Gangliosides as Signaling Regulators in Cancer
Abstract
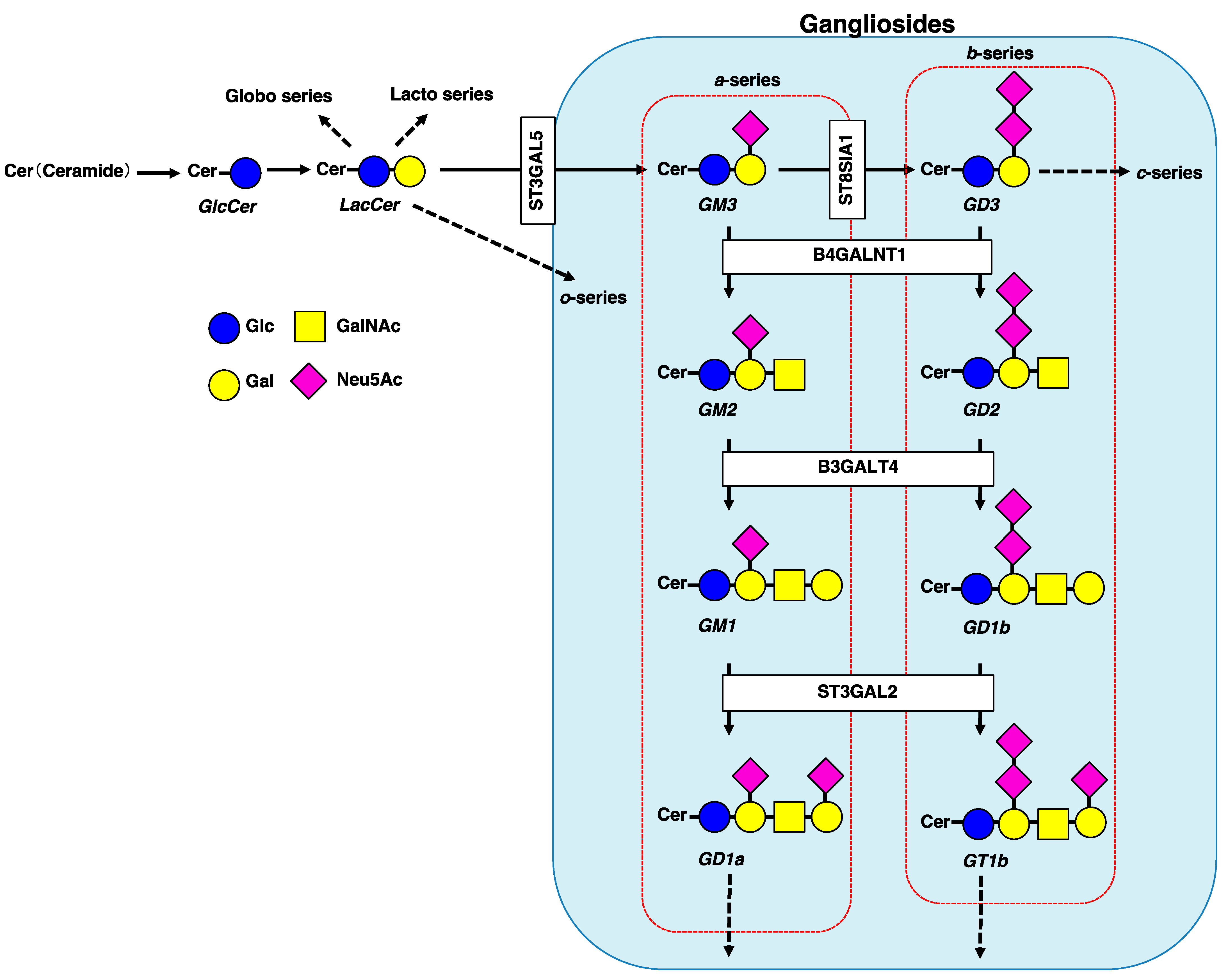
1. Introduction
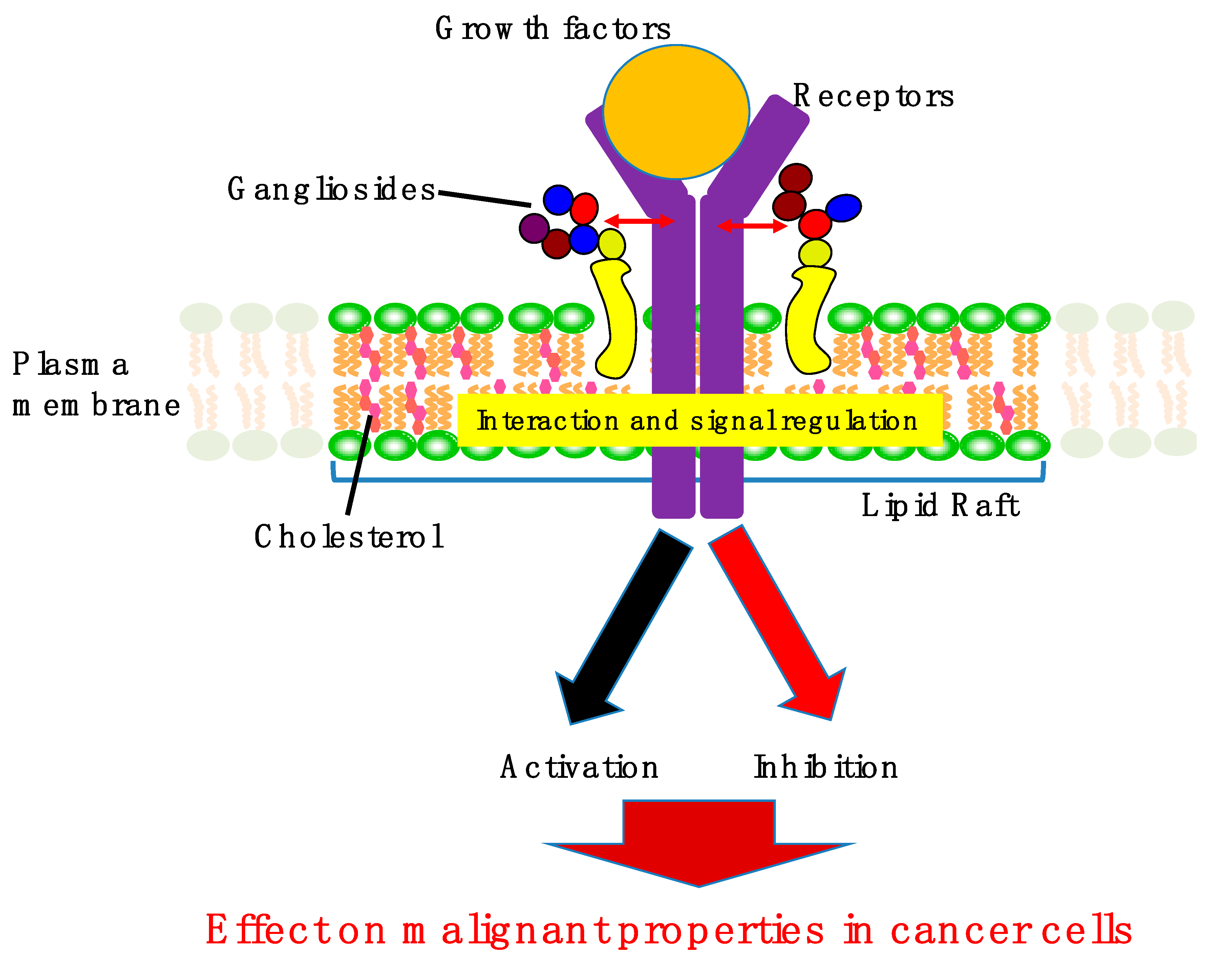
2. Involvement of Gangliosides in Cancer Cell Signaling
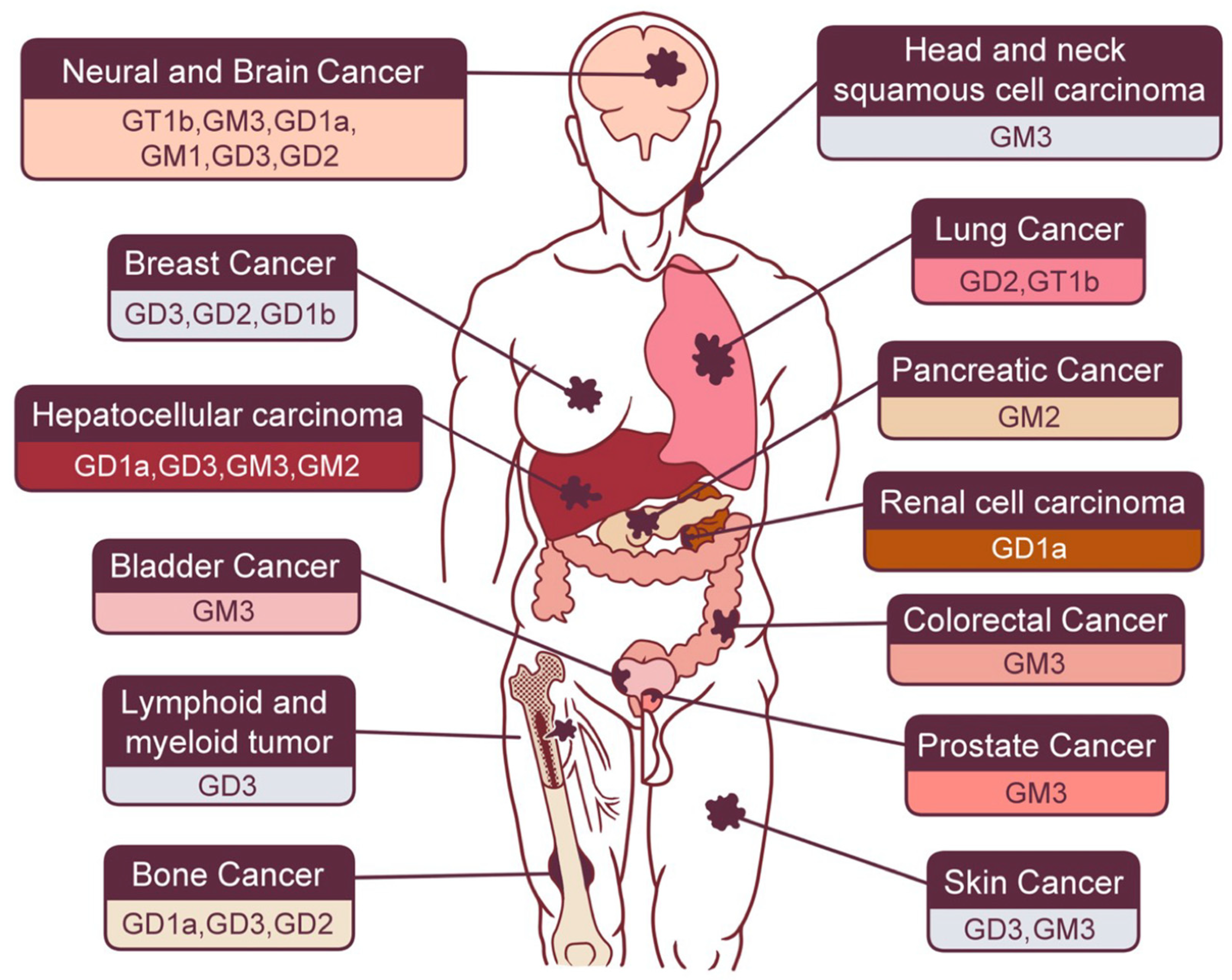
2.1. Gastrointestinal Cancers
2.1.1. Hepatocellular Carcinoma (HCC)
2.1.2. Pancreatic Cancer
2.1.3. Colorectal Cancer
2.2. Neural and Brain Cancer
2.3. Skin Cancer
2.4. Sex Hormone-Related Cancer
2.5. Bone Cancer
2.6. Lung Cancer
2.7. Renal Urinary Cancer
2.8. Other Types of Cancer
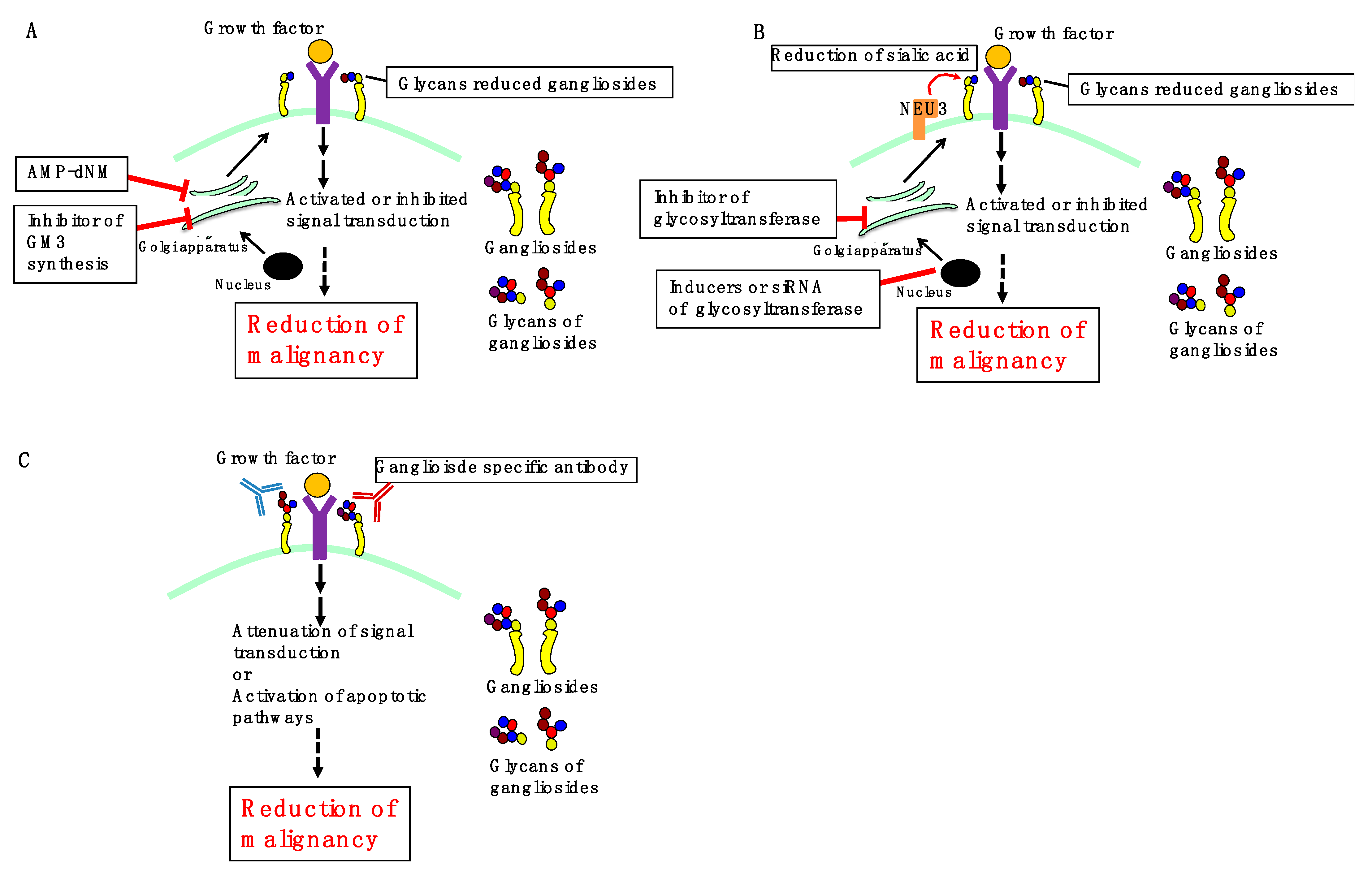
3. Perspectives
3.1. Inhibition of Ganglioside Synthesis
3.2. Regulation of Ganglioside Expression
3.3. Ganglioside-Specific Antibodies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Sonnino, S.; Chiricozzi, E.; Grassi, S.; Mauri, L.; Prioni, S.; Prinetti, A. Gangliosides in membrane organization. Prog. Mol. Biol. Transl. Sci. 2018, 156, 83–120. [Google Scholar] [CrossRef]
- Zhuo, D.; Guan, F. Ganglioside GM1 promotes contact inhibition of growth by regulating the localization of epidermal growth factor receptor from glycosphingolipid-enriched microdomain to caveolae. Cell Prolif. 2019, 52, e12639. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Gangliosides contribute to vascular insulin resistance. Int. J. Mol. Sci. 2019, 20, 1819. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. Ganglioside designation. Adv. Exp. Med. Biol. 1980, 125, 11. [Google Scholar] [CrossRef]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The double-edge sword of neuro-ectodermal derived tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Balmaña, M.; Macedo, J.A.; Poças, J.; Fernandes, Â.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhães, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell. Immunol. 2018, 333, 46–57. [Google Scholar] [CrossRef]
- Bremer, E.G.; Schlessinger, J.; Hakomori, S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 1986, 261, 2434–2440. [Google Scholar] [CrossRef]
- Russo, D.; Parashuraman, S.; D’Angelo, G. Glycosphingolipid-protein interaction in signal transduction. Int. J. Mol. Sci. 2016, 17, 1732. [Google Scholar] [CrossRef] [PubMed]
- Gajate, C.; Mollinedo, F. Lipid rafts and raft-mediated supramolecular entities in the regulation of CD95 death receptor apoptotic signaling. Apoptosis 2015, 20, 584–606. [Google Scholar] [CrossRef]
- De Maria, R.D.; Lenti, L.; Malisan, F.; d’Agostino, F.; Tomassini, B.; Zeuner, A.; Rippo, M.R.; Testi, R. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science 1997, 277, 1652–1655. [Google Scholar] [CrossRef]
- Giammarioli, A.M.; Garofalo, T.; Sorice, M.; Misasi, R.; Gambardella, L.; Gradini, R.; Fais, S.; Pavan, A.; Malorni, W. GD3 glycosphingolipid contributes to Fas-mediated apoptosis via association with ezrin cytoskeletal protein. FEBS Lett. 2001, 506, 45–50. [Google Scholar] [CrossRef]
- Hyuga, S.; Kawasaki, N.; Hyuga, M.; Ohta, M.; Shibayama, R.; Kawanishi, T.; Yamagata, S.; Yamagata, T.; Hayakawa, T. Ganglioside GD1a inhibits HGF-induced motility and scattering of cancer cells through suppression of tyrosine phosphorylation of c-Met. Int. J. Cancer 2001, 94, 328–334. [Google Scholar] [CrossRef]
