
| 22 | Ephrin B Signaling | 0.004 | NaN | GNB1, EPHB2, GNG13, GNG3 | [61] (Tkatchenko et al., 2018) | N/A |
| 23 | Gαi Signaling | 0.004 | NaN | PRKACB, GNB1, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) [66] (Srinivasalu et al., 2018) | N/A |
| 24 | Purine Nucleotides De Novo Biosynthesis II | 0.004 | NaN | ADSS, PAICS | [60] (Tkatchenko et al., 2019) | N/A |
| 25 | RhoGDI Signaling | 0.004 | NaN | GNB1, RHOC, PAK2, GNG13, GNG3, ACTA1 | [60] (Tkatchenko et al., 2019) | N/A |
| 26 | Gustation Pathway | 0.004 | NaN | PRKACB, GNB1, GNG13, PDE6B, PRKAR1A | [61] (Tkatchenko et al., 2018) | N/A |
| 27 | Oleate Biosynthesis II (Animals) | 0.005 | NaN | SCD5, CYB5A | [60] (Tkatchenko et al., 2019) | N/A |
| 28 | Signaling by Rho Family GTPases | 0.005 | 1.000 | GNB1, RHOC, PAK2, MAPK9, GNG13, GNG3, ACTA1 | [60] (Tkatchenko et al., 2019) | N/A |
| 29 | P2Y Purigenic Receptor Signaling Pathway | 0.006 | NaN | PRKACB, GNB1, GNG13, GNG3, PRKAR1A | [60] (Tkatchenko et al., 2019) | N/A |
| 30 | Protein Kinase A Signaling | 0.007 | 1.633 | PRKACB, GNB1, CAMK2A, CAMK2D, GNG13, GNG3, GRK1, PDE6B, PRKAR1A | [60] (Tkatchenko et al., 2019)[61] (Tkatchenko et al., 2018) | N/A |
| 31 | ILK Signaling | 0.007 | 0.816 | PPP2R2A, RHOC, ILK, MAPK9, ACTN4, ACTA1 | [60] (Tkatchenko et al., 2019)[67] (Wu et al., 2018) | N/A |
| 32 | IL-8 Signaling | 0.007 | 1.000 | GNB1, RHOC, PAK2, MAPK9, GNG13, GNG3 | [60] (Tkatchenko et al., 2019) | N/A |
| 33 | Clathrin-mediated Endocytosis Signaling | 0.007 | NaN | EPN1, SYNJ1, RAB11B, EPHB2, AP2A2, ACTA1 | [60] (Tkatchenko et al., 2019) | N/A |
| 34 | D-myo-inositol (1,4,5)-trisphosphate Degradation | 0.010 | NaN | SYNJ1, BPNT1 | [60] (Tkatchenko et al., 2019) | N/A |
| 35 | CDK5 Signaling | 0.010 | −1.000 | PRKACB, PPP2R2A, MAPK9, PRKAR1A | [60] (Tkatchenko et al., 2019) | N/A |
| 36 | Regulation of eIF4 and p70S6K Signaling | 0.011 | NaN | RPS7, EIF4EBP2, PPP2R2A, EIF2B2, EIF3M | [60] (Tkatchenko et al., 2019)[61] (Tkatchenko et al., 2018) [68] (Zelinka et al., 2016) | N/A |
| 37 | EIF2 Signaling | 0.012 | NaN | RPS7, RPL8, EIF2B2, ACTA1, RPLP0, EIF3M | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) [ [69](Yang et al., 2015) [67] (Wu et al., 2018) | N/A |
| 38 | Spermine Biosynthesis | 0.017 | NaN | SMS | [61] (Tkatchenko et al., 2018) | |
| 39 | Ephrin Receptor Signaling | 0.017 | NaN | GNB1, EPHB2, PAK2, GNG13, GNG3 | [60] (Tkatchenko et al., 2019)[61] (Tkatchenko et al., 2018) | N/A |
| 40 | Super-pathway of D-myo-inositol (1,4,5)-trisphosphate Metabolism | 0.018 | NaN | SYNJ1, BPNT1 | [60] (Tkatchenko et al., 2019) | N/A |
| 41 | Synaptic Long Term Potentiation | 0.020 | 1.000 | PRKACB, CAMK2A, CAMK2D, PRKAR1A | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | N/A |
| 42 | RAR Activation | 0.024 | NaN | PRKACB, SRA1, MAPK9, CRABP1, PRKAR1A | [60] (Tkatchenko et al., 2019) [70] (Huang et al., 2011) | [70] (Huang et al., 2011) [71] (Huo et al., 2013) |
| 43 | S-methyl-5-thio-α-D-ribose 1-phosphate Degradation | 0.026 | NaN | APIP | [61] (Tkatchenko et al., 2018) | N/A |
| 44 | Inosine-5’-phosphate Biosynthesis II | 0.026 | NaN | PAICS | [60] (Tkatchenko et al., 2019) | N/A |
| 45 | NRF2-mediated Oxidative Stress Response | 0.026 | NaN | AKR1A1, MAPK9, DNAJA1, MGST3, ACTA1 | [60] (Tkatchenko et al., 2019) [67] (Wu et al., 2018) | N/A |
| 46 | Antiproliferative Role of Somatostatin Receptor 2 | 0.027 | NaN | GNB1, GNG13, GNG3 | [60] (Tkatchenko et al., 2019)[61] (Tkatchenko et al., 2018) | N/A |
| 47 | BMP signaling pathway | 0.028 | NaN | PRKACB, MAPK9, PRKAR1A | [72] (Li et al., 2015) | [73] (Li et al., 2016) |
