Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa
Abstract
1. Introduction
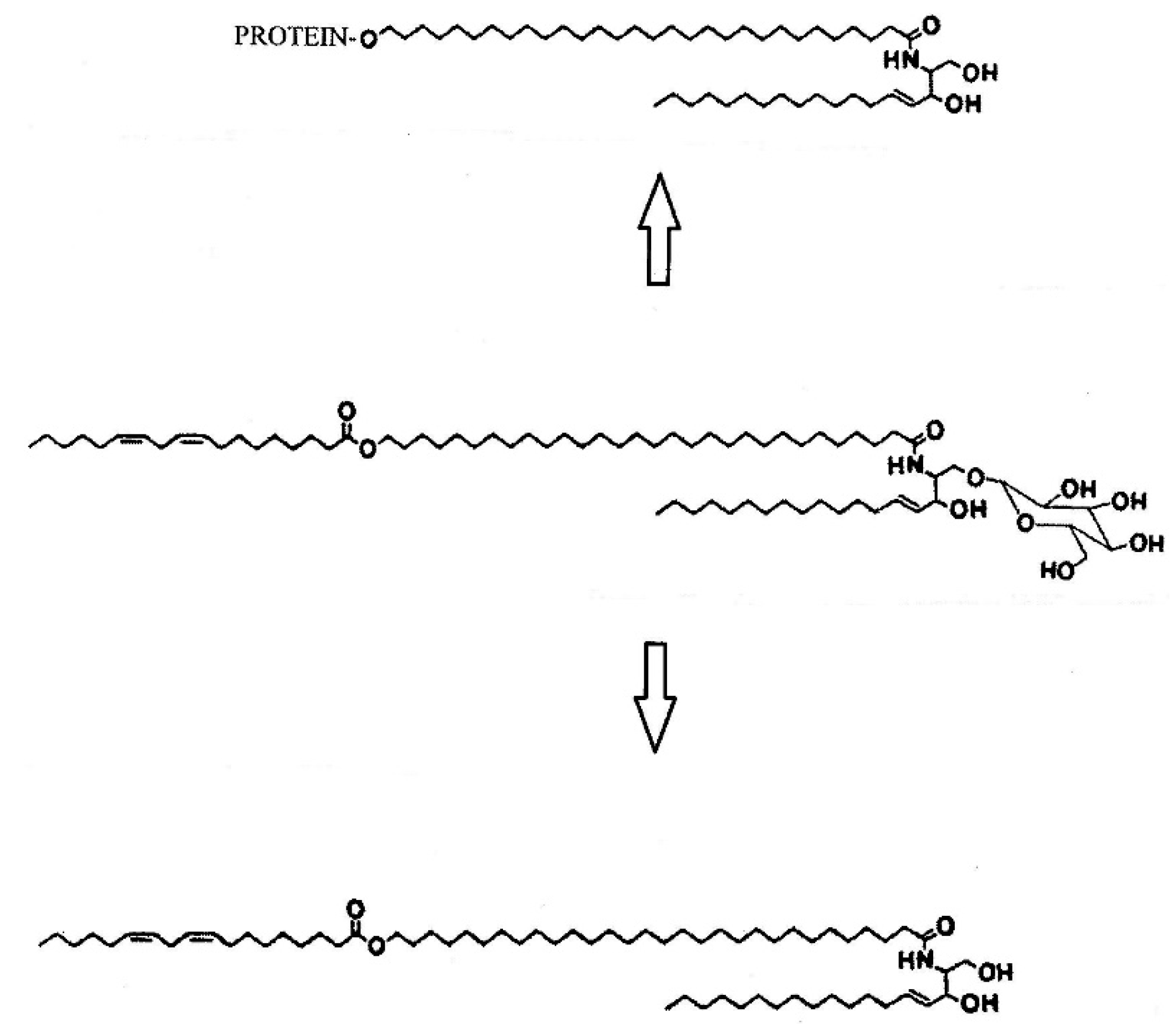
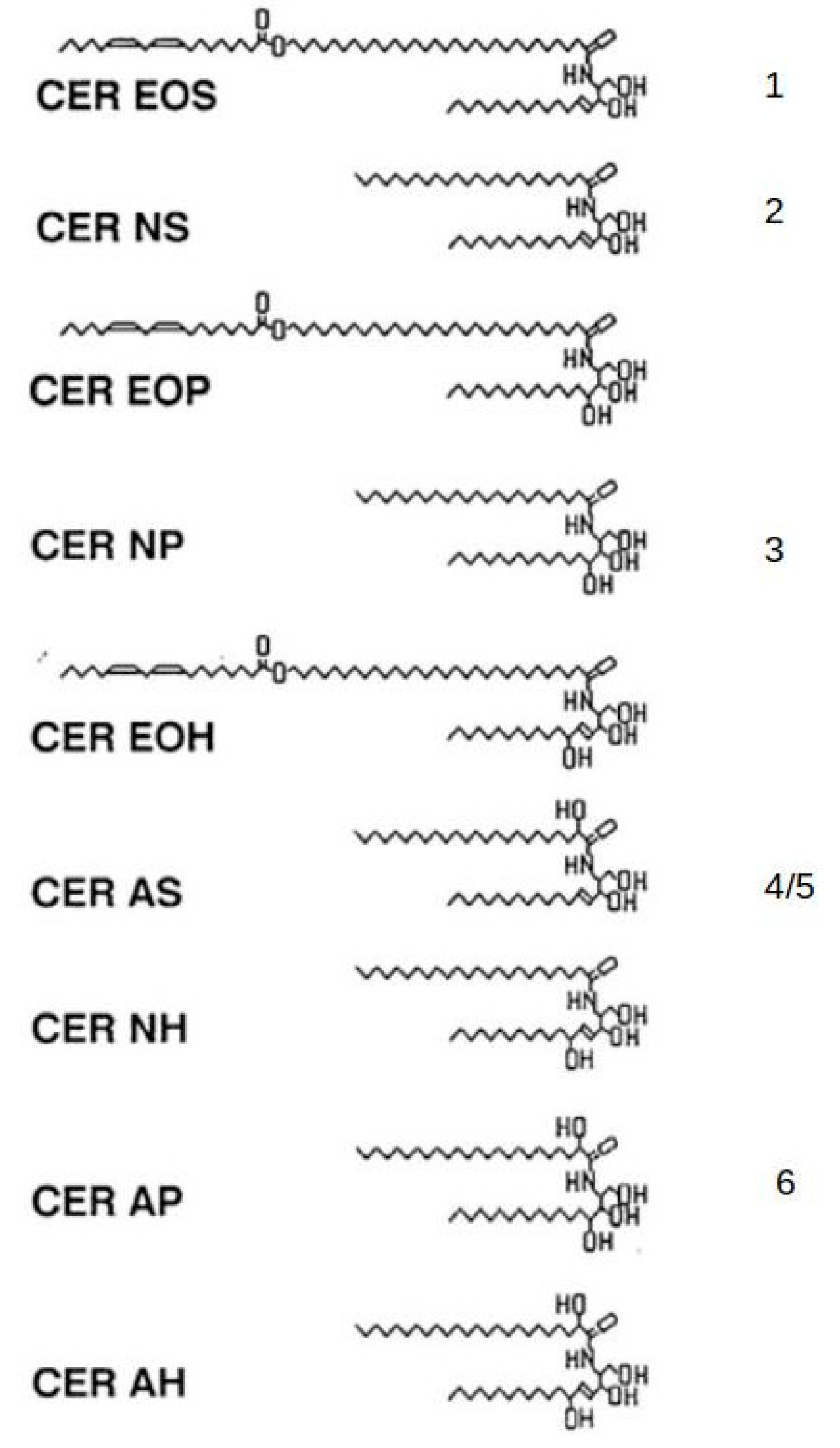
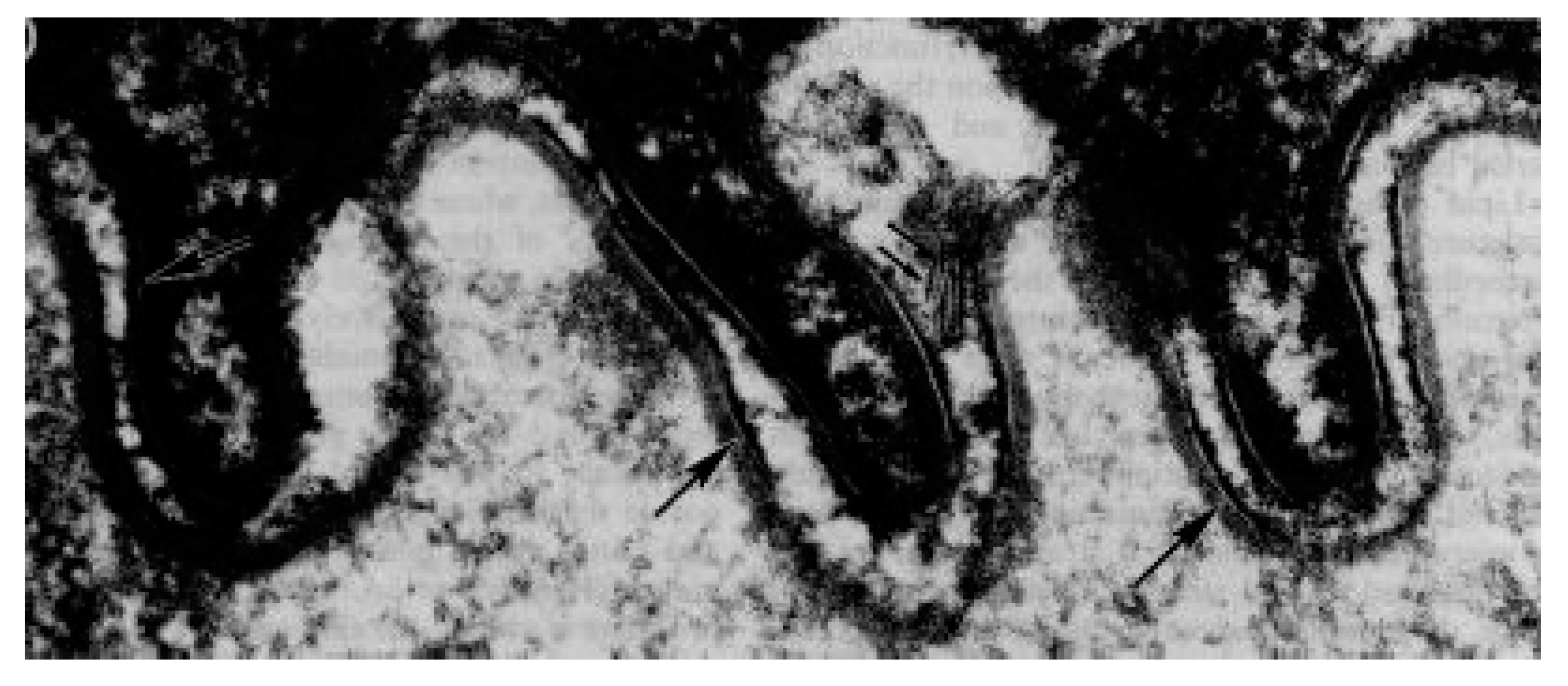
2. Skin
3. Keratinized Oral Mucosa
4. Nonkeratinized Oral Mucosa
5. Transdermal Drug Delivery
6. Skin Disease and the Barrier
7. Buccal Absorption
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wertz, P.W. Lipids and the permeability and antimicrobial barriers of the skin. J. Lipids 2018. [Google Scholar] [CrossRef]
- Schmitt, T.; Neubert, R.H.H. State of the art in stratum corneum research: The biophysical properties of ceramides. Chem. Phys. Lipids 2018, 216, 91–103. [Google Scholar] [CrossRef]
- Crumrine, D.; Khnykin, D.; Krieg, P.; Man, M.-Q.; Celli, A.; Mauro, T.M.; Wakefield, J.S.; Menon, G.; Mauldin, E.; Miner, J.H.; et al. Mutations in recessive congenital ichthoses illuminate the origin and functions of the corneocyte lipid envelope. J. Investig. Dermatol. 2019, 139, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W. Lipid metabolic events underlying the formation of the corneocyte lipid envelope. Skin Pharmacol. Physiol. 2021, 34, 38–50. [Google Scholar] [PubMed]
- Attenborough, D. Life on Earth, 1st ed.; Little Brown & Company: Boston, MA, USA, 1980. [Google Scholar]
- Baker, H.; Kligman, A.M. A simple in vivo method for studying the permeability of the human stratum corneum. J. Investig. Dermatol. 1967, 48, 273–274. [Google Scholar] [CrossRef]
- Yardley, H.J. Epidermal lipids. Int. J. Cosmet. Sci. 1987, 9, 13–19. [Google Scholar] [CrossRef]
- Diaz-Del Consuelo, I.; Jacques, Y.; Pizzolato, G.-P.; Guy, R.H.; Falson, F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch. Oral Biol. 2005, 50, 981–987. [Google Scholar] [CrossRef]
- Pinto, S.; Pintado, M.E.; Sarmento, B. In Vivo, ex vivo and in vitro assessment of buccal permeation of drugs from delivery systems. Expert Opin. Drug Deliv. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Scheuplein, R.J. Permeability of the skin: A review of major concepts and some new developments. J. Investig. Dermatol. 1976, 67, 672–676. [Google Scholar] [CrossRef]
- Manganaro, A.M. Review of transmucosal drug delivery. Militar. Med. 1997, 162, 27–30. [Google Scholar] [CrossRef]
- Nemanic, M.K.; Elias, P.M. In Situ precipitation: A novel cytochemical technique for visualization of permeability pathways in mammalian stratum corneum. J. Histochem. Cytochem. 1980, 28, 573–578. [Google Scholar] [CrossRef]
- Squier, C.A.; Lesch, C.A. Penetration pathways of different compounds through epidermis and oral epithelia. J. Oral Pathol. 1988, 17, 512–516. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J. Drug delivery to hair follicles. Expert Opin. Drug Deliv. 2013, 10, 787–797. [Google Scholar] [CrossRef]
- Gorsky, M.; Buchner, A.; Fundoianu-Dayan, D.; Cohen, C. Fordyce’s granules in the oral mucosa of adult Israeli Jews. Commun. Dent. Oral Epidemiol. 1986, 14, 231–232. [Google Scholar] [CrossRef]
- Olivier, J.H. Fordyce granules on the prolabial and oral mucous membranes of a selected population. S. Afr. Dent. J. 2006, 61, 72–74. [Google Scholar]
- Nicolaides, N. Skin Lipids. II. Lipid class composition of samples from various species and anatomical locations. J. Am. Oil Chem. Soc. 1965, 42, 691–702. [Google Scholar] [CrossRef]