- Paris, R.; Morales, A.; Coll, O.; Sánchez-Reyes, A.; García-Ruiz, C.; Fernández-Checa, J.C. Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J. Biol. Chem. 2002, 277, 49870–49876. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zhang, J.; Li, Y.; Ma, K. Synergistic inhibition of cell migration by tetraspanin CD82 and gangliosides occurs via the EGFR or cMet-activated Pl3K/Akt signalling pathway. Int. J. Biochem. Cell Biol. 2013, 45, 2349–2358. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zhong, W.; Zhang, J.; Ma, K. Ganglioside GM3 promotes HGF-stimulated motility of murine hepatoma cell through enhanced phosphorylation of cMet at specific tyrosine sites and PI3K/Akt-mediated migration signaling. Mol. Cell. Biochem. 2013, 382, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Wang, C.; Li, Y.; Luan, M.; Ma, K. Ganglioside GM3 exerts opposite effects on motility via epidermal growth factor receptor and hepatocyte growth factor receptor-mediated migration signaling. Mol. Med. Rep. 2015, 11, 2959–2966. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Zhang, J.; Xu, Y.; Tian, Y.; Ma, K. Ganglioside GM3 inhibits hepatoma cell motility via down-regulating activity of EGFR and PI3K/AKT signaling pathway. J. Cell. Biochem. 2013, 114, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Hirabayashi, K.; Michishita, M.; Takahashi, K.; Hasegawa, F.; Gomi, F.; Itakura, Y.; Nakamura, N.; Toyoda, M.; Ishiwata, T. Ganglioside GM2, highly expressed in the MIA PaCa-2 pancreatic ductal adenocarcinoma cell line, is correlated with growth, invasion, and advanced stage. Sci. Rep. 2019, 9, 19369. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Chung, T.W.; Kang, S.K.; Lee, Y.C.; Ko, J.H.; Kim, J.G.; Kim, C.H. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology 2006, 16, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Choi, H.J.; Kim, S.J.; Kwak, C.H.; Song, K.H.; Jin, U.H.; Chang, Y.C.; Chang, H.W.; Lee, Y.C.; Ha, K.T.; et al. The ganglioside GM3 is associated with cisplatin-induced apoptosis in human colon cancer cells. PLoS ONE 2014, 9, e92786. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hosono, M.; Sato, I.; Hata, K.; Wada, T.; Yamaguchi, K.; Nitta, K.; Shima, H.; Miyagi, T. Sialidase NEU3 contributes neoplastic potential on colon cancer cells as a key modulator of gangliosides by regulating Wnt signaling. Int. J. Cancer 2015, 137, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, B.L.; Clark, S.H.; Zhang, C. Inhibition of human neuroblastoma cell proliferation and EGF receptor phosphorylation by gangliosides GM1, GM3, GD1A and GT1B. Cell Prolif. 2002, 35, 105–115. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Biase, E.D.; Maggioni, M.; Lunghi, G.; Fazzari, M.; Pomè, D.Y.; Casellato, R.; Loberto, N.; Mauri, L.; Sonnino, S. GM1 promotes TrkA-mediated neuroblastoma cell differentiation by occupying a plasma membrane domain different from TrkA. J. Neurochem. 