| 48 | Dopamine Receptor Signaling | 0.028 | NaN | PRKACB, PPP2R2A, PRKAR1A | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | [74] (Feldkaemper and Schaeffel, 2013) [75] (Chen et al., 2017) [76] (Zhou et al., 2017) |
| 49 | Insulin Receptor Signaling | 0.033 | 0.000 | PRKACB, SYNJ1, EIF2B2, PRKAR1A | [60] (Tkatchenko et al., 2019) [77] (He et al., 2014) [78] (Penha et al., 2011) | [79] (Ritchey et al., 2012) |
| 50 | Xenobiotic Metabolism Signaling | 0.037 | NaN | SRA1, CAMK2A, CAMK2D, PPP2R2A, MAPK9, MGST3 | [60] (Tkatchenko et al., 2019) | N/A |
| 51 | HIPPO signaling | 0.039 | NaN | TJP2, PPP2R2A, DLG4 | [60] (Tkatchenko et al., 2019) [61] (Tkatchenko et al., 2018) | N/A |
| 52 | Integrin Signaling | 0.041 | −0.447 | RHOC, PAK2, ILK, ACTN4, ACTA1 | [60] (Tkatchenko et al., 2019)[61] (Tkatchenko et al., 2018) [80] (Tian et al., 2013) | N/A |
| 53 | cAMP-mediated signaling | 0.044 | 0.447 | PRKACB, CAMK2A, CAMK2D, PDE6B, PRKAR1A | [61] (Tkatchenko et al., 2018) [81] (Tao et al., 2013) | [82](Chun et al., 2015) |
| 54 | Regulation of Actin-based Motility by Rho | 0.044 | NaN | RHOC, PAK2, ACTA1 | [60] (Tkatchenko et al., 2019) [40] (Wu et al., 2018a) | N/A |
| 55 | Netrin Signaling | 0.044 | NaN | PRKACB, PRKAR1A | [60] (Tkatchenko et al., 2019) | N/A |
| 56 | Gαq Signaling | 0.049 | NaN | GNB1, RHOC, GNG13, GNG3 | [60] (Tkatchenko et al., 2019) | N/A |
| No | UniProt Accession | Gene Name | Protein Description | SWATH | MRM | |||
|---|---|---|---|---|---|---|---|---|
| Protein FC (T/C) | p Value | Protein FC (T/C) | p Value | Confidence | ||||
| 1 | H0VS95 | SLC6A6 | Sodium- and chloride-dependent taurine transporter | 1.33 | 0.030 | 1.16 | 0.039 * | 2 |
| 2 | A0A286XMC0 | PTGES2 | Prostaglandin E synthase 2 | 1.23 | 0.030 | 1.13 | 0.040 * | 2 |
| 3 | A0A286XCE4 | CP | Ceruloplasmin | 1.23 | 0.050 | 1.12 | 0.527 | 1 |
| 4 | A0A286XGK4 | CDS1 | Phosphatidate cytidylyltransferase 1 | 1.30 | 0.010 | 1.31 | 0.083 | 1 |
| 5 | H0VSK3 | SPTLC1 | Serine palmitoyltransferase 1 | 1.28 | 0.010 | 1.05 | 0.352 | 1 |
| 6 | H0WDS3 | CCDC22 | Coiled-coil domain-containing protein 22 | 1.25 | 0.030 | 1.12 | 0.350 | 1 |
| 7 | H0UU62 | GLA | Alpha-galactosidase A | 1.22 | 0.030 | 1.08 | 0.356 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, J.; Sze, Y.-H.; Tse, D.Y.-Y.; To, C.-H.; McFadden, S.A.; Lam, C.S.-Y.; Li, K.-K.; Lam, T.C. SWATH Based Quantitative Proteomics Reveals Significant Lipid Metabolism in Early Myopic Guinea Pig Retina. Int. J. Mol. Sci. 2021, 22, 4721. https://doi.org/10.3390/ijms22094721
Bian J, Sze Y-H, Tse DY-Y, To C-H, McFadden SA, Lam CS-Y, Li K-K, Lam TC. SWATH Based Quantitative Proteomics Reveals Significant Lipid Metabolism in Early Myopic Guinea Pig Retina. International Journal of Molecular Sciences. 2021; 22(9):4721. https://doi.org/10.3390/ijms22094721
Chicago/Turabian StyleBian, Jingfang, Ying-Hon Sze, Dennis Yan-Yin Tse, Chi-Ho To, Sally A. McFadden, Carly Siu-Yin Lam, King-Kit Li, and Thomas Chuen Lam. 2021. "SWATH Based Quantitative Proteomics Reveals Significant Lipid Metabolism in Early Myopic Guinea Pig Retina" International Journal of Molecular Sciences 22, no. 9: 4721. https://doi.org/10.3390/ijms22094721
APA StyleBian, J., Sze, Y.-H., Tse, D. Y.-Y., To, C.-H., McFadden, S. A., Lam, C. S.-Y., Li, K.-K., & Lam, T. C. (2021). SWATH Based Quantitative Proteomics Reveals Significant Lipid Metabolism in Early Myopic Guinea Pig Retina. International Journal of Molecular Sciences, 22(9), 4721. https://doi.org/10.3390/ijms22094721