- Gray, G.M.; White, R.J. Glycosphingolipids and ceramides in human and pig epidermis. J. Investig. Dermatol. 1978, 70, 336–341. [Google Scholar] [CrossRef]
- Gray, G.M.; Yardley, H.J. Different populations of pig epidermal cells: Isolation and lipid composition. J. Lipid Res. 1975, 16, 441–447. [Google Scholar] [CrossRef]
- Wertz, P.W.; Swartzendruber, D.C.; Madison, K.C.; Downing, D.T. Composition and morphology of epidermal cyst lipids. J. Investig. Dermatol. 1987, 89, 419–424. [Google Scholar] [CrossRef]
- Gray, G.M.; White, R.J.; Majer, J.R. 1-(3′-)-acyl-beta-glucosyl-N-dihydropentatriacontadienoyl-sphingosine of pig and human epidermis. Biochim. Biophys. Acta 1978, 528, 127–137. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Acylglucosylceramides of pig epidermis: Structure determination. J. Lipid Res. 1983, 24, 753–758. [Google Scholar] [CrossRef]
- Abraham, W.; Wertz, P.W.; Downing, D.T. Linoleate-rich acylglucosylceramides of pig epidermis: Structure determination by proton magnetic resonance. J. Lipid Res. 1985, 26, 761–766. [Google Scholar] [CrossRef]
- Bowser, P.A.; Nugteren, D.H.; White, R.J.; Houtsmuller, U.M.; Prottey, C. Identification, isolation and characterization of epidermal lipids containing linoleic acid. Biochim. Biophys. Acta 1985, 843, 419–428. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Glucosylceramides of pig epidermis: Structure determination. J. Lipid Res. 1983, 24, 1135–1139. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Ceramides of pig epidermis: Structure determination. J. Lipid Res. 1983, 24, 759–765. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T.; Freinkel, R.K.; Traczyk, T.N. Sphingolipids of the stratum corneum and lamellar granules of fetal rat epidermis. J. Investig. Dermatol. 1984, 83, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Grayson, S.; Johnson-Winegar, A.G.; Wintraub, B.U.; Isseroff, R.R.; Epstein, E.H.; Elias, P.M. Lamellar body-enriched fractions from neonatal mice: Preparative techniques and partial characterization. J. Investig. Dermatol. 1985, 85, 289–294. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Covalently bound omega-hydroxyacylsphingosine in the stratum corneum. Biochim. Biophys. Acta 1987, 917, 108–111. [Google Scholar] [CrossRef]
- Swartzendruber, D.C.; Wertz, P.W.; Madison, K.C.; Downing, D.T. Evidence that the corneocyte has a chemically bound lipid envelope. J. Investig. Dermatol. 1987, 88, 709–713. [Google Scholar] [CrossRef]
- Madison, K.C.; Swartzendruber, D.C.; Wertz, P.W.; Downing, D.T. Presence of intact intercellular lipid lamellae in the upper layers of the stratum corneum. J. Investig. Dermatol. 1987, 88, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Gooris, G.S.; van der Spek, J.A.; Bras, W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J. Investig. Dermatol. 1991, 97, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Gooris, G.S.; Dubbelaar, F.E.; Weerheim, A.M.; Izerman, A.P.; Ponec, M. Role of ceramide 1 in the organization of the stratum corneum lipids. J. Lipid Res. 1998, 39, 186–196. [Google Scholar] [CrossRef]
- Kuempel, D.; Swartzendruber, D.C.; Squier, C.A.; Wertz, P.W. In Vitro reconstitution of stratum corneum lipid lamellae. Biochim. Biophys. Acta 1998, 1372, 135–140. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Gooris, G.S.; van der Spek, J.A.; Lavrijsen, S.; Bras, W. The lipid and protein structure of mouse stratum corneum: A wide and small angle diffraction study. Biochim. Biophys. Acta 1994, 1212, 183–192. [Google Scholar] [CrossRef]