2019, 149, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, T.; Furukawa, K.; Fukumoto, S.; Miyazaki, H.; Urano, T.; Furukawa, K. Overexpression of ganglioside GM1 results in the dispersion of platelet-derived growth factor receptor from glycolipid-enriched microdomains and in the suppression of cell growth signals. J. Biol. Chem. 2002, 277, 11239–11246. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, Y.; Momota, H.; Kato, A.; Hashimoto, N.; Tsuda, Y.; Kotani, N.; Honke, K.; Suzumura, A.; Furukawa, K.; Ohmi, Y.; et al. Ganglioside GD3 enhances invasiveness of gliomas by forming a complex with platelet-derived growth factor receptor α and yes kinase. J. Biol. Chem. 2015, 290, 16043–16058. [Google Scholar] [CrossRef]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.H.; Furukawa, K.; Furukawa, K. Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Hamamura, K.; Tsuji, M.; Hotta, H.; Ohkawa, Y.; Takahashi, M.; Shibuya, H.; Nakashima, H.; Yamauchi, Y.; Hashimoto, N.; Hattori, H.; et al. Functional activation of Src family kinase yes protein is essential for the enhanced malignant properties of human melanoma cells expressing ganglioside GD3. J. Biol. Chem. 2011, 286, 18526–18537. [Google Scholar] [CrossRef]
- Furukawa, K.; Kambe, M.; Miyata, M.; Ohkawa, Y.; Tajima, O.; Furukawa, K. Ganglioside GD3 induces convergence and synergism of adhesion and hepatocyte growth factor/Met signals in melanomas. Cancer Sci. 2014, 105, 52–63. [Google Scholar] [CrossRef]
- Makino, Y.; Hamamura, K.; Takei, Y.; Bhuiyan, R.H.; Ohkawa, Y.; Ohmi, Y.; Nakashima, H.; Furukawa, K.; Furukawa, K. A therapeutic trial of human melanomas with combined small interfering RNAs targeting adaptor molecules p130Cas and paxillin activated under expression of ganglioside GD3. Biochim. Biophys. Acta 2016, 1860, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Nishimiya, Y.; Takahata, S.; Nakayama, K.I. Induction of glycosphingolipid GM3 expression by valproic acid suppresses cancer cell growth. J. Biol. Chem. 2016, 291, 21424–21433. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.; Bobowski, M.; Rombouts, Y.; Lefebvre, J.; Steenackers, A.; Popa, I.; Guérardel, Y.; Le Bourhis, X.L.; Tulasne, D.; Delannoy, P. The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 2012, 22, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Wang, C.Y.; Wang, I.A.; Chen, Y.W.; Li, L.T.; Lin, C.Y.; Ho, M.Y.; Chou, T.L.; Wang, Y.H.; Chiou, S.P.; et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget 2017, 8, 47454–47473. [Google Scholar] [CrossRef]
- Nguyen, K.; Yan, Y.; Yuan, B.; Dasgupta, A.; Sun, J.; Mu, H.; Do, K.A.; Ueno, N.T.; Andreeff, M.; Battula, V.L. ST8SIA1 regulates tumor growth and metastasis in TNBC by activating the FAK-AKT-mTOR signaling pathway. Mol. Cancer Ther. 2018, 17, 2689–2701. [Google Scholar] [CrossRef]
- Ha, S.H.; Lee, J.M.; Kwon, K.M.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Cho, S.H.; Lee, K.; Chang, Y.C.; Lee, Y.C.; et al. Exogenous and endogeneous disialosyl ganglioside GD1b induces apoptosis of MCF-7 human breast cancer cells. Int. J. Mol. Sci. 2016, 17, 652. [Google Scholar] [CrossRef]
- Kawamura, S.; Sato, I.; Wada, T.; Yamaguchi, K.; Li, Y.; Li, D.; Zhao, X.; Ueno, S.; Aoki, H.; Tochigi, T.; et al. Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell Death Differ. 2012, 19, 170–179. [Google Scholar] [CrossRef]
- Shibuya, H.; Hamamura, K.; Hotta, H.; Matsumoto, Y.; Nishida, Y.; Hattori, H.; Furukawa, K.; Ueda, M.; Furukawa, K. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012, 103, 1656–1664. [Google Scholar] [CrossRef]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar]
- Aixinjueluo, W.; Furukawa, K.; Zhang, Q.; Hamamura, K.; Tokuda, N.; Yoshida, S.; Ueda, R.; Furukawa, K. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: Roles of anoikis. J. Biol. Chem. 2005, 280, 29828–29836. [Google Scholar] [CrossRef]
- Hwang, J.H.; Sung, J.S.; Kim, J.M.; Chung, Y.H.; Park, J.S.; Lee, S.H.; Jang, I.S. Caveolin-1-dependent and -independent uPAR signaling pathways contribute to ganglioside GT1b induced early apoptosis in A549 lung cancer cells. Am. J. Cancer Res. 2014, 4, 801–810. [Google Scholar]
- Tringali, C.; Lupo, B.; Silvestri, I.; Papini, N.; Anastasia, L.; Tettamanti, G.; Venerando, B. The plasma membrane sialidase NEU3 regulates the malignancy of renal carcinoma cells by controlling β1 integrin internalization and recycling. J. Biol. Chem. 2012, 287, 42835–42845. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Isaji, T.; Satoh, M.; Li, D.; Arai, Y.; Gu, J. Antitumor effects of exogenous ganglioside GM3 on bladder cancer in an orthotopic cancer model. Urology 2013, 81, 210.e11–210.e15. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Takahashi, K.; Sato, I.; Kato, K.; Saijo, S.; Moriya, S.; Hosono, M.; Miyagi, T. Upregulation of sialidase NEU3 in head and neck squamous cell carcinoma associated with lymph node metastasis. Cancer Sci. 2015, 106, 1544–1553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, K.D.; Hu, M.Y.; Zhou, K. SF/HGF-c-Met autocrine and paracrine promote metastasis of hepatocellular carcinoma. World J. Gastroenterol. 2001, 7, 816–820. [Google Scholar] [CrossRef]
- Wang, H.; Rao, B.; Lou, J.; Li, J.; Liu, Z.; Li, A.; Cui, G.; Ren, Z.; Yu, Z. The function of the HGF/c-met axis in hepatocellular carcinoma. Front. Cell Dev. Biol. 2020, 8, 55. [Google Scholar] [CrossRef]
- Park, S.-Y.; Yoon, S.-J.; Freire-de-Lima, L.; Kim, J.-H.; Hakomori, S.-I. Control of cell motility by interaction of gangliosides, tetraspanins, and epidermal growth factor receptor in A431 versus KB epidermoid tumor cells. Carbohydr. Res. 2009, 344, 1479–1486. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Melzer, C.; Hass, R.; von der Ohe, J.; Lehnert, H.; Ungefroren, H. The role of TGF-β and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun. Signal. 2017, 15, 19. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Shichi, Y.; Sasaki, N.; Michishita, M.; Hasegawa, F.; Matsuda, Y.; Arai, T.; Gomi, F.; Aida, J.; Takubo, K.; Toyoda, M.; et al. Enhanced morphological and functional differences of pancreatic cancer with epithelial or mesenchymal characteristics in 3D culture. Sci. Rep. 2019, 9, 10871. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Hosoi, H.; Kuwahara, Y.; Kikuchi, K.; Otabe, O.; Izumi, M.; Tsuchiya, K.; Iehara, T.; Gotoh, T.; Sugimoto, T. Induction of apoptosis by an inhibitor of EGFR in neuroblastoma cells. Biochem. Biophys. Res. Commun. 2007, 358, 226–232. [Google Scholar] [CrossRef]