- Pilgram, G.S.; Engeksma-van Pelt, A.M.; Bouwstra, J.A.; Koerten, H.K. Electron diffraction provides new information on human stratum corneum lipid organization studied in relation to depth and temperature. J. Investig. Dermatol. 1999, 113, 403–409. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Pilgram, G.S.; Gooris, G.S.; Koerten, H.K.; Ponec, M. New aspects of the skin barrier organization. Skin Pharmacol. Physiol. 2001, 14 (Suppl. 1), 52–62. [Google Scholar] [CrossRef]
- Groen, D.; Poole, D.S.; Gooris, G.S.; Bouwstra, J.A. Is an orthorhombic lateral packing and a proper lateral organization important for the skin barrier function? Biochim. Biophys. Acta 2011, 1808, 1529–1537. [Google Scholar] [CrossRef]
- Pilgram, G.S.; Vissers, D.C.; van der Meulen, H.; Pavel, S.; Larvijsen, S.P.; Bouwstra, J.A.; Koerten, H.K. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J. Investig. Dermatol. 2001, 117, 710–717. [Google Scholar] [CrossRef]
- Elias, P.M.; Cullander, C.; Mauro, T.; Rassner, U.; Komuves, L.; Brown, B.E.; Menon, G.K. The secretary granular cell: The outermost granular cell as a specialized secretory cell. J. Investig. Dermatol. Symp. Proc. 1998, 3, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Matoltsy, A.G. Keratinization. J. Investig. Dermatol. 1976, 67, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Hara, M.; Nishio, H.; Sidransky, E.; Inoue, S.; Otsuka, F.; Suzuki, A.; Elias, P.M.; Holleran, W.M. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 2000, 41, 2071–2082. [Google Scholar] [CrossRef]
- Hamanaka, S.; Hara, M.; Nishio, H.; Otsuka, F.; Suzuki, A.; Uchida, Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J. Investig. Dermatol. 2002, 119, 416–423. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Feingold, K.R.; Jain, M.; Elias, P.M. Extracellular processing of phospholipids is required for permeability barrier homeostasis. J. Lipid Res. 1995, 36, 1925–1935. [Google Scholar] [CrossRef]
- Ilic, D.; Bollinger, J.M.; Gelb, M.; Mauro, T.M. sPLA2 and the epidermal barrier. Biochim. Biophys. Acta 2014, 1841, 416–421. [Google Scholar] [CrossRef]
- Landmann, L. Epidermal permeability barrier: Transformation of lamellar granule-disks into intercellular sheets by a membrane-fusion process, a freeze-fracture study. J. Investig. Dermatol. 1986, 87, 202–209. [Google Scholar] [CrossRef]
- Ponec, M.; Weerheim, A.; Lankhorst, P.; Wertz, P. New acylceramide in native and reconstructed epidermis. J. Investig. Dermatol. 1989, 92, 581–588. [Google Scholar] [CrossRef]
- Motta, S.; Monti, M.; Sesana, S.; Caputo, R.; Carelli, S.; Ghidoni, R. Ceramide composition of the psoriatic scale. Biochim. Biophys. Acta Mol. Basis Dis. 1993, 1182, 147–151. [Google Scholar] [CrossRef]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Nat. Cancer Instit. Monogr 2001, 29, 7–15. [Google Scholar] [CrossRef]
- Hayward, A.F.; Hackemann, M. Electron microscopy of membrane-coating granules and a cell surface coat in keratinized and nonkeratinized human oral epithelium. J. Ultrastruc. Res. 1973, 43, 205–219. [Google Scholar] [CrossRef]
- Squier, C.A. Zinc iodide-osmium staining of membrane-coating granules in keratinized and non-keratinized mammalian oral epithelium. Arch. Oral Biol. 1982, 27, 377–382. [Google Scholar] [CrossRef]
- Schroeder, H.E. Differentiation of Human Oral Stratified Epithelia, 1st ed.; Karger: Basel, Switzerland, 1981. [Google Scholar]
- Law, S.; Wertz, P.W.; Swartzendruber, D.C.; Squier, C.A. Regional variation in content, composition and organization of porcine epithelial barrier lipids revealed by thin-layer chromatography and transmission electron microscopy. Arch. Oral Biol. 1995, 40, 1085–1091. [Google Scholar] [CrossRef]
- Hill, J.R.; Wertz, P.W. Structures of the ceramides from porcine palatal stratum corneum. Lipids 2009, 44, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Groen, D.; Gooris, G.S.; Bouwstra, J.A. Model membranes prepared with ceramide EOS, cholesterol and free fatty acids form a unique lamellar phase. Langmuir 2010, 26, 4168–4175. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Gooris, G.S.; Groen, D.; Barlow, D.J.; Lawrence, M.J.; Deme, B.; Bouwstra, J.A. Stratum corneum lipid matrix: Localization of ceramide and cholesterol in the unit cell of the long periodicity phase. Biochim. Biophys. Acta 2016, 1858, 1926–1936. [Google Scholar] [CrossRef]
- Swartzendruber, D.C.; Manganaro, A.; Madison, K.C.; Kremer, M.; Wertz, P.W.; Squier, C.A. Organization of the intercellular spaces of porcine epidermal and palatal stratum corneum: A quantitative study employing ruthenium tetroxide. Cell Tissue Res. 1995, 279, 271–276. [Google Scholar] [CrossRef]
- Chang, F.; Swartzendruber, D.C.; Wertz, P.W.; Squier, C.A. Covalently bound lipids in keratinizing epithelia. Biochim. Biophys. Acta 1993, 1150, 98–102. [Google Scholar] [CrossRef]
- Lesch, C.A.; Squier, C.A.; Cruchley, A.; Williams, D.M.; Speight, P. The permeability of human oral mucosa and skin to water. J. Dent. Res. 1989, 68, 1345–1349. [Google Scholar] [CrossRef]
- Squier, C.A.; Hall, B.K. The permeability of skin and oral mucosa to water and horseradish peroxidase as related to the thickness of the permeability barrier. J. Investig. Dermatol. 1985, 84, 176–179. [Google Scholar] [CrossRef]
- Squier, C.A. The permeability of keratinized and nonkeratinized oral epithelium to horseradish peroxidase. J. Ultrastruc. Res. 1973, 43, 160–177. [Google Scholar] [CrossRef]
- Squier, C.A.; Rooney, L. The permeability of keratinized and nonkeratinized oral epithelium to lanthanum in vivo. J. Ultrastruc. Res. 1976, 54, 286–295. [Google Scholar] [CrossRef]
- Squier, C.A. Membrane coating granules in nonkeratinizing epithelium. J. Ultrastruc. Res. 1977, 60, 212–220. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Uchida, N.; Yanagi, M.; Hamada, H. Physical enhancement? Nanocarrier? Current progress in transdermal drug delivery. Nanomaterials 2021, 11, 335. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschstein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert. Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- Daftardar, S.; Bahl, D.; Boddu, S.H.S.; Altorok, N.; Kahaleh, B. Ultrasound-mediated topical delivery of econazole nitrate with potential for treating Raynaud’s phenomenon. Int. J. Pharm. 2020, 580, 119229. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.; Shende, P. Potential of sonophoresis as a skin penetration technique in the treatment of rheumatoid arthritis with transdermal patch. AAPS PharmSciTech 2020, 21, 180. [Google Scholar] [CrossRef]