- Ho, R.; Minturn, J.E.; Hishiki, T.; Zhao, H.; Wang, Q.; Cnaan, A.; Maris, J.; Evans, A.E.; Brodeur, G.M. Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res. 2005, 65, 9868–9875. [Google Scholar] [CrossRef]
- da Motta, L.A.; Galli, P.; Piva, F.; Maggi, R. Effects of epidermal growth factor on the, 3H]-thymidine uptake in the SK-N-SH and SH-SY5Y human neuroblastoma cell lines. Arq. Neuro Psiquiatr. 1997, 55, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, A.N.; Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef]
- Lokker, N.A.; Sullivan, C.M.; Hollenbach, S.J.; Israel, M.A.; Giese, N.A. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002, 62, 3729–3735. [Google Scholar] [PubMed]
- Lind, C.R.P.; Gray, C.W.; Pearson, A.G.; Cameron, R.E.; O’Carroll, S.J.; Narayan, P.J.; Lim, J.; Dragunow, M. The mitogen-activated/extracellular signal-regulated kinase kinase 1/2 inhibitor U0126 induces glial fibrillary acidic protein expression and reduces the proliferation and migration of C6 glioma cells. Neuroscience 2006, 141, 1925–1933. [Google Scholar] [CrossRef]
- Petterson, S.A.; Dahlrot, R.H.; Hermansen, S.K.; Munthe, S.K.A.; Gundesen, M.T.; Wohlleben, H.; Rasmussen, T.; Beier, C.P.; Hansen, S.; Kristensen, B.W. High levels of c-Met is associated with poor prognosis in glioblastoma. J. Neurooncol. 2015, 122, 517–527. [Google Scholar] [CrossRef]
- Furue, M.; Ito, T.; Wada, N.; Wada, M.; Kadono, T.; Uchi, H. Melanoma and immune checkpoint inhibitors. Curr. Oncol. Rep. 2018, 20, 29. [Google Scholar] [CrossRef]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar]
- Lugović-Mihić, L.; Ćesić, D.; Vuković, P.; Novak Bilić, G.N.; Šitum, M.; Špoljar, S. Melanoma development: Current knowledge on melanoma pathogenesis. Acta Dermatovenerol. Croat. 2019, 27, 163–168. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Dawson, S.J.; Provenzano, E.; Caldas, C. Triple negative breast cancers: Clinical and prognostic implications. Eur. J. Cancer 2009, 45 (Suppl. 1), 27–40. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Hui, Y.; Zhang, H.; Sun, Y.; Liu, X.; Zhao, X.; Zhao, Z.; Yang, Q.; Wang, F.; et al. Clinicopathological and prognostic significance of c-Met overexpression in breast cancer. Oncotarget 2017, 8, 56758–56767. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The osteosarcoma microenvironment: A complex but targetable ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef]
- Coltella, N.; Manara, M.C.; Cerisano, V.; Trusolino, L.; Di Renzo, M.F.D.; Scotlandi, K.; Ferracini, R. Role of the MET/HGF receptor in proliferation and invasive behavior of osteosarcoma. FASEB J. 2003, 17, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Lu, X.; Yao, N.; Chen, Y.; Yang, A.; Chen, H.; Zhang, J.; Wu, S.; Shi, X.; Wang, C.; et al. Focal adhesion kinase overexpression and its impact on human osteosarcoma. Oncotarget 2015, 6, 31085–31103. [Google Scholar] [CrossRef]
- Byers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef]