- Mitragoti, S.; Blankschstein, D.; Langer, R. Ultrasound-mediated transdermal protein delivery. Science 1995, 269, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, L.; Song, W.; Liu, J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: An overview of recent progress. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Eastman, R.C.; Pitzer, K.; Ackerman, N.R.; Fermi, S.J. The GlucoWatch biographer: A frequent automatic and noninvasive glucose monitor. Ann. Med. 2000, 32, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Sieg, A.; Guy, R.H.; Delgado-Charro, M.B. Noninvasive and minimally invasive methods for transdermal glucose monitoring. Diabetes Ther. 2005, 7, 174–197. [Google Scholar] [CrossRef]
- Giri, T.K.; Chakrabarty, S.; Ghosh, B. Transdermal reverse iontophoresis: A novel technique for therapeutic drug monitoring. J. Control. Release 2017, 28, 230–246. [Google Scholar] [CrossRef]
- Nawrocki, S.; Cha, J. The etiology, diagnosis, and management of hyperhydrosis: A comprehensive review: Therapeutic options. J. Am. Acad. Dermatol. 2019, 81, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Dermol-Cerne, J.; Pirc, E.; Miklavcic, D. Mechanistic view of skin electroporation—models and dosimetry for successful applications: An expert review. Expert. Opin. Drug Deliv. 2020, 17, 689–704. [Google Scholar] [CrossRef]
- Campana, L.G.; Miklavcic, D.; Bertino, G.; Marcanato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumors—Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta. Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef]
- Halder, J.; Gupta, S.; Kumari, R.; Das Gupta, G.; Rai, V.K. Microneedle array: Applications, Recent Advances, and Clinical Pertinence in Transdermal Drug Delivery. J. Pharm. Innov. 2020, 8, 1–8. [Google Scholar]
- Dabholkar, N.; Gorantla, S.; Waghule, T.; Rapalli, V.K.; Kothuru, A.; Goel, S.; Singvi, G. Biodegradable microneedles with carbohydrates and proteins: Revolutionary approach for transdermal drug delivery. Int. J. Biol. Macromol. 2021, 170, 621–802. [Google Scholar] [CrossRef] [PubMed]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, C.; Fontana, F.; Cheng, R.; Santos, H.A. Development of vaccine formulations: Past, present, and future. Drug Deliv. Transl. Res. 2021, 11, 353–372. [Google Scholar] [CrossRef]
- Korkmaz, E.; Balmert, S.C.; Sumpter, T.L.; Carey, C.D.; Erdos, G.; Falo, L.D. Microarray patches enable the development of skin-targeted vaccines against COVID-19. Adv. Drug Deliv. Rev. 2021, 171, 164–186. [Google Scholar] [CrossRef] [PubMed]
- Koutsonanos, D.G.; Esser, E.S.; McMaster, S.R.; Kalluri, P.; Lee, J.-W.; Prausnitz, M.R.; Skountzou, I.; Denning, T.L.; Kohlmeier, J.E.; Compans, R.W. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine 2015, 33, 4675–4682. [Google Scholar] [CrossRef]
- Epstein, W.L. Occupational poison ivy and oak dermatitis. Dermatol. Clin. 1994, 12, 511–516. [Google Scholar] [CrossRef]
- Kim, Y.; Flamm, A.; ElSohly, M.A.; Kaplan, D.H.; Hage, R.J.; Hamann, C.P.; Marks, J.G. Poison Ivy, Oak and Sumac dermatitis: What is known and what is new? Dermatitis 2019, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Maibach, H.I. Transepidermal water loss and skin surface hydration in the non invasive assessment of stratum corneum. Derm. Beruf. Unwelt 1990, 38, 50–53. [Google Scholar]