- Deng, W.; Li, R.; Ladisch, S. Influence of cellular ganglioside depletion on tumor formation. J. Natl. Cancer Inst. 2000, 92, 912–917. [Google Scholar] [CrossRef][Green Version]
- Aerts, J.M.; Ottenhoff, R.; Powlson, A.S.; Grefhorst, A.; van Eijk, M.V.; Dubbelhuis, P.F.; Aten, J.; Kuipers, F.; Serlie, M.J.; Wennekes, T.; et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 2007, 56, 1341–1349. [Google Scholar] [CrossRef]
- van Eijk, M.; Aten, J.; Bijl, N.; Ottenhoff, R.; van Roomen, C.P.; Dubbelhuis, P.F.; Seeman, I.; Ghauharali-van der Vlugt, K.; Overkleeft, H.S.; Arbeeny, C.; et al. Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS ONE 2009, 4, e4723. [Google Scholar] [CrossRef][Green Version]
- Yu, Z.; Peng, Q.; Huang, Y. Potential therapeutic targets for atherosclerosis in sphingolipid metabolism. Clin. Sci. (Lond.) 2019, 133, 763–776. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Kim, S.J.; Kim, C.H.; Son, S.W.; Kim, K.S.; Lee, J.H.; Do, S.I.; Lee, Y.C. Triptolide downregulates human GD3 synthase (hST8Sia I) gene expression in SK-MEL-2 human melanoma cells. Exp. Mol. Med. 2010, 42, 849–855. [Google Scholar] [CrossRef]
- Sarkar, T.R.; Battula, V.L.; Werden, S.J.; Vijay, G.V.; Ramirez-Peña, E.Q.; Taube, J.H.; Chang, J.T.; Miura, N.; Porter, W.; Sphyris, N.; et al. GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene 2015, 34, 2958–2967. [Google Scholar] [CrossRef]
- Kakugawa, Y.; Wada, T.; Yamaguchi, K.; Yamanami, H.; Ouchi, K.; Sato, I.; Miyagi, T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl. Acad. Sci. USA 2002, 99, 10718–10723. [Google Scholar] [CrossRef]
- Doronin, I.I.; Vishnyakova, P.A.; Kholodenko, I.V.; Ponomarev, E.D.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014, 14, 295. [Google Scholar] [CrossRef]
- Tsao, C.Y.; Sabbatino, F.; Cheung, N.K.V.; Hsu, J.C.F.; Villani, V.; Wang, X.; Ferrone, S. Anti-proliferative and pro-apoptotic activity of GD2 ganglioside-specific monoclonal antibody 3F8 in human melanoma cells. Oncoimmunology 2015, 4, e1023975. [Google Scholar] [CrossRef]
- Durbas, M.; Horwacik, I.; Boratyn, E.; Kamycka, E.; Rokita, H. GD2 ganglioside specific antibody treatment downregulates PI3K/Akt/mTOR signaling network in human neuroblastoma cell lines. Int. J. Oncol. 2015, 47, 1143–1159. [Google Scholar] [CrossRef]
- Furman, W.L.; Shulkin, B.L.; Federico, S.M.; McCarville, M.B.; Davidoff, A.M.; Krasin, M.J.; Wu, J.; Brennan, R.C.; Bishop, M.W.; Helmig, S.E.; et al. Early response rates and Curie scores at end of induction: An update from a phase II study of an anti-GD2 monoclonal antibody (mAb) with chemotherapy (CT) in newly diagnosed patients (pts) with high-risk (HR) neuroblastoma (NB). J. Clin. Oncol. 2017, 35, 10534. [Google Scholar] [CrossRef]
- Perez Horta, Z.P.; Goldberg, J.L.; Sondel, P.M. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy 2016, 8, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Doronin, I.I.; Kholodenko, I.V.; Zubareva, A.A.; Yarygin, K.N.; Deev, S.M.; Kholodenko, R.V. Involvement of actin filaments in the cytotoxic effect of GD2-specific antibodies. Bull. Exp. Biol. Med. 2019, 166, 541–547. [Google Scholar] [CrossRef]