- Melnik, B.; Hollmann, J.; Hofmann, U.; Yub, M.S.; Plewig, G. Lipid composition of outer stratum corneum and nails in atopic and control subjects. Arch. Dermatol. Res. 1990, 282, 549–551. [Google Scholar] [CrossRef]
- Yamamoto, A.; Serizawa, S.; Ito, M.; Sato, Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch. Dermatol. Res. 1991, 283, 219–223. [Google Scholar] [CrossRef]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Wertz, P.; Gianetti, A.; Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 1998, 78, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Cutting edge of the pathogenesis of atopic dermatitis: Sphingomyelin deacylase, the enzyme involved in its ceramide deficiency, plays a pivotal role. Int. J. Mol. Sci. 2021, 22, 1613. [Google Scholar] [CrossRef]
- Van Smeden, J.; Janssens, M.; Kaye, E.C.J.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014, 23, 45–52. [Google Scholar] [CrossRef]
- Van Smeden, J.; Janssens, M.; Boiten, W.A.; van Drongelen, V.; Furio, L.; Vreeken, R.J.; Hovnanian, A.; Bouwstra, J.A. Intercellular skin barrier lipid composition and organization in Netherton syndrome patients. J. Investig. Dermatol. 2014, 134, 1238–1245. [Google Scholar] [CrossRef]
- Edsley, S.M.; Olesen, C.M.; Norreslet, L.B.; Ingham, A.C.; Iversen, S.; Lilje, B.; Clausen, M.L.; Jensen, J.S.; Stegger, M.; Agner, T.; et al. Staphylococcal communities on skin associated with atopic dermatitis and disease severity. Microorganisms 2021, 9, 432. [Google Scholar] [CrossRef]
- Arikawa, J.; Ishibashi, M.; Kawashima, M.; Tagaki, Y.; Ichikawa, Y.; Imokawa, G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Investig. Dermatol. 2002, 119, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, H.; Nakagawa, H.; Kuzukawa, M.; Mori, H.; Imokawa, G. Deficient production of hexadecanoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatology 2005, 211, 240–248. [Google Scholar] [CrossRef]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Dudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernandez-Hogueras, M.-V.; Perez-Rodriguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernandez-Gonzalez, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tooks to assess disease severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.; Monti, M.; Sesana, S.; Mellesi, L.; Ghidoni, R.; Caputo, R. Abnormality of water barrier function in psoriasis. Role of ceramide fractions. Arch. Dermatol. 1994, 130, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.; Sesani, S.; Ghidoni, R.; Monti, M. Content of the different lipid classes in psoriatic scale. Arch. Dermatol. Res. 1995, 287, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Lew, B.-L.; Cho, Y.; Kim, J.; Sim, W.-Y.; Kim, N.-I. Ceramides and signaling molecules in psoriatic epidermis: Reduced levels of ceramides, PKC-α, and JNK. J. Korean Med. Sci. 2006, 21, 95–99. [Google Scholar] [CrossRef]
- Hong, K.-K.; Cho, H.-R.; Ju, W.-C.; Cho, Y.; Kim, N.-I. A study on altered expression of serinepalmitoyl transferase (SPT) and ceramidase in psoriatic skin lesion. J. Korean Med. Sci. 2007, 22, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Kiatsurayanon, C.; Ogawa, H.; Niyonsaba, F. The role of host defense peptide human β-defensin in the maintenance of the skin barriers. Curr. Pharmaceut. Des. 2018, 24, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Demerjian, M.; Crumrine, D.A.; Milstone, L.M.; Williams, M.L.; Elias, P.M. Barrier disfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin-Dorfman syndrome). J. Investig. Dermatol. 2006, 126, 2032–2038. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakamichi, S.; Ohkuni, A.; Kamiyama, N.; Naoe, A.; Tsujimura, H.; Yokose, U.; Sugiura, K.; Ishikawa, J.; Akiyama, M.; et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formatuion. Proc. Natl. Acad. Sci. USA 2015, 112, 7707–7712. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, H.; Boeglin, W.E.; Elias, P.M.; Crumrine, D.; Beier, D.R.; Brash, A.R. Lipoxygenases mediate the effect of essential fatty acid in skinj barrier formation: A proposed role in releasing 0mega-hydroxyceramide for construction of the corneocyte lipid envelope. J. Biol. Chem. 2011, 286, 4046–4056. [Google Scholar] [CrossRef] [PubMed]
- Nemes, Z.; Marekov, L.N.; Festis, L.; Steinert, P.M. A novel function for transglutaminase 1: Attachment of omega-hydroxyceramide to involucrin by ester bond formation. Proc. Nat. Acad. Sci. USA 1999, 96, 8402–8407. [Google Scholar] [CrossRef]
- Russel, L.J.; DiGiovanna, J.J.; Rogers, G.R.; Steinert, P.M.; Hashem, N.; Compton, J.G.; Bale, S.J. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat. Genet. 1995, 9, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, T. SDR9C7 plays an essential role in skin barrier function by dehydrogenating acylceramide for attachment to proteins. J. Dermatol. Sci. 2020, 98, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Akayama, M. Epidemiology, medical genetics, diagnosis and treatment of harlequin ichthyosis in Japan. Pediatr. Int. 2015, 57, 516–522. [Google Scholar] [CrossRef]
- Elias, P.M.; Williams, M.L.; Holleran, W.M.; Jiang, Y.J.; Schmuth, M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: Inherited disorders of lipid metabolism. J. Lipid Res. 2008, 49, 697–714. [Google Scholar] [CrossRef]
- Wells, R.S.; Kerr, C.B. Genetic classification of ichthyosis. Arch. Dermatol. 1965, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.J.; Weiss, R.; Webster, D.; France, J.T. X-linked ichthyosis due to steroid sulfatase deficiency. Lancet 1978, 1, 70–72. [Google Scholar] [CrossRef]
- Long, S.A.; Wertz, P.W.; Strauss, J.S.; Downing, D.T. Human stratum corneum polar lipids and desquamation. Arch. Dermatol. Res. 1985, 277, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Ranasingh, A.W.; Wertz, P.W.; Downing, D.T.; Mackenzie, I.C. Lipid composition of cohesive and desquamated corneocytes from mouse ear skin. J. Investig. Dermatol. 1986, 86, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; Elias, P.M. Stratum corneum lipids in disorders of cornification. J. Clin. Investig. 1981, 68, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Denda, M.; Nakanishi, J.; Nomura, J.; Koyama, J. Cholesterol sulfate inhibits proteases that are involved in desquamation of stratum corneum. J. Investig. Dermatol. 1998, 111, 189–193. [Google Scholar] [CrossRef]
- Elias, P.M.; Crumrine, D.; Rassner, U.; Hachem, J.-P.; Menon, G.K.; Man, W.; Choy, M.H.W.; Leypoldt, L.; Feingold, K.R.; Williams, M.L. Basis for abnormal desquamation and permeability barrier disfunction in RXLI. J. Investig. Dermatol. 2004, 122, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Squier, C.A. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Therap. Drug Carr. Syst. 1991, 8, 237–269. [Google Scholar]