- Zhu, W.; Mao, X.; Wang, W.; Chen, Y.; Li, D.; Li, H.; Dou, P. Anti-ganglioside GD2 monoclonal antibody synergizes with cisplatin to induce endoplasmic reticulum-associated apoptosis in osteosarcoma cells. Pharmazie 2018, 73, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Bando, H.; Takeuchi, S.; Kita, K.; Li, Q.; Wang, W.; Akinaga, S.; Nishioka, Y.; Sone, S.; Yano, S. Genetically engineered humanized anti-ganglioside GM2 antibody against multiple organ metastasis produced by GM2-expressing small-cell lung cancer cells. Cancer Sci. 2011, 102, 2157–2163. [Google Scholar] [CrossRef]
- He, D.; Fan, X.; Liu, B.; Tian, Y.; Zhang, X.; Kang, L.; Tai, Y.; Liu, S.; Wang, Q.; Li, Q. Generation and characterization of a IgG monoclonal antibody specific for GM3 (NeuGc) ganglioside by immunizing β3Gn-T5 knockout mice. Sci. Rep. 2018, 8, 2561. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Morrisey, D.; Panageas, K.S.; Williams, L.; Lewis, J.J.; Israel, R.J.; Hamilton, W.B.; Livingston, P.O. Vaccination with a bivalent G(M2) and G(D2) ganglioside conjugate vaccine: A trial comparing doses of G(D2)-keyhole limpet hemocyanin. Clin. Cancer Res. 2000, 6, 4658–4662. [Google Scholar]
- Ragupathi, G.; Livingston, P.O.; Hood, C.; Gathuru, J.; Krown, S.E.; Chapman, P.B.; Wolchok, J.D.; Williams, L.J.; Oldfield, R.C.; Hwu, W.-J. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin. Cancer Res. 2003, 9, 5214–5220. [Google Scholar] [PubMed]
- Myrianthopoulos, V.; Evangelou, K.; Vasileiou, P.V.S.; Cooks, T.; Vassilakopoulos, T.P.; Pangalis, G.A.; Kouloukoussa, M.; Kittas, C.; Georgakilas, A.G.; Gorgoulis, V.G. Senescence and senotherapeutics: A new field in cancer therapy. Pharmacol. Ther. 2019, 193, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Gomi, F.; Yoshimura, H.; Yamamoto, M.; Matsuda, Y.; Michishita, M.; Hatakeyama, H.; Kawano, Y.; Toyoda, M.; Korc, M.; et al. FGFR4 inhibitor BLU9931 attenuates pancreatic cancer cell proliferation and invasion while inducing senescence: Evidence for senolytic therapy potential in pancreatic cancer. Cancers 2020, 12, 2976. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
| Types of Cancer | Types of Cells | Types of Gangliosides | Functional Roles | References |
|---|---|---|---|---|
| Hepatocellular Carcinoma | human hepatoma HepG2 | GD1a | Inhibition of HGF/c-Met signaling | [13] |
| human hepatoma HepG2 | GD3 | Inhibition of NF-kB signaling | [14] | |
| mouse hepatocellular carcinoma Hepa1-6 | GM3 or GM2 and GM3 | Inhibition of HGF signaling in CD82-upregulated cells | [15] | |
| mouse hepatic cancer cell lines (Hca/A2, Hca/16A3, and Hepa1-6) | GM3 | Promotion of HGF/c-Met and PI3K/Akt signaling | [16,17] | |
| mouse hepatocellular carcinoma Hepa1-6 | GM3 | Inhibition of EGF signaling | [17] | |
| mouse ascites hepatoma cell line HcaF | GM3 | Inhibition of phosphorylation of Akt and EGFR | [18] | |
| Pancreatic Cancer | human pancreatic cancer MIA PaCa-2 | GM2 | Promotion of TGF-b1 signaling | [19] |
| Colorectal Cancer | human colon cancer HCT116 | GM3 | Inhibition of the PI3K/Akt/MDM2 signaling | [20] |