- Pacheco, M.S.; Barbieri, D.; da Silva, C.F.; de Morares, M.A. A review on orally disintegrating films (ODFs) made from natural polymers such as pullulan, maltodextrin, starch, and others. Int. J. Biol. Macromol. 2021, 178, 504–513. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal permeation enhancers—How do they really work? J. Control. Release 2005, 105, 1–15. [Google Scholar] [CrossRef]
- Sanz, R.; Calpena, A.C.; Mallandrich, M.; Clares, B. Enhancing topical analgesic administration: Review and prospect for transdermal and transbuccal drug delivery systems. Curr. Pharmaceut. Des. 2015, 21, 2867–2882. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; Hincal, A.A. Drug permeation enhancement via buccal route: Possibilities and limitations. J. Control. Release 2001, 72, 133–144. [Google Scholar] [CrossRef]
- Kontogiannidou, E.; Andreadis, D.A.; Zografos, A.L.; Nazar, H.; Klepetsanis, P.; van der Merwe, S.M.; Fatouros, D.G. Ex vivo drug delivery of ropinirole hydrochloride in the presence of permeation enhancers: The effect of charge. Pharm. Develop. Technol. 2017, 22, 1017–1021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Junginger, H.E.; Hoogstraate, J.A.; Verhoef, J.C. Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. J. Control. Release 1999, 62, 149–159. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Wang, N.; Pei, X.; Guo, Y.; Wang, J.; Barth, S.; Yu, F.; Lee, S.J.; He, H.; et al. Cell-penetrating peptide enhanced insulin buccal absorption. Int. J. Pharm. 2020. [Google Scholar] [CrossRef]
- Vaidya, A.; Mitragoti, S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J. Control. Release 2020, 327, 26–34. [Google Scholar] [CrossRef]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2020, 25, 901–908. [Google Scholar] [CrossRef]
- Matos, B.N.; Pereira, M.N.; de Oliveira Bravo, M.; Cunha-Filho, M.; Saldanha-Araujo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loaded with oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-J.; Cha, H.-R.; Hwang, S.J.; Kim, D.-S.; Choi, Y.-J.; Kim, Y.-S.; Shin, Y.-R.; Nguyen, T.T.; Choi, S.-O.; Lee, J.M.; et al. Ovalbumin and cholera toxin delivery to buccal mucosa for immunization using microneedles and comparison of immunological response to transmucosal delivery. Drug. Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef] [PubMed]

| Drug | MW | Log (K) |
|---|---|---|
| nicotine | 162 | 1.2 |
| nitroglycerin | 227 | 1.6 |
| lidocaine | 234 | 2.3 |
| estradiol | 272 | 4.0 |
| testosterone | 288 | 3.3 |
| scopolamine | 303 | 1.0 |
| fentanyl | 336 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. https://doi.org/10.3390/ijms22105229
Wertz PW. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. International Journal of Molecular Sciences. 2021; 22(10):5229. https://doi.org/10.3390/ijms22105229
Chicago/Turabian StyleWertz, Philip W. 2021. "Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa" International Journal of Molecular Sciences 22, no. 10: 5229. https://doi.org/10.3390/ijms22105229
APA StyleWertz, P. W. (2021). Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. International Journal of Molecular Sciences, 22(10), 5229. https://doi.org/10.3390/ijms22105229