| human colon cancer HCT116 | GM3 | Promotion of oxidative stress-mediated mitochondrial pathway | [21] | |
| human colon cancer HCT116 and HT-29 | GM3 | Inhibition of Wnt/b-catenin signaling | [22] | |
| Neural and Brain Cancer | human neuroblastoma cell line NBL-W | GT1b, GM3, or GD1a | Inhibition of EGF signaling | [23] |
| neuroblastoma | GM1 | Activation of the TrkA receptor | [24] | |
| human glioma | GM1 | Inhibition of PDGF signaling | [25] | |
| human glioma | GD3 | Promotion of PDGF signaling | [26] | |
| glioma cell line U-251MG | GD3 and GD2 | Promotion of ERK1/2 and Akt pathway | [27] | |
| glioblastoma multiform cell line | GD3 | Promotion of c-Met signaling | [28] | |
| Skin Cancer | human melanoma | GD3 | Activation of Src family kinase | [29] |
| human melanoma | GD3 | Promotion of HGF signaling | [30] | |
| human melanoma | GD3 | Promotion of p130Cas and paxillin pathway | [31] | |
| human epidermoid carcinoma A431 | GM3 | Inhibition of EGF signaling | [32] | |
| Sex Hormone-Related Cancer | breast cancer cell MDA-MB231 | GD2 | Promotion of c-Met signaling | [33] |
| breast cancer cell MDA-MB468 | GD3 | Promotion of EGF signaling | [34] | |
| triple-negative breast cancer | GD2 | Promotion of FAK-Akt-mTOR signaling | [35] | |
| human breast cancer MCF-7 | GD1b | Activation of apoptotic pathway | [36] | |
| prostate cancer PC-3 and LNCaP | GM3 | Inhibition of EGF signaling | [37] | |
| Bone Cancer | mouse osteosarcoma cell variant FBJ-LL | GD1a | Inhibition of HGF/c-Met signaling | [13] |
| human osteosarcoma | GD3 and GD2 | Promotion of p130Cas, FAK and paxillin pathway | [38] | |
| Lung Cancer | small cell lung cancer | GD2 | Inhibition of growth and induction of apoptosis by anti-GD2 mAb | [39] |
| small cell lung cancer | GD2 | Promotion of FAK pathway | [40] | |
| A549 lung adenocarcinoma | GT1b | Inhibition of fibronectin-a5b1-integrin-ERK signaling | [41] | |
| Renal Urinary Cancer | human renal cell carcinoma cell | GD1a | Inhibition of the FAK/Akt signaling | [42] |
| human bladder cancer YTS-1, T24, 5637, and KK47 | GM3 | Inhibition of EGF signaling | [43] | |
| Other Types of Cancer | lymphoid and myeloid tumor cells | GD3 | Activation of CD95-mediated apoptotic pathway | [11] |
| squamous carcinoma HSC-2 and SAS | GM3 | Inhibition of EGF signaling | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, N.; Toyoda, M.; Ishiwata, T. Gangliosides as Signaling Regulators in Cancer. Int. J. Mol. Sci. 2021, 22, 5076. https://doi.org/10.3390/ijms22105076
Sasaki N, Toyoda M, Ishiwata T. Gangliosides as Signaling Regulators in Cancer. International Journal of Molecular Sciences. 2021; 22(10):5076. https://doi.org/10.3390/ijms22105076
Chicago/Turabian StyleSasaki, Norihiko, Masashi Toyoda, and Toshiyuki Ishiwata. 2021. "Gangliosides as Signaling Regulators in Cancer" International Journal of Molecular Sciences 22, no. 10: 5076. https://doi.org/10.3390/ijms22105076
APA StyleSasaki, N., Toyoda, M., & Ishiwata, T. (2021). Gangliosides as Signaling Regulators in Cancer. International Journal of Molecular Sciences, 22(10), 5076. https://doi.org/10.3390/ijms22105076